Introduction
Among malignancies, ovarian cancer accounts for the
seventh most common cancer in females globally (1). As per GLOBOCAN 2018 data, there were
295,414 new ovarian cancer cases with 184,799 cancer deaths (1.9%
of all cancers) (2). In Indian
females, it is the third most common cancer (new cases: 36,170,
6.2%) (2). A 5-year survival rate
of ~45% (3), indicates a low
prognosis rate compared with other gynecological cancers. The high
mortality rate of ovarian cancer can be attributed to the delayed
disease diagnosis leading to increased cancer stage or metastasis
of the disease in most (~75%) of the patients (4).
More than 95% ovarian cancers are of epithelial type
arising from ovarian surface, fallopian tube or peritoneum
(5,6). The mainstay of ovarian cancer
management include cytoreduction or surgical debulking followed by
chemotherapy. The cornerstone of chemotherapy for advanced disease
include the use of platinum-agents containing regimens. The
landmark GOG-111 and EORTC studies in advanced ovarian cancer have
reported significantly improved OS with combination of paclitaxel
and a platinum agent (7,8). Docetaxel, owing to its favorable
neurotoxicity profile than paclitaxel, was evaluated as a
first-line therapy with platinum agents in ovarian cancer (9,10). In
platinum-sensitive/resistant cases, docetaxel has also been
assessed as a single agent for second-line therapy (11), or as a combination therapy with
cisplatin (12), carboplatin
(13), or cyclophosphamide
(14).
The conventional docetaxel formulation has several
toxicity concerns which are greatly related to the formulation
vehicles polysorbate 80 and ethanol (15-19).
To overcome the toxicity issues, nanosomal docetaxel lipid
suspension (NDLS, DoceAqualip) formulation was developed, which
does not contain formulation vehicles polysorbate 80 and ethanol.
Using the patented ‘NanoAqualip’ technology (20), NDLS was developed with lipids
generally regarded as safe (GRAS) by the US FDA. Nanosomal
lipid-based particles (<100 nm) of NDLS may increase delivery of
docetaxel to tumor tissues due to damaged tumor vasculature, and
may result in enhanced permeability and retention (EPR) effect,
leading to increased docetaxel systemic availability, (20,21)
hence, enhanced therapeutic outcomes are anticipated (22). In addition, polysorbate 80 and
ethanol related toxicity issues can be circumvented, thus improving
overall outcomes. NDLS is approved in India for advanced gastric
adenocarcinoma, androgen independent (hormone refractory)
metastatic prostate cancer (HRPC), locally advanced or metastatic
breast cancer (MBC) after failure of prior chemotherapy, non-small
cell lung cancer (NSCLC) after failure of prior chemotherapy, and
for the induction treatment of locally advanced squamous cell
carcinoma of head and neck (LA SCCHN).
NDLS has demonstrated efficacy and safety in several
cancers including breast, gastric, cervical, penile, HRPC, NSCLC
and sarcoma (23-27).
Although NDLS is not approved for ovarian cancer treatment, its
effectiveness and safety is reported for ovarian cancer management
in the published literature (26,28).
We report here a real-life clinical experience on NDLS use for
managing metastatic epithelial ovarian carcinoma.
Methods
Study design
Medical charts of adult women with metastatic
epithelial ovarian carcinoma were reviewed in this retrospective
study. The women should have received NDLS based chemotherapy for
clinical care between from September 2014 to September 2018. The
efficacy parameters were the overall response rate (ORR), disease
control rate (DCR) and overall survival (OS). The ORR was defined
as the proportion of patients achieving complete response (CR,
disappearance of all target lesions or reduction to <10 mm in
short axis of any lymph nodes) and partial response (PR, ≥30%
decrease in tumor diameter). The DCR was defined as the proportion
of patients achieving CR, PR, and stable disease (SD, no ≥30%
decrease or ≥20 increase in tumor diameter). The OS was calculated
as time from initiating the treatment to death due to any cause.
Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 was used
for tumor response assessment (29). The medical charts of the patients
were screened for documented adverse events (AEs), and the
incidence of AEs were recorded and graded per Common Terminology
Criteria for Adverse Events (as per CTCAE) 5.0 criteria (30).
Ethics statement
The study was conducted after due approval from OM
ethics committee (ECR/1168/Inst/GJ/2018), in accordance with the
ethical principles of Declaration of Helsinki, International
Council for Harmonistion (ICH) Good Clinical Practice (GCP)
guidelines, and protocol requirements. Patient consent to review
their medical records was not required by the ethics committee as
this was a retrospective study where patients received NDLS as part
of natural course of their treatment. In addition, the data
presented in the current study was analyzed data without
identifying any patient. Throughout the data analysis and
manuscript preparation, patient confidentiality was completely
maintained and data were anonymized.
Statistical analyses
Descriptive statistics were used for demographic and
baseline characteristics whereas frequency and percentage were
provided for categorical variables. Count, mean, standard deviation
(SD), median, minimum and maximum were provided for continuous
variables. The frequency and % of patients were reported for
response rate. Kaplan-Meier estimates were used for calculating OS.
The AEs were summarized as frequencies and percentages by type of
reactions. All statistical analyses were done using SAS®
version 9.4 (SAS Institute, Inc.).
Results
Patient disposition and
demographics
Data of thirteen women with metastatic epithelial
ovarian carcinoma, who received NDLS based chemotherapy, was
retrospectively reviewed. Table I
summarizes the baseline patient and disease characteristics.
 | Table IPatient disposition and baseline
characteristics. |
Table I
Patient disposition and baseline
characteristics.
| Parameter | Ovarian carcinoma
(n=13) |
|---|
| Age, years
(range) | 52.9±9.9a (42-70) |
| BSA,
m2 | 1.4±0.2a |
| Female, n (%) | |
|
Premenopausal | 5 (38.5) |
|
Post-menopausal | 8 (61.5) |
| Metastasis site, n
(%)b | |
|
Lymph
node | 7 (53.8) |
|
Liver | 2 (15.4) |
|
Peritoneum | 5 (38.5) |
| ECOG score, n
(%) | |
|
0 | 2 (15.4) |
|
1 | 8 (61.5) |
|
2 | 2 (15.4) |
|
3 | 1 (7.7) |
| Line of therapy, n
(%) | |
|
First-line | 6 (46.2) |
|
Second-line | 7 (53.8) |
|
Platinum-sensitive
patients | 4 (57.1) |
|
Platinum-resistant
patients | 3 (42.9) |
| Comorbid disease, n
(%) | |
|
Hypertension | 4 (30.8) |
|
Diabetes | 3 (23.1) |
|
Hypothyroidism | 2 (15.4) |
|
Other | 3 (23.1) |
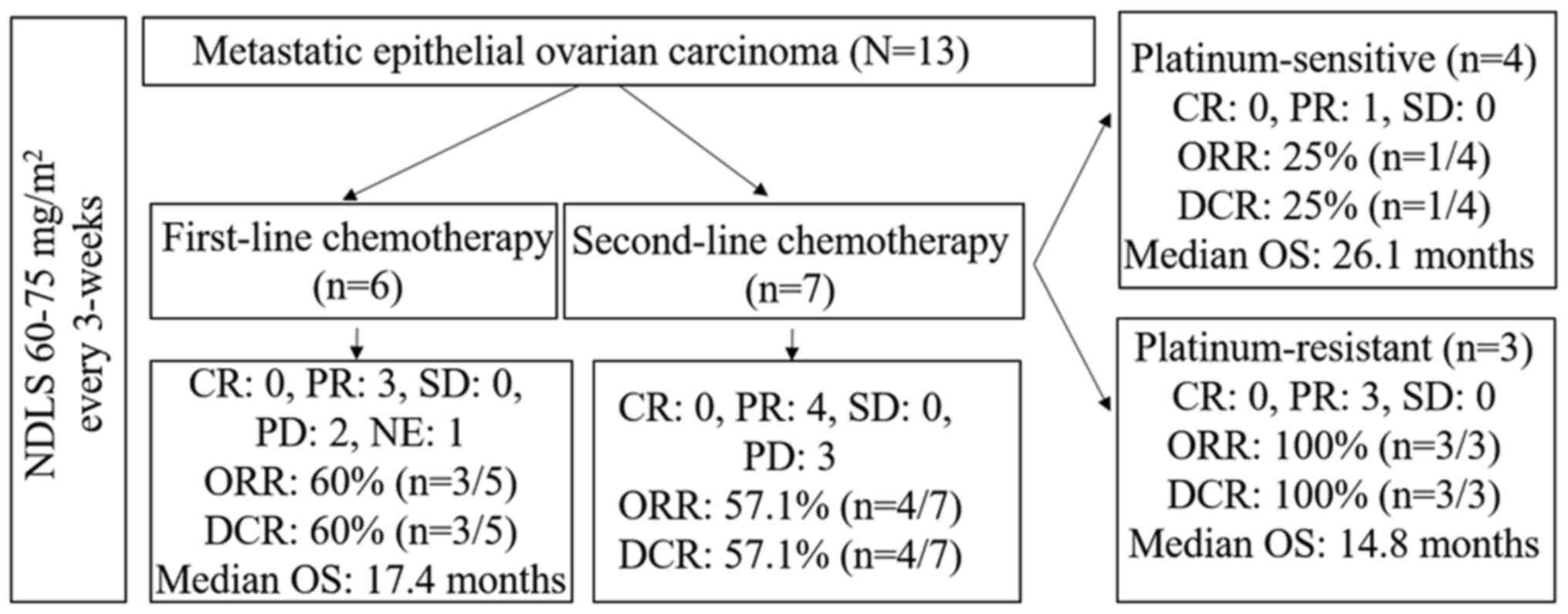
In all patients, NDLS was given as 1-hour infusion
in 3-weekly cycles. The NDLS doses of 60 mg/m2 or 75
mg/m2 were administered in 23.1% (3/13) and 76.9%
(10/13) patients, respectively. Overall, 46.2% (n=6/13) patients
received NDLS based first-line combination chemotherapy
(NDLS/gemcitabine, n=2; NDLS/cisplatin, NDLS/oxaliplatin,
NDLS/liposomal doxorubicin, NDLS/oxaliplatin/bevacizumab, n=1
each); of the remaining 53.8% (n=7/13) patients who received NDLS
based second-line combination chemotherapy, 4 patients were
platinum-sensitive (NDLS/cisplatin, n=2; NDLS/carboplatin, n=1;
NDLS/oxaliplatin/bevacizumab, n=1), and 3 patients were
platinum-resistant (NDLS monotherapy, n=2 and
NDLS/cyclophosphamide, n=1). The median number of NDLS based
chemotherapy cycles were: 5.5 (range: 3-6) for first-line therapy,
6 for platinum-sensitive cases (all 4 patients received 6 cycles)
and 6 (range: 2-6) for platinum-resistant cases. All the patients
were administered granulocyte-colony stimulating factor (GCSF)
support as primary prophylaxis.
Efficacy
Of 13 patients, efficacy data was available for 12
patients. For patients receiving NDLS based first-line and
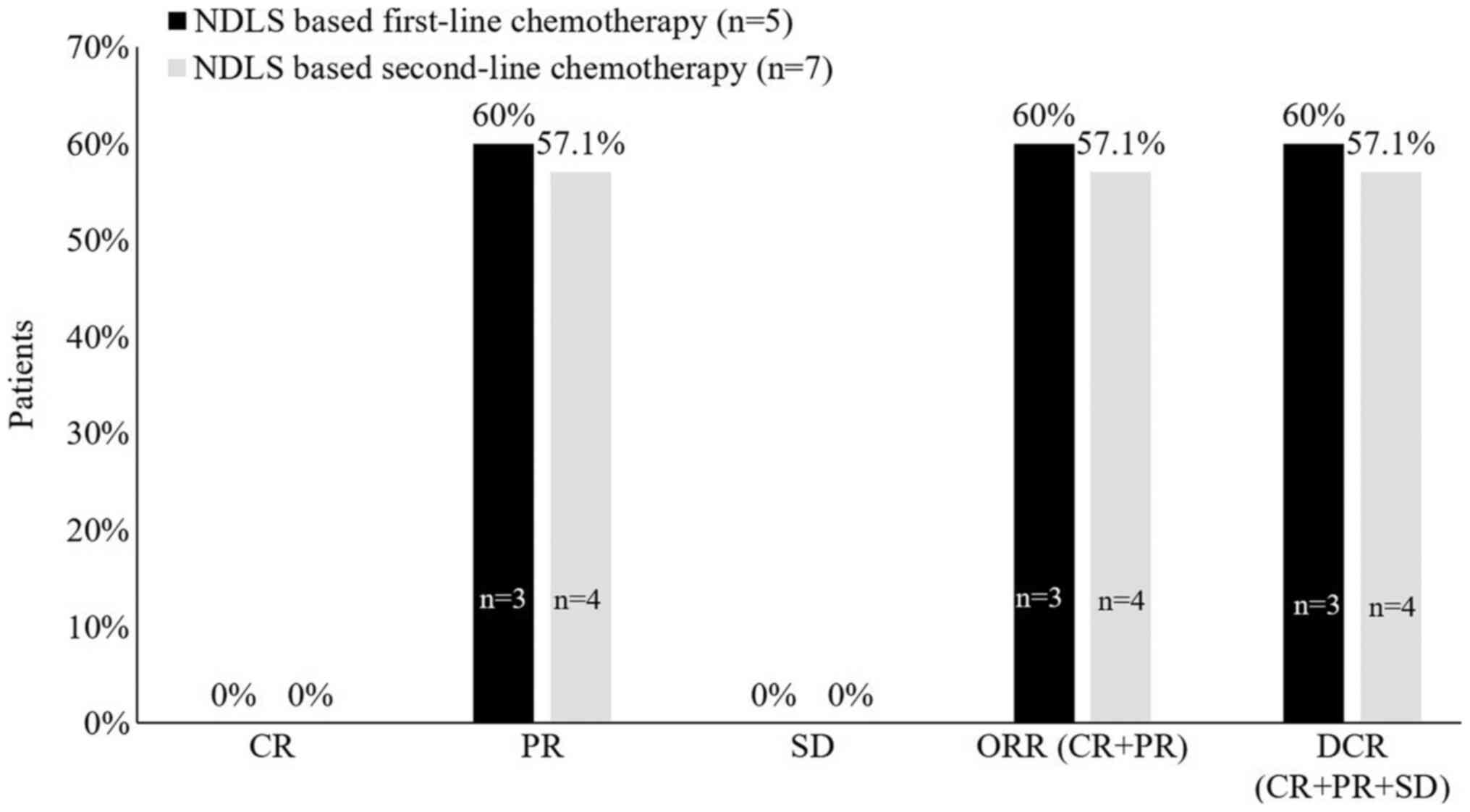
second-line chemotherapy, the ORRs were 60% (PR, n=3/5) and 57.1%
(PR, n=4/7), respectively (Fig. 1).
For patients receiving NDLS based second-line chemotherapy, the ORR
was 25% (PR, n=1/4) for platinum-sensitive and 100% (PR, n=3/3) for
platinum-resistant patients.
Overall survival
The patient survival data was collected from the
initiation of NDLS treatment till the date the patient was
followed-up and the date of death for patients who succumbed. OS
was censored at the last follow-up date for alive patients at the
time of data analysis or who were lost to follow-up, At a median
follow-up duration of 15.1 months (range: 4.3-49.4 months), there
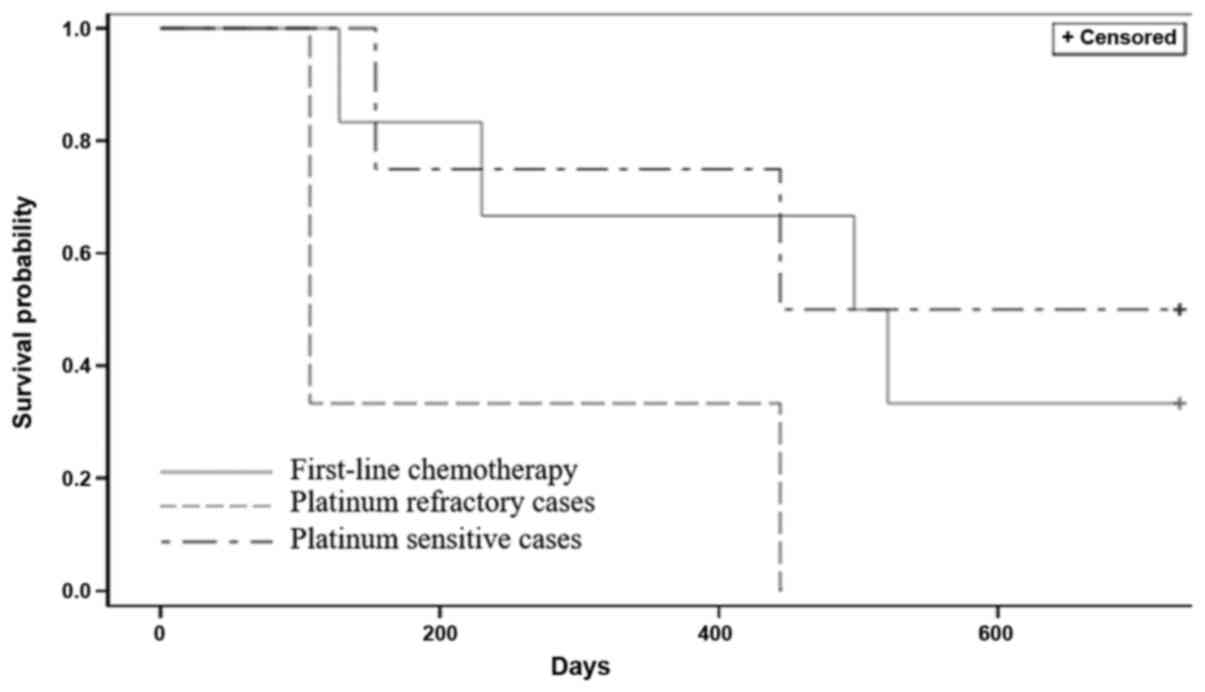
were 7 (53.8%) deaths. For patients receiving NDLS based first-line
chemotherapy, the estimated median OS was 17.4 months (follow-up
duration: 4.3-49.4 months). Similarly, for platinum-sensitive
cases, the estimated median OS was 26.1 months (follow-up duration:
5.1-37.5 months). For platinum-resistant cases, the estimated
median OS was 14.8 months (follow-up duration: 3.5-14.8 months)
(Fig. 2). As the sample size in the
subgroups of platinum-sensitive (n=4) and platinum-resistant (n=3)
cases were very low, comparing OS would not yield clinically
meaningful data, and hence, they were not compared. Fig. 3 enlists the key details of the study
and its findings.
Safety
There were no grade III/IV AEs reported. Eleven
(84.6%) patients had ≥1 AEs. Grade I AEs were reported in 84.6%
(11/13) and grade II in 30.8% (4/13) patients. Hematological AEs
included anemia, lymphopenia thrombocytopenia, and neutropenia
while the non-hematological AEs were hyperglycemia, nausea,
vomiting and edema (Table II).
 | Table IISafety profile of NDLS based
chemotherapy in metastatic ovarian cancer (n=13). |
Table II
Safety profile of NDLS based
chemotherapy in metastatic ovarian cancer (n=13).
| A, Hematological
AEs |
|---|
| AE | All grades, n
(%) | Grade I, n (%) | Grade II, n
(%) |
|---|
| Anemia | 7 (53.8) | 6 (46.2) | 4 (30.8) |
| Lymphopenia | 4 (30.8) | 3 (23.1) | 1 (7.7) |
|
Thrombocytopenia | 4 (30.8) | 4 (30.8) | - |
| Neutropenia | 2 (15.4) | 2 (15.4) | - |
| B,
Non-hematological AEs |
| AE | All grades, n
(%) | Grade I, n (%) | Grade II, n
(%) |
| Hyperglycemia | 3 (23.1) | 3 (23.1) | - |
| Nausea | 1 (7.7) | - | - |
| Vomiting | 1 (7.7) | - | - |
| Edema | 1 (7.7) | 1 (7.7) | - |
Discussion
The combination of taxane with platinum compounds
has been the cornerstone of ovarian cancer management. The landmark
GOG-111 study first demonstrated significantly higher response
rates and patient survival with cisplatin plus paclitaxel versus
cisplatin plus cyclophosphamide (7), which was further confirmed by the
EORTC trial (8). Docetaxel has been
effective and well-tolerated in combination with a platinum agent
for managing ovarian carcinoma (6,31).
Docetaxel monotherapy or docetaxel plus carboplatin are recommended
palliative treatment options for managing ovarian carcinoma for
platinum-sensitive or platinum-resistant ovarian cancers (30).
NDLS evaluated for metastatic ovarian carcinoma
treatment in this study resulted in ORR/DCR rates of 60% and 57.1%
when used as first- and second-line therapies, respectively. In
patients receiving NDLS based first-line chemotherapy, the
estimated median OS was 17.4 months. First-line therapy with
docetaxel and cisplatin was evaluated by the Scottish
Gynaecological Cancer Trials Group (n=100), which showed an ORR of
69% (6). In Asian patients (n=44),
Mokhlesuddin et al, demonstrated a response rate of 80% with
the 2-year OS at 62% with docetaxel-cisplatin regimen (32). In our study, NDLS and cisplatin
based first-line therapy evaluated in 1 patient resulted in a PR.
First-line therapy with docetaxel and gemcitabine followed by
carboplatin (n=44) evaluated in SCOTROC 2A study reported an ORR of
77.3% (33). NDLS-gemcitabine based
chemotherapy was used in 2 patients in our study and reported a PR
in one patient while the other patient had disease progression.
NDLS/liposomal doxorubicin, NDLS/bevacizumab/oxaliplatin and
NDLS/oxaliplatin were the other regimens used as first-line
treatment.
Second-line chemotherapy with docetaxel based
regimens has shown an OS between 8-10.4 months in ovarian cancer
(34). In our study, patients
receiving NDLS based chemotherapy as a second-line treatment showed
an ORR/DCR of 57.1% (platinum-sensitive cases: 25% and
platinum-resistant cases: 100%). The estimated median OS was 26.1
months for platinum-sensitive cases (follow-up duration: 5.1-37.5
months). Ota et al, treated 39 patients with irinotecan plus
cisplatin/nedaplatin and 21 patients with docetaxel/cisplatin and
divided in two groups: Group A-29 patients refractory to initial
platinum-based chemotherapy; group B-31 platinum-sensitive
patients. The study reported a response rate of 41.9% with the
median OS at 9.23 months, in group B (12). In our study, two patients with
platinum-sensitive disease, were administered NDLS/cisplatin
therapy and resulted in a PR in one patient.
The estimated median OS was 14.8 months for
platinum-resistant cases (follow-up duration: 3.5-14.8 months).
Docetaxel/cyclophosphamide use reported the remission of ovarian
cancer in a patient with platinum-resistant disease (14). In our study, NDLS/cyclophosphamide
used in one patient of platinum-resistant disease and reported a
PR. Kavanagh et al, reported a response rate of 40% in
patients with failed platinum chemotherapy (n=55) (11). In our study, NDLS monotherapy showed
partial responses in all 2 patients with platinum-resistant
disease.
In the current safety analysis no severe AEs (grade
III/IV) were reported. The hematological AEs included anemia,
lymphopenia, thrombocytopenia, and neutropenia while the
non-hematological AEs were hyperglycemia, nausea, vomiting and
edema. Previous studies have highlighted the potential role of
formulation vehicles polysorbate 80 and ethanol with AEs generally
occurring with docetaxel such as acute hypersensitivity reactions,
cumulative fluid retention, peripheral neuropathy (15), severe nonimmunologic anaphylactoid
reactions (16), injection-site
reactions (17), and alcohol
intoxication (18,19). Furthermore, these AEs can still be
observed despite corticosteroid and antihistamine premedication,
generally used to limit these toxicities (35). In our study, AEs like neurotoxicity,
fluid retention and acute hypersensitivity reactions were not
reported with NDLS based chemotherapy. In previous studies with
docetaxel and cisplatin combination, neutropenia was the major
toxicity. First-line therapy with docetaxel-cisplatin (n=100)
reported grade 3/4 neutropenia in >75% of patients with advanced
ovarian cancer. The AEs leading to treatment discontinuations were
neurotoxicity (n=6), nephrotoxicity (n=3), neutropenia (n=2),
hypersensitivity, diarrhea and vomiting, skin rash, and clinical
deterioration (n=1, each) (6).
Another study evaluating docetaxel-cisplatin as a first-line
chemotherapy showed grade III neutropenia (25%) among other AEs
(32). For docetaxel based
triple-drug combination as a first-line therapy, neutropenia
(42.4%) followed by leukopenia (13.6%), hypertension (8.3%),
fatigue and nausea (6.1% each) were most common grade III/IV AEs
(36). Docetaxel-based chemotherapy
in platinum-sensitive ovarian cancer showed that neutropenia was
the most common grade III/IV hematologic AE (60%) and diarrhea the
non-hematologic AE (12%). Hypersensitivity reaction led to dose
reduction in one patient (13). In
platinum-refractory disease, neutropenia (98%) was the main
toxicity whereas the AE of cumulative fluid retention required dose
modification. The common AEs reported with docetaxel monotherapy
were alopecia (100%), anemia (87%), dermatitis (67%),
gastrointestinal disorders (53%), stomatitis (49%), neurotoxicity
(45%), excessive lacrimation (33%), and hypersensitivity reactions
(11%) (11).
Corticosteroids premedication is routinely
administered in patients receiving conventional docetaxel to
mitigate the toxicity issues such as hypersensitivity and fluid
retention (35). In a recent study,
Obradović and colleagues used transcriptional profiling of tumors
and matched metastases in mice with patient-derived xenograft
models and suggested a possible role of glucocorticoid receptor
(GR) activation resulting in breast cancer progression and
metastasis (37). Corticosteroid
premedication is not warranted with NDLS formulation as it does not
contain the solvent polysorbate 80, hence, it may potentially help
in circumventing the risk of disease progression.
As this is a retrospective data collection study,
lack of completeness of data for safety as well as survival data is
a major limitation. The data on disease progression and the serial
scans for most of the patients were not available for most of the
timepoints for analysis of progression free-survival (PFS) as this
was a real-world study, which is a major limitation.
In conclusion, NDLS regimens were effective and
well-tolerated in the management of metastatic ovarian cancer. This
data provides valuable insights into the effectiveness and safety
of NDLS in the management of ovarian cancer in clinical practice.
Prospective studies with a larger patient pool are required to
confirm these results.
Acknowledgements
The authors would like to thank Mr. Shreekant
Sharma, MPharm, ISMPP CMPPTM (Intas Pharmaceuticals
Ltd.) for providing writing assistance and Dr Venugopal
Madhusudhana, MBBS, EPBM, ISMPP CMPPTM (Intas
Pharmaceuticals Ltd.) for additional editorial assistance for the
development of this manuscript.
Funding
The current study was funded by Intas Pharmaceutical Ltd.,
India, towards statistical analysis and manuscript writing.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS and AS researched patient data, were involved in
data acquisition and critically revised the manuscript for
intellectual content. NJ, JS, MAK and IA designed the current
study, interpreted the data and critically revised the manuscript
for intellectual content. IA and MAK confirm the authenticity of
all the raw data. All authors had full access to all the data in
the study and take responsibility for integrity of the data and the
accuracy of the data analysis. All authors meet ICMJE criteria and
all those who fulfilled those criteria are listed as authors. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was conducted after due approval
from the OM Ethics Committee, in accordance with the ethical
principles of Declaration of Helsinki, ICH GCP guidelines and
protocol requirements. Patient consent to review their medical
records was not required by the ethics committee as this was a
retrospective study where patients received NDLS as part of natural
course of their treatment. In addition, the data presented in the
current study was analyzed data without identifying any patient.
Throughout the data analysis and manuscript preparation, patient
confidentiality was completely maintained and data were
anonymized.
Patient consent for publication
Not applicable
Competing interests
MAK, NJ and JS and are employees of Intas
Pharmaceutical Ltd., India. IA is an employee of Jina
Pharmaceutical Inc. (USA). The remaining authors had no competing
interests.
References
|
1
|
Reid BM, Permuth JB and Sellers TA:
Epidemiology of ovarian cancer: A review. Cancer Biol Med. 14:9–32.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Basu P, De P, Mandal S, Ray K and Biswas
J: Study of ‘patterns of care’ of ovarian cancer patients in a
specialized cancer institute in Kolkata, eastern India. Indian J
Cancer. 46:28–33. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Desai A, Xu J, Aysola K, Qin Y, Okoli C,
Hariprasad R, Chinemerem U, Gates C, Reddy A, Danner O, et al:
Epithelial ovarian cancer: An overview. World J Transl Med. 3:1–8.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vasey PA, Paul J, Birt A, Junor EJ, Reed
NS, Symonds RP, Atkinson R, Graham J, Crawford SM, Coleman R, et
al: Docetaxel and cisplatin in combination as first-line
chemotherapy for advanced epithelial ovarian cancer. Scottish
gynaecological cancer trials group. J Clin Oncol. 17:2069–2080.
1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Piccart MJ, Bertelsen K, James K, Cassidy
J, Mangioni C, Simonsen E, Stuart G, Kaye S, Vergote I, Blom R, et
al: Randomized intergroup trial of cisplatin-paclitaxel versus
cisplatin-cyclophosphamide in women with advanced epithelial
ovarian cancer: Three-year results. J Natl Cancer Inst. 92:699–708.
2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Markman M, Kennedy A, Webster K, Peterson
G, Kulp B and Belinson J: Combination chemotherapy with carboplatin
and docetaxel in the treatment of cancers of the ovary and
fallopian tube and primary carcinoma of the peritoneum. J Clin
Oncol. 19:1901–1905. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dieras V, Guastalla JP, Ferrero JM, Cure
H, Weber B, Winckel P, Lortholary A, Mayer F, Paraiso D, Magherini
E and Pujade-Lauraine E: A multicenter phase II study of cisplatin
and docetaxel (Taxotere) in the first-line treatment of advanced
ovarian cancer: A GINECO study. Cancer Chemother Pharmacol.
53:489–495. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kavanagh JJ, Kudelka AP, de Leon CG,
Tresukosol D, Hord M, Finnegan MB, Kim EE, Varma D, Forman A, Cohen
P, et al: Phase II study of docetaxel in patients with epithelial
ovarian carcinoma refractory to platinum. Clin Cancer Res.
2:837–842. 1996.PubMed/NCBI
|
|
12
|
Ota T, Takeshima N and Takizawa K:
Second-line chemotherapy for carboplatin/paclitaxel-refractory
ovarian cancer: Are multi-agent chemotherapies of little value
truly? Eur J Gynaecol Oncol. 32:471–475. 2011.PubMed/NCBI
|
|
13
|
Strauss HG, Henze A, Teichmann A, Karbe I,
Baumgart A, Thomssen C and Koelbl H: Phase II trial of docetaxel
and carboplatin in recurrent platinum-sensitive ovarian, peritoneal
and tubal cancer. Gynecol Oncol. 104:612–616. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Benjapibal M, Kudelka AP, Vasuratna A,
Edwards CL, Verschraegen CF, Valero V, Vadhan-Raj S and Kavanagh
JJ: Docetaxel and cyclophosphamide induced remission in platinum
and paclitaxel refractory ovarian cancer. Anticancer Drugs.
9:577–579. 1998.PubMed/NCBI View Article : Google Scholar
|
|
15
|
ten Tije AJ, Verweij J, Loos WJ and
Sparreboom A: Pharmacological effects of formulation vehicles:
Implications for cancer chemotherapy. Clin Pharmacokinet.
42:665–685. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Coors EA, Seybold H, Merk HF and Mahler V:
Polysorbate 80 in medical products and nonimmunologic anaphylactoid
reactions. Ann Allergy Asthma Immunol. 95:593–599. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schwartzberg LS and Navari RM: Safety of
polysorbate 80 in the oncology setting. Adv Ther. 35:754–767.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
FDA Drug Safety Communication (2014, June
20). FDA warns that cancer drug docetaxel may cause symptoms of
alcohol intoxication after treatment. Retrieved from: https://www.fda.gov/Drugs/DrugSafety/ucm401752.htm.
Accessed April 21, 2020.
|
|
19
|
Mirza A and Mithal N: Alcohol intoxication
with the new formulation of docetaxel. Clin Oncol (R Coll Radiol).
23:560–561. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ahmad A, Sheikh S, Ali SM, Ahmad MU,
Paithankar M, Saptarishi D, Maheshwari K, Kumar K, Singh J and
Patel G: Development of aqueous based formulation of docetaxel:
Safety and pharmacokinetics in patients with advanced solid tumors.
J Nanomed Nanotechnol. 6:2015.
|
|
21
|
Lewis LD, Miller AA, Rosner GL, Dowell JE,
Valdivieso M, Relling MV, Egorin MJ, Bies RR, Hollis DR, Levine EG,
et al: A comparison of the pharmacokinetics and pharmacodynamics of
docetaxel between African-American and Caucasian cancer patients:
CALGB 9871. Clin Cancer Res. 13:3302–3311. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ahmad A, Sheikh S, Taran R, Srivastav SP,
Prasad K, Rajappa SJ, Kumar V, Gopichand M, Paithankar M, Sharma M,
et al: Therapeutic efficacy of a novel nanosomal docetaxel lipid
suspension compared with taxotere in locally advanced or metastatic
breast cancer patients. Clin Breast Cancer. 14:177–181.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ashraf M, Sajjad R, Khan MA, Shah M, Bhat
Y and Wani ZA: 1046-Efficacy and safety of a novel nanosomal
docetaxel lipid suspension (NDLS) as an anti cancer agent-a
retrospective study. Ann Oncol. 27 (Suppl 9):ix46–ix51. 2016.
|
|
24
|
Naik R and Khan MA: Doceaqualip in a
patient with prostate cancer who had an allergic reaction to
conventional docetaxel: A case report. Mol Clin Oncol. 6:341–343.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Prasanna R, Bunger D and Khan MA: Efficacy
and safety of DoceAqualip in a patient with locally advanced
cervical cancer: A case report. Mol Clin Oncol. 8:296–299.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vyas V, Joshi N and Khan MA: Novel
docetaxel formulation (NDLS) in low cardiac reserve ovarian cancer.
Open Access J Cancer Oncol. 2(000122)2018.
|
|
27
|
Gupta S, Pawar SS and Bunger D: Successful
downstaging of locally recurrent penile squamous cell carcinoma
with neoadjuvant nanosomal docetaxel lipid suspension (NDLS) based
regimen followed by curative surgery. BMJ Case Rep: Nov 12, 2017
(Epub ahead of print). doi: 10.1136/bcr-2017-220686.
|
|
28
|
Ashraf QM, Sajad QR, Khan MA, Wani ZA,
Mujeeb S, Bhat YM and Joshi N: Efficacy and safety of a novel
nanosomal docetaxel lipid suspension as an anticancer-A
retrospective study. J Cancer Oncol. 2(000132)2018.
|
|
29
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
National Comprehensive Cancer Network
(NCCN) Clinical Practice Guidelines in Oncology (NCCN
Guidelines®). Ovarian cancer including fallopian tube
cancer and primary peritoneal cancer. Version 2.2020-January 12,
2021. [cited 25 Feb 2021]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
|
|
31
|
Vasey PA, Atkinson R, Coleman R, Crawford
M, Cruickshank M, Eggleton P, Fleming D, Graham J, Parkin D, Paul
J, et al: Docetaxel-carboplatin as first line chemotherapy for
epithelial ovarian cancer. Br J Cancer. 84:170–178. 2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mokhlesuddin M, Akhter PS, Ahmed DU, Khan
MA, Rahman MA and Islam T: Clinical experience of using
docetaxel-cisplatin in the treatment of advanced ovarian cancer: A
Bangladesh perspective. J Clin Oncol. 24 (Suppl
18)(S15070)2006.
|
|
33
|
Vasey PA, Atkinson R, Osborne R, Parkin D,
Symonds R, Paul J, Lewsley L, Coleman R, Reed NS, Kaye S and Rustin
GJ: SCOTROC 2A: Carboplatin followed by docetaxel or
docetaxel-gemcitabine as first-line chemotherapy for ovarian
cancer. Br J Cancer. 94:62–68. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Katsumata N: Docetaxel: An alternative
taxane in ovarian cancer. Br J Cancer. 89 (Suppl 3):S9–S15.
2003.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Weiss RB, Donehower RC, Wiernik PH, Ohnuma
T, Gralla RJ, Trump DL, Baker JR Jr, Van Echo DA, Von Hoff DD and
Leyland-Jones B: Hypersensitivity reactions from taxol. J Clin
Oncol. 8:1263–1268. 1990.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Herzog TJ, Monk BJ, Rose PG, Braly P,
Hines JF, Bell MC, Wenham RM, Secord AA, Roman LD, Einstein MH, et
al: A phase II trial of oxaliplatin, docetaxel, and bevacizumab as
first-line therapy of advanced cancer of the ovary, peritoneum, and
fallopian tube. Gynecol Oncol. 132:517–525. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Obradović MMS, Hamelin B, Manevski N,
Couto JP, Sethi A, Coissieux MM, Münst S, Okamoto R, Kohler H,
Schmidt A and Bentires-Alj M: Glucocorticoids promote breast cancer
metastasis. Nature. 567:540–544. 2019.PubMed/NCBI View Article : Google Scholar
|