Introduction
Chromosomal translocations resulting in gene fusions
are one mechanism underlying tumorigenesis, and some are more
frequent in certain cancer entities. The Ewing sarcoma breakpoint
region 1 gene (EWSR1), found on chromosome 22q12.2, has a
tendency to fuse with the transcription factor cyclic AMP response
element binding (CREB) family genes like CREB1, cAMP
response element modulator (CREM), or activating
transcription factor 1 (ATF1) (1,2). A
group of neoplasms are associated with EWSR1-CREB1 and/or
EWSR1-ATF1 gene fusions, including angiomatoid fibrous
histiocytoma (AFH) but also, clear cell sarcoma, clear cell
sarcoma-like tumor of the gastrointestinal tract, primary pulmonary
myxoid sarcoma, hyalinizing clear cell carcinoma of the salivary
gland, and soft tissue myoepithelial tumor (3). AFH is a rare soft tissue tumor
described initially as ‘angiomatoid malignant fibrous histiocytoma’
by Enzinger in 1979(4). It is now
described as an indolent tumor with a favorable prognosis by the
2013 World Health Organization classification (5). It is a rare tumor of soft tissue
(<0.5%) and mostly occurs superficially, in the extremities of
children and young adults (6). Most
AFHs are indolent, with a 15 percent regional recurrence rate and a
metastatic rate <5%, most frequently involving regional nodes
(7). Pathological diagnostic of AHF
remain difficult, especially in other sites than skin. As
immunohistochemical phenotype is not specific, molecular analysis
is useful to confirm the diagnosis and distinguish this entity from
likeness tumors. AFH is associated with 3 characteristic
translocations: t(2;22)(q33;q12) EWSR1/CREB1 being the most
common, t(12;22)(q13;q12) EWSR1/ATF1, and t(12;16)(q13;p11)
FUS/ATF1 (3,8). The intracranial location represents a
rare primary site, with six conventional AFH cases reported
(9-13)
and fifteen myxoid AFH (14-23)
described as a novel tumor entity. The EWSR1/CREB1 fusion is
reported in the myxoid variant but never in the classical
non-myxoid component. None of the patients received chemotherapy
for this lesion. Herein we describe a case of a classical
intracranial non-myxoid AFH with ESWR1-CREB1 transcript
fusion treated with doxorubicin as a single agent chemotherapy,
inducing a prolonged stable disease fifteen months after treatment
discontinuation.
Case report
Clinical history and histological
findings
A 40-year-old male referred to the emergency
department in 2012 for an intracranial hypertension syndrome with
headaches and diplopia. Otherwise, his personal and familial
medical clinical history was unremarkable. The magnetic resonance
imaging (MRI) of the brain revealed a suspicious lesion of the
splenium of the corpus callosum and the pineal region, with
hypointense T1 signal, hyperintense T2 signal, and with strong
enhancement following gadolinium administration. The lesion was
associated with bi-parietal edema. Large increase in lipid and
choline without apparent necrosis were showed on spectrometry.
There was no sign of neoangiogenesis. The original diagnosis was
thought to be lymphoma. He had three needle biopsies between 2012
and 2014 but none of them confirmed this diagnosis. He was then
started on corticosteroids but the symptoms got worse with
seizures, papillary edema, right homonymous hemianopsia, and
agnostic alexia requiring a ventriculoperitoneal shunt. Finally, in
March 2014, the patient underwent a left parieto-occipital
craniotomy and a subtotal (60%) resection (Fig. 1). The surgical approach was decided
because of the retrospenial origin of the tumor and the inferior
repulse of the deep venous system. Postoperatively, the patient
maintained a right homonymous hemianopsia. He was started on
radiation therapy for a total planned dose of 36 grays distributed
into 20 sessions, but stopped after 12 sessions for his convenience
(i.e. radiotherapy risks and consequences). Chemotherapy was not
added to the treatment because of the lack of evidence of its
benefits in this case.
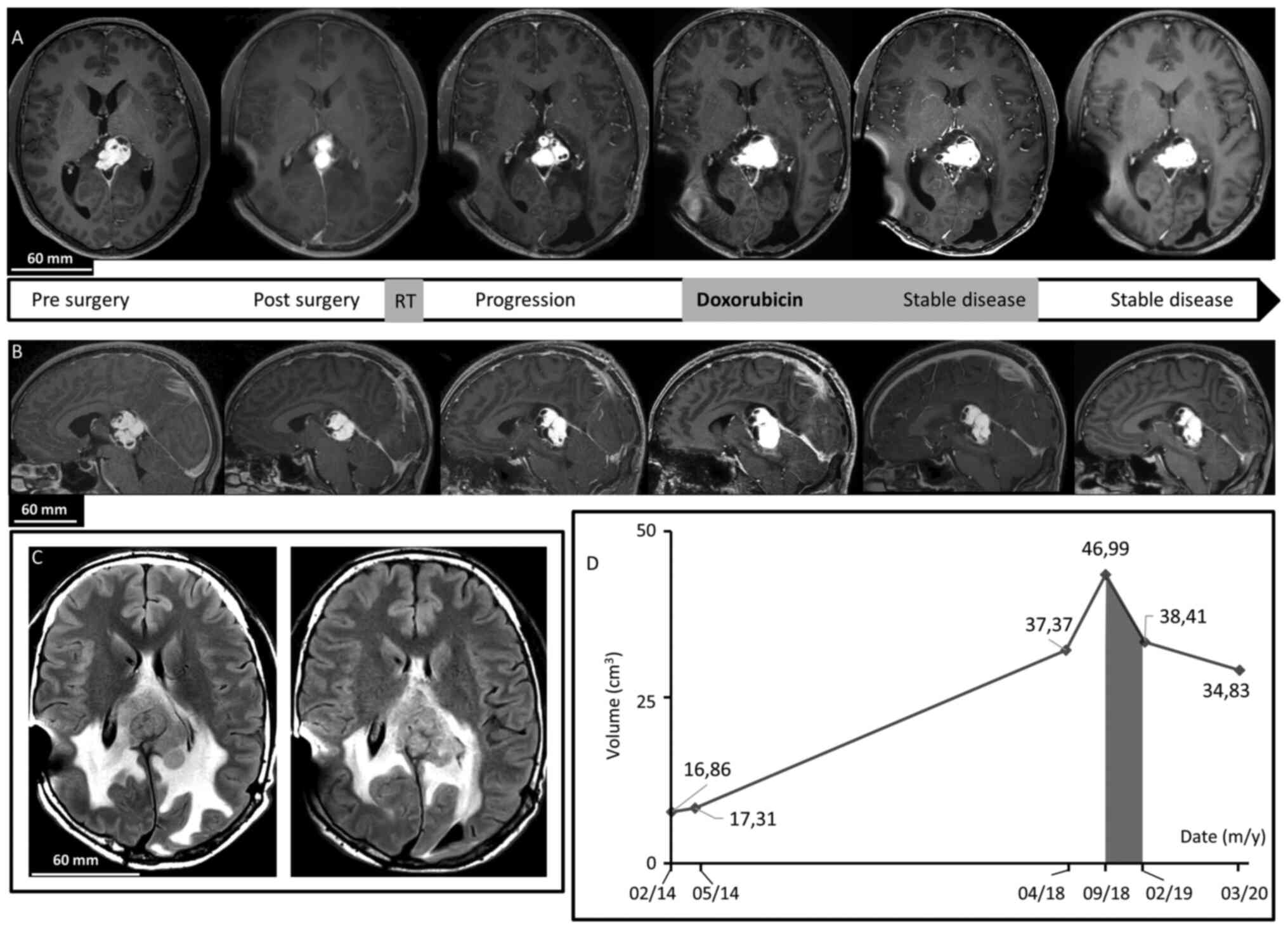 | Figure 1Brain MRI. (A) Axial after gadolinium
injection T1-weighted imaging revealed dominant, patchy intense
enhancing lesion of the splenium of the corpus callosum and the
pineal region. In chronological order: Before surgery, after
surgery showing residual tumor, 4 years after surgery demonstrating
progression of the disease, at the beginning of Doxorubicin regimen
treatment, just after treatment discontinuation confirming <50%
decrease in tumor size, and last follow-up 15 months after
treatment discontinuation demonstrating a stable disease. Note the
cystic component. (B) Sagittal after gadolinium injection
T1-weighted imaging revealed dominant, patchy intense enhancing
lesion of the splenium of the corpus callosum and the pineal region
before surgery, after surgery, 4 years after surgery, at the
beginning of Doxorubicin treatment, after treatment, and at last
follow-up. (C) Axial FLAIR T2-weighted images revealing peritumoral
edema by large heterogeneous hyperintense signal before treatment
with Doxorubicin and at last follow-up. (D) Diagram of evolution of
tumor volume on MRI revealed a decrease during and after the
Doxorubicin period (grey). |
From tumor specimens obtained after fine needle
biopsy and after surgery, several formalin-zinc fixed
paraffin-embedded (FFPE) blocks and Hematoxylin-Eosin-Saffron
stained slides were submitted to histological examination after
obtaining written informed and signed consent. It revealed a
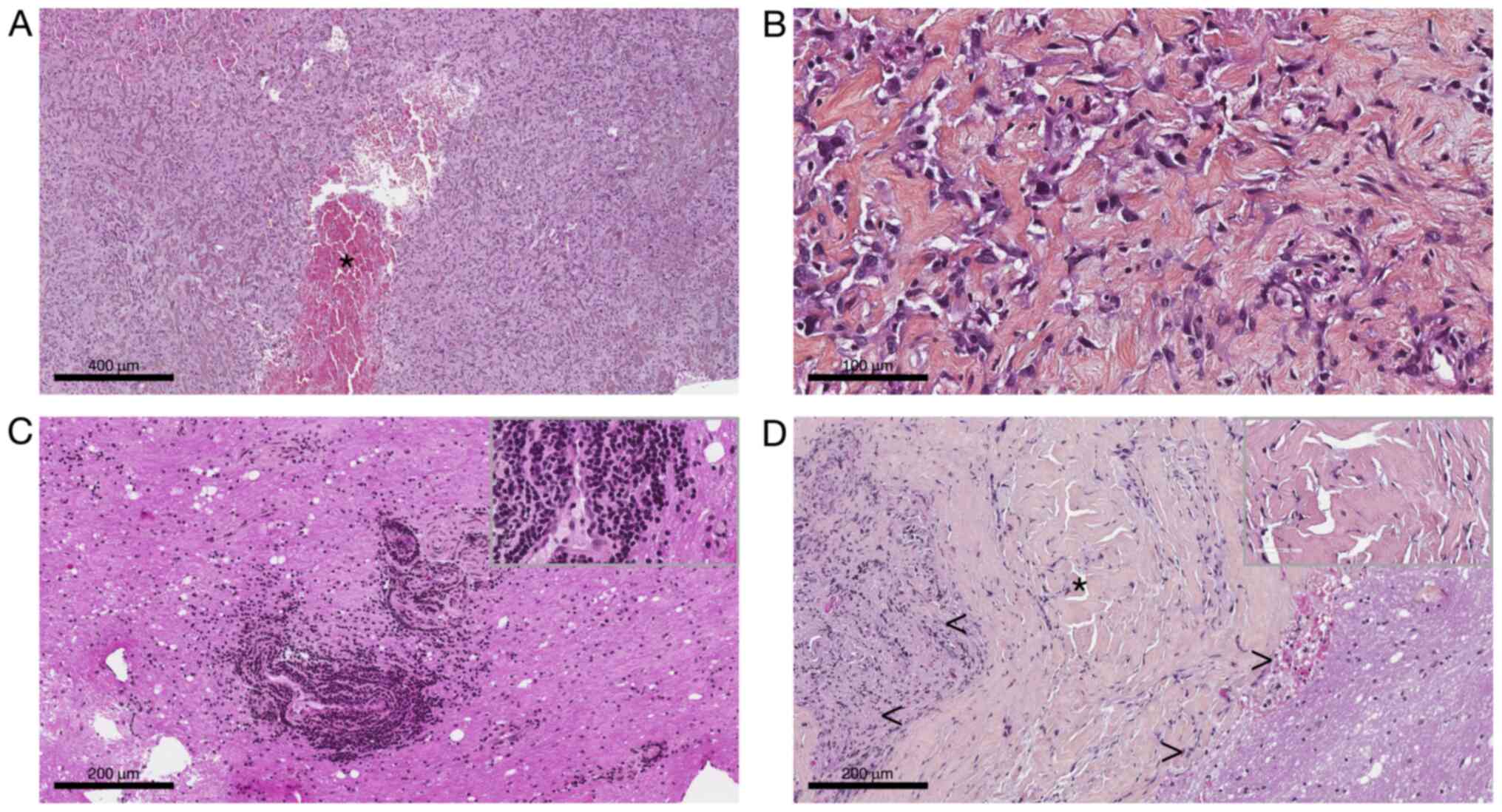
pathological tissue dominated by thick organized collagen fibers
mixed with spindle or epithelioid cells. The nuclei were bland with
open chromatin resembling those of macrophages or histiocytes
(Fig. 2A and B). There was no pseudo syncytial growth
pattern. The tumor exhibit large blood-filled pseudo-vascular
spaces (Fig. 2A).
Lympho-plasmocytic cuffs as well as thick fibrous pseudocapsule
were not seen anymore compared to the second and third biopsy where
they were respectively present (Fig.
2C and D). There was no myxoid
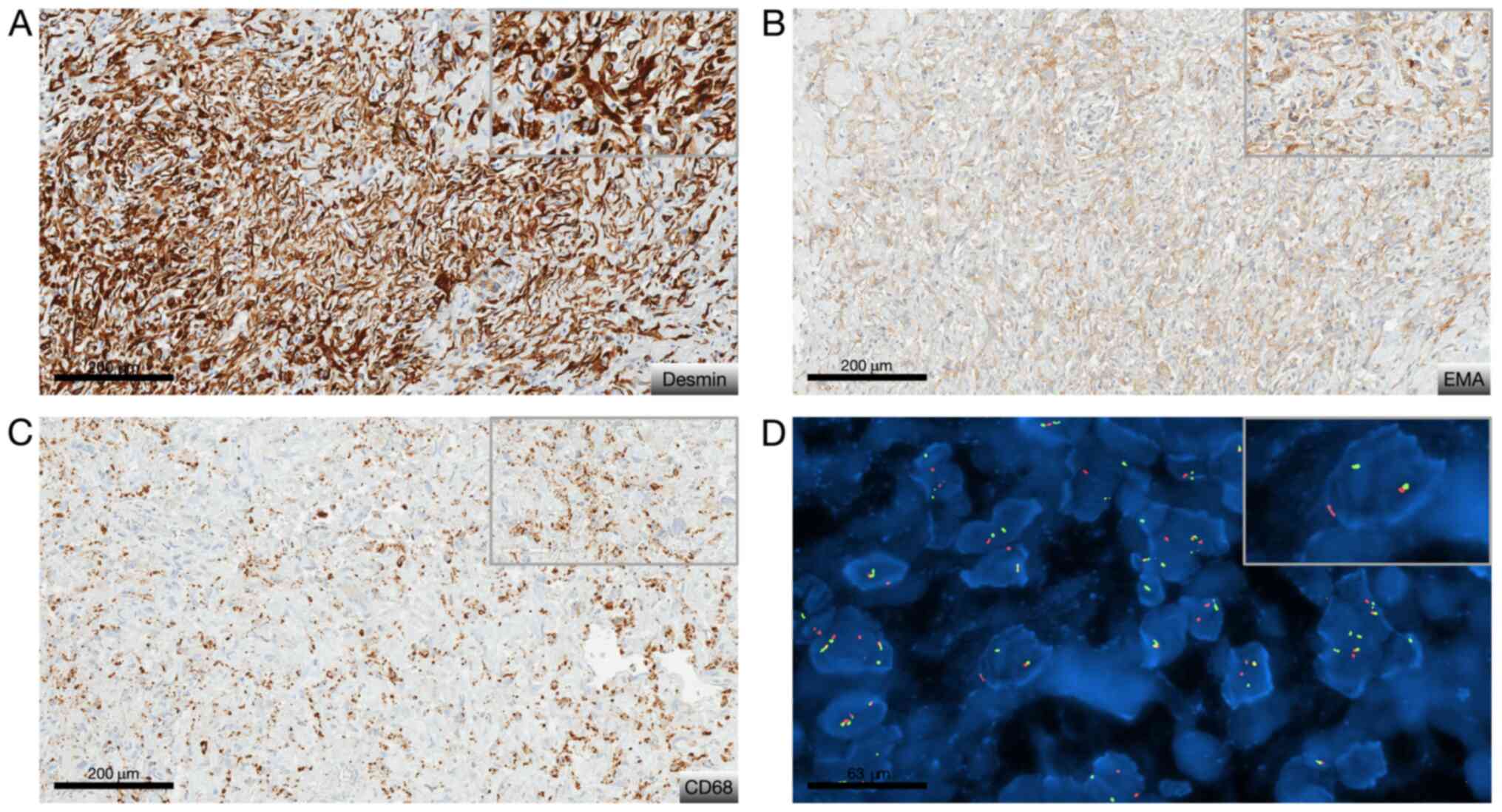
feature nor necrosis, and no mitosis. Immunohistochemically was
carried out on 4-µm-thick FFPE tissue section using UltraView
Ventana Universal DAB Detection Kit® (F. Hoffmann-La
Roche AG, Switzerland). There was only cytoplastic diffuse staining
for desmin, patchy staining for EMA and CD68. The Ki-67 labeling
index was low (<7%) (Fig. 3A-C).
Fluorescence in situ hybridization (FISH) analysis was
performed on tumoral nuclei of paraffin embedded 4-µm-sections
using ZytoLight FISH-Tissue Implementation Kit with EWSR1
Dual Color Break Apart Probe (Z-2096-50; ZytoVision), specific for
EWSR1 at 22q12.2. The number of orange and green dots were
then counted (centromeric probe in 5' to the breakpoint and
telomeric probe in 3' to the breakpoint, respectively), both into
intron 4 of EWSR1, after DNA were counterstained with DAPI,
using a fluorescence microscope. Fifty non-overlapping intact
nuclei were examined for EWSR1 rearrangement. Eighty percent
of them presented in this case a split signal also called break
apart signal meaning that separated orange and green dots or single
orange dots were seen consistent with a EWSR1 rearrangement
(>20% of rearranged nuclei) (Fig.
3D). To look for its fusion partner, a
retrotranscriptase-quantitative polymerase chain reaction was then
performed in two different molecular departments on FFPE and frozen
tissue and confirmed the presence of EWSR1/CREB1 fusion
transcript. Diagnosis of classical non-myxoid angiomatoid fibrous
histiocytoma with the fusion transcript EWSR1/CREB1 was made
by the association of morphological, immunohistochemical and
molecular data (5).
Patient management and outcomes
The patient was monitored for four years until MRI
demonstrated tumor progression. The last MRI before progression in
August 2017 showed a lesion of 35x28x29 mm.
On the MRI of April 2018, the lesion of the pineal
region was heterogeneous and measure 40 mm of height, 27 mm of
anteroposterior length, and 38 mm of width. It had a hypointense T1
signal, heterogeneous hyperintense T2 signal, and strong patchy
enhancement following gadolinium administration. There were some
cystic components, with the largest in the right anterior superior
part with a diameter of 26 mm (Fig.
1). There was also peritumoral edema. At this time, surgical
resection was considered too risky without possibility of complete
removal and the patient could not be re-irradiated. The
interdisciplinary tumor board decided to treat him as if he had a
sarcoma-like tumor. In September 2018, as the tumor was still on
progression (Fig. 1), he was
started on intravenously doxorubicin 60 mg/m² every three weeks for
a total of seven injections. Less than a month after treatment
cessation, the patient was neurologically stable and brain MRI
showed a <50% decrease in tumor size, considered as stable
disease by the Response Assessment in Neuro-Oncology criteria
(Fig. 1). Toxicities were measured
by the Common Terminology Criteria for Adverse Events v5.0. The
patient had a grade III constipation requiring a short-term
hospitalization and a treatment with osmotic laxatives and
mechanical removal. Otherwise, treatment was well tolerated; by the
end, he had grade II alopecia, grade I asthenia, anorexia, and oral
mycosis treated with oral bicarbonate. He had no feared
anthracycline complication, meaning neither cardiac failure nor
hepatotoxicity. Six months after stopping doxorubicin, he recovered
from toxicities and MRI showed no signs of progression. Fourteen
months after doxorubicin discontinuation in March 2020, MRI and
neurological examination showed stable disease (Fig. 1). He still had some mild blurred
vision and alexia. Although he was not cured from the disease, the
tumor's progression was stopped and both his neuro-cognitive
functions and quality of life were preserved.
Discussion
To our knowledge, this is the first case of a
patient with classical non-myxoid intracranial AFH treated with
single chemotherapy inducing prolonged stable disease. Even if the
location is extremely rare, AFH was here confirmed by the
integration of radiological, morphological and immunohistochemical
data with the molecular analysis. The latter demonstrated the
original EWSR1-CREB1 fusion, heretofore only described in
intracranial myxoid mesenchymal variant. Indeed, even if
EWSR1-CREB1 is the most frequently described fusion
transcript in this entity (24), it
is the first reported description in intracranial classical AFH.
Most soft tissue AFH are indolent with 15 percent risk of local
recurrence and less than 5 percent risk of metastases,
predominantly to regional lymph nodes (7,25,26).
It accounts for 0.3% of all soft tissue tumors and usually occurs
in children and young adult (6).
The intracranial location is extremely rare and only cases reports
are described. This tumor has been reported in twenty-one previous
instances: Six conventional AFH (9-13)
and fifteen myxoid AFH (14-23).
Characteristics of these tumors and their outcomes are listed in
Table I. Unlike the present case,
medium reported age at diagnosis is 26-year-old with a female
predominance. Long-term outcomes (<1 year) are not available in
11 cases but the recurrence rate for the others is 60% (9-19,23).
The scarcity of this location makes the diagnosis difficult. When
utilizing imaging results, the most frequently established
diagnosis is meningioma or lymphoma (12,18,23).
Histologically, the diagnostic of intracranial AFH is difficult:
The tumor is well delimited with lobulated or multinodular borders
and thick fibrous pseudocapsule. In up to 80% of tumors, dense
lymphoplasmacytic infiltrate or cuffs can be seen, resembling those
of schwannoma. Multifocal hemorrhage is seen in most cases, forming
blood-filled cystic spaces of variable size. Mostly half of the
AFHs express desmin, without positivity for myogenin or MyoD1. Many
express EMA and CD68(3). In our
case, diagnosis was complicated due to repeated intracranial biopsy
sampling of the tumor that not only gives little insights of its
morphological characteristics but also induces changes in
morphological features (like fibrosis, hemorrhage or tissue
distortion). This tumor expressed desmin, EMA and CD68, which
finally made us suspect AFH diagnosis and ask for the molecular
analyses that confirmed it (25).
 | Table IComprehensive list of reported cases
of angiomatoid fibrous histiocytoma (with myxoid component or not)
and their treatment with reported outcomes. |
Table I
Comprehensive list of reported cases
of angiomatoid fibrous histiocytoma (with myxoid component or not)
and their treatment with reported outcomes.
| AFH | Authors | Age (years)/sex | Location | Symptoms/signs
(clinical features) | MRI | Surgery | IHC | Genetic marker | Treatment
post-surgery | Time to
recurrence | Total follow
up/recurrence | (Refs.) |
|---|
| Conventional AFH | Dunham et al,
2008 | 25/M | Occipital lobe | Visual problems,
headaches | Cystic component,
heterogeneously enhancement | GTR | EMA+,
desmin+ |
EWSR1/ATF1 | None | NM | NM | (9) |
| | Ochalski et
al, 2010 | 35/M | Temporal lobe | Headaches, facial
weakness | Minimal
enhancement | GTR | Desmin+,
EMA NM | Rearranged
EWSR1 gene | GKSa | 0.8 months | 49
months/multipleb | (10) |
| | Hansen et al,
2015 | 17/F | Parieto-occipital
lobe | Headaches | Heterogeneously
enhancement, edema | GTR | EMA+,
desmin+ | Negative | None | NA | 3 months/none | (11) |
| | Alshareef et
al, 2016 | 58/F | Porous
trigeminus | Facial weakness | Heterogeneously
enhancement, edema | GTR | NM | Rearranged
EWSR1 gene | None | NA | 6 months/none | (12) |
| | Konstantinidis
et al, 2019 | 13/F | Frontal lobe | Headaches | Cystic component,
enhancement, edema | GTR | Desmin+,
EMA NM |
EWSR1/ATF1 | None | 5 years
(surgery) | 11 years/yes | (13) |
| | Konstantinidis
et al, 2019 | 12/F | Frontal lobe | Visual problems,
headaches | Cystic
component, | STR | EMA+,
desmin+ |
EWSR1/CREM | None | 28 months | 28 months/yes | (13) |
| Myxoid
mesenchymal | Kao et al,
2017 | 15/F | Meninges | NM | NM | NM | EMA+,
desmin+ |
EWSR1/CREM | None | NA | 17 months/none | (14) |
| AFH | Kao et al,
2017 | 23/F | Meninges
(occipital) | NM | NM | NM | EMA+,
desmin+ |
EWSR1/CREB1 | None | NM | NM | (14) |
| | Kao et al,
2017 | 20/M | Frontal lobe | NM | NM | NM | EMA+,
desmin+ |
EWSR1/CREB1 | None | NM | NM | (14) |
| | Kao et al,
2017 | 12/M | Frontal lobe | Seizures | NM | NM | EMA+,
desmin- |
EWSR1/ATF1 | None | NM | NM | (14) |
| | Bale et al,
2018 | 12/M | Posterior
cerebellar fossa | Headaches | Heterogeneously
enhancement | STR | EMA+,
desmin+ |
EWSR1/CREB1 | None | NA | 12 months/none | (17) |
| | Bale et al,
2018 | 14/F |
Intraventricular | Headaches, visual
problems | Heterogeneously
enhancement, edema | STR | EMA+,
desmin+ |
EWSR1/CREB1 | None | NA | 12 months/none | (17) |
| | Bale et al,
2018 | 18/M | Frontal lobe | Seizures | Enhancement,
edema | STR | EMA+,
desmin+ |
EWSR1/CREM | None | NA | 12 months/none | (17) |
| | Sciot et al,
2018 | 17/F | Frontal lobe | Hemiparesis,
seizures | Cystic component,
minimal enhancement | GTR | EMA+,
desmin- |
EWSR1/ATF1 | RT after 2nd
surgery | 3 months (surgery
and RT) | 7 years/two | (16) |
| | Gareton et
al, 2018 | 19/M | Tentorium
cerebelli | Seizures | NM | GTR | EMA+,
desmin- |
EWSR1/CREM | 6 API/AI and
RT | 10 years | 10 years/yes | (15) |
| | Spatz et al,
2018 | 22/F | Occipital lobe | Visual problems,
headaches, seizure | Heterogeneously
enhancement, edema | STR | EMA+,
desmin+ | NM | None | NA | 3 months/none | (23) |
| | Ghanbari et
al, 2019 | 58/F | Parafalcine | Seizure | Homogenous
enhancement, edema | STR | EMA+,
desmin+ |
EWSR1/CREB1 | None | NA | 3 months/none | (18) |
| | Gunness et
al, 2019 | 32/F | Temporal lobe | Headaches | Cystic component,
heterogeneously enhancement | STR | NM | NM | None | 1 year
(surgery) | 2 years/yes | (19) |
| | White et al,
2019 | 9/M | Frontal lobe | Fatigue,
abulia | Cystic component,
enhancement | GTR |
Desmin+/-, EMA NM |
EWSR1/CREM | None | 6 months (surgery
and RT) | 6 months/yes | (20) |
| | Ballester et
al, 2020 | 67/M | Temporal lobe | Aphasia,
confusion | Cystic component,
enhancement, edema | STR | EMA+,
desmin+ |
EWSR1/ATF1 | None | NA | 3.5
months/none | (21) |
| | Komatsu et
al, 2020 | 53/F | Third
ventricle | Headache,
dizziness | Homogenous
enhancement | STR | EMA+,
desmin+ |
EWSR1/CREB1 | GKS | NA | 3 months/none | (22) |
All the reports support gross total resection as the
gold standard treatment at presentation and recurrence. Indeed
there is a lack of proof regarding radiotherapy and chemotherapy.
In metastatic soft tissue AFH, anthracycline based chemotherapy has
previously been used successfully in two cases suggesting the
likely usefulness of such treatment in patient with unresectable
and/or metastatic disease (26,27).
Also, one of the latest case reports of intracranial AFH suggests
that use of chemotherapy as an adjuvant therapy could be considered
if surgical resection was vain or deleterious to the patient
(13). Actually, one patient was
treated with 6 cycles of API/AI type chemotherapy but it was for a
myxoid-variant AFH and after complete tumor removal. None of the
patients presented in the cases was challenged with chemotherapy at
tumor progression.
This case report confirms that the diagnostic of
intracranial AFH can be problematic and a gross total resection,
when possible, must be considered. Besides, an integrated approach
using morphology, immunohistochemistry and molecular analysis is
recommended to support the diagnosis of this rare entity. The
potential relation with the myxoid component needs to be further
studied. Treatments options after surgery for recurrent or
progressive intracranial AFH, myxoid or not, are scarce and the
optimal treatment sequence is unknown. Here we present the first
case of intracranial conventional AFH with EWSR1/CREB1
fusion transcript, treated with doxorubicin at progression,
inducing prolonged stable disease fourteen months after treatment
discontinuation.
In conclusion, in the absence of gold standard
management for such cases, the present case suggests that
chemotherapy should be considered in intracranial AFH when surgery
is not an option. Desmin staining and EWSR1 gene fusions
should be searched for in all cases possibly compatible with
intracranial AFH especially in EMA positive spindle cell tumors
without typical meningioma features. A single institute
observational study is currently ongoing in Italy. All the medical
records, radiological imaging, and histological slides are being
reviewed to identify the best therapeutic approach (NCT03759327).
Moreover, radiation therapy with or without chemotherapy
combination (including doxorubicin) or targeted therapy before
surgery are currently being explored for patients with newly
diagnosed non-rhabdomyosarcoma soft tissue sarcomas (comprising
AFH) (NCT02180867), which could give us clues to the best treatment
for intracranial AFH patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LG instructed and participated in the treatment of
the patient, performed the literature search, and mainly wrote the
manuscript. LG and TF created the figures and tables. JH and FD
instructed and participated in the treatment of the patient. TF, JH
and FD provided critical revisions of the manuscript for important
intellectual content. TF, DM and DP carefully reviewed the
pathological findings. RA carefully reviewed the radiology
findings. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The patient provided written informed consent prior
to treatment.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lemaigre FP, Ace CI and Green MR: The cAMP
response element binding protein, CREB, is a potent inhibitor of
diverse transcriptional activators. Nucleic Acids Res.
21:2907–2911. 1993.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gonzalez GA and Montminy MR: Cyclic AMP
stimulates somatostatin gene transcription by phosphorylation of
CREB at serine 133. Cell. 59:675–680. 1989.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thway K and Fisher C: Tumors with
EWSR1-CREB1 and EWSR1-ATF1 fusions: The current status. Am J Surg
Pathol. 36(11)2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Enzinger FM: Angiomatoid malignant fibrous
histiocytoma. A distinct fibrohistiocytic tumor of children and
young adults simulating a vascular neoplasm. Cancer. 44:2147–2157.
1979.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Antonescu CR and Rossi S: WHO
Classification of Tumours of Soft Tissue and Bone. Vol 5. 4th
edition. Fletcher CD, Bridge JA, Hogendoorn PC and Mertens F (eds).
IARC, Lyon, pp204-205, 2013.
|
|
6
|
Saito K, Kobayashi E, Yoshida A, Araki Y,
Kubota D, Tanzawa Y, Kawai A, Yanagawa T, Takagishi K and Chuman H:
Angiomatoid fibrous histiocytoma: A series of seven cases including
genetically confirmed aggressive cases and a literature review. BMC
Musculoskelet Disord. 18(31)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fanburg-Smith JC and Miettinen M:
Angiomatoid ‘malignant’ fibrous histiocytoma: A clinicopathologic
study of 158 cases and further exploration of the myoid phenotype.
Hum Pathol. 30:1336–1343. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huret JL: EWSR1 (Ewing sarcoma breakpoint
region 1). Atlas Genet Cytogenet Oncol Haematol. 15:395–407.
2011.
|
|
9
|
Dunham C, Hussong J, Seiff M, Pfeifer J
and Perry A: Primary Intracerebral angiomatoid fibrous
histiocytoma: Report of a case with a t(12;22)(q13;q12) causing
type 1 fusion of the EWS and ATF-1 genes. Am J Surg Pathol.
32:478–484. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ochalski PG, Edinger JT, Horowitz MB,
Stetler WR, Murdoch GH, Kassam AB and Engh JA: Intracranial
angiomatoid fibrous histiocytoma presenting as recurrent multifocal
intraparenchymal hemorrhage. J Neurosurg. 112:978–982.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hansen JM, Larsen VA, Scheie D, Perry A
and Skjøth-Rasmussen J: Primary intracranial angiomatoid fibrous
histiocytoma presenting with anaemia and migraine-like headaches
and aura as early clinical features. Cephalalgia. 35:1334–1336.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alshareef MA, Almadidy Z, Baker T, Perry
A, Welsh CT and Vandergrift WA III: Intracranial angiomatoid
fibrous histiocytoma: Case report and literature review. World
Neurosurg. 96:403–409. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Konstantinidis A, Cheesman E, O'Sullivan
J, Pavaine J, Avula S, Pizer B and Kilday JP: Intracranial
angiomatoid fibrous histiocytoma with EWSR1-CREB family fusions: A
report of 2 pediatric cases. World Neurosurg. 126:113–119.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kao YC, Sung YS, Zhang L, Chen CL,
Vaiyapuri S, Rosenblum MK and Antonescu CR: EWSR1 fusions with CREB
family transcription factors define a novel myxoid mesenchymal
tumor with predilection for intracranial location. Am J Surg
Pathol. 41:482–490. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gareton A, Pierron G, Mokhtari K, Tran S,
Tauziède-Espariat A, Pallud J, Louvel G, Meary E, Capelle L,
Chrétien F and Varlet P: ESWR1-CREM fusion in an intracranial
myxoid angiomatoid fibrous histiocytoma-like tumor: A case report
and literature review. J Neuropathol Exp Neurol. 77:537–541.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sciot R, Jacobs S, Calenbergh FV, Demaerel
P, Wozniak A and Debiec-Rychter M: Primary myxoid mesenchymal
tumour with intracranial location: Report of a case with a
EWSR1-ATF1 fusion. Histopathology. 72:880–883. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bale TA, Oviedo A, Kozakewich H, Giannini
C, Davineni PK, Ligon K and Alexandrescu S: Intracranial myxoid
mesenchymal tumors with EWSR1-CREB family gene fusions: Myxoid
variant of angiomatoid fibrous histiocytoma or novel entity? Brain
Pathol. 28:183–191. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ghanbari N, Lam A, Wycoco V and Lee G:
Intracranial myxoid variant of angiomatoid fibrous histiocytoma: A
case report and literature review. Cureus. 11(e4261)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gunness VR, Munoz I, González-López P,
Alshafai N, Mikalkova A and Spears J: Intracranial angiomatoid
fibrous histiocytoma with Hodgkin lymphoma. Med J Malaysia.
74:234–236. 2019.PubMed/NCBI
|
|
20
|
White MD, McDowell MM, Pearce TM,
Bukowinski AJ and Greene S: Intracranial Myxoid mesenchymal tumor
with rare EWSR1-CREM translocation. Pediatr Neurosurg. 54:347–353.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ballester LY, Meis JM, Lazar AJ, Prabhu
SS, Hoang KB, Leeds NE and Fuller GN: Intracranial myxoid
mesenchymal tumor with EWSR1-ATF1 Fusion. J Neuropathol Exp Neurol.
79:347–351. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Komatsu M, Yoshida A, Tanaka K, Matsuo K,
Sasayama T, Kojita Y, Kanda T, Kodama Y, Itoh T and Hirose T:
Intracranial myxoid mesenchymal tumor with EWSR1-CREB1 gene fusion:
A case report and literature review. Brain Tumor Pathol. 37:76–80.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Spatz M, Nussbaum ES, Lyons L, Greenberg
S, Kallmes KM and Nussbaum LA: Primary intracranial angiomatoid
fibrous histiocytoma: A case report and literature review. Br J
Neurosurg: Mar 15, 2018 (Epub ahead of print). doi:
10.1080/02688697.2018.1451823.
|
|
24
|
Antonescu CR, Dal Cin P, Nafa K, Teot LA,
Surti U, Fletcher CD and Ladanyi M: EWSR1-CREB1 is the predominant
gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosomes
Cancer. 46:1051–1060. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Thway K and Fisher C: Angiomatoid fibrous
histiocytoma: The current status of pathology and genetics. Arch
Pathol Lab Med. 139:674–682. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ogden S, Harave S, McPartland J, Brennan
B, Jeys L, Losty P and Pizer B: Angiomatoid fibrous histiocytoma: A
case of local recurrence and metastases to loco-regional lymph
nodes that responded to chemotherapy. Pediatr Blood Cancer: 64,
2017 doi: 10.1002/pbc.26376.
|
|
27
|
Bernini JC, Fort DW, Pritchard M, Rogers
BB and Winick NJ: Adjuvant chemotherapy for treatment of
unresectable and metastatic angiomatoid malignant fibrous
histiocytoma. Cancer. 74:962–964. 1994.PubMed/NCBI View Article : Google Scholar
|