Introduction
Liver cancer is the sixth-most commonly diagnosed
cancer and the fourth leading cause of cancer death worldwide
(1). Hepatocellular carcinoma (HCC)
accounts for 90% of primary liver cancers, and hepatitis B virus
(HBV) and hepatitis C virus (HCV) infection as well as alcohol
consumption and non-alcoholic steatohepatitis (NASH) are known risk
factors (2-4).
The early detection of HCC by regular surveillance
may lead to curative treatment and improve the prognosis (5). Several methods developed for the
diagnosis of HCC, including the evaluation of serum markers,
ultrasonography (US), computed tomography (CT), and magnetic
resonance imaging (MRI), have been tested clinically. Among these
methods, US is simple with a low invasiveness, but its accuracy
depends on the skill of the examiner. Contrast-enhanced CT and MRI
are useful for the diagnosis but are invasive. The most widely used
markers are α-fetoprotein (AFP) and des-γ carboxy prothrombin
(DCP), serum proteins that are elevated in HCC. Although routine
screening offers the best chance for early tumor detection, the
reported sensitivities and specificities of elevated serum AFP and
DCP levels vary significantly (6-12).
In 2009, the highly sensitive Lens culinaris
agglutinin-reactive fraction of AFP (hs-AFP-L3) assay was
developed, and AFP-L3 measurement became possible, even in cases
with AFP <20 ng/ml (13).
However, biomarkers that contribute to hepatocarcinogenesis during
the long-term observation of patients with chronic liver disease
are still unclear. We previously examined the clinical utility of
hs-AFP-L3 in patients with chronic liver disease (9). Seven years have passed since that
study, so we examined the clinical utility of hs-AFP-L3 in a
long-term observation.
Materials and methods
Study population
Frozen serum samples were collected from 117
patients with chronic liver disease without HCC who visited our
hospital between December 1, 2006, and March 31, 2011. The analysis
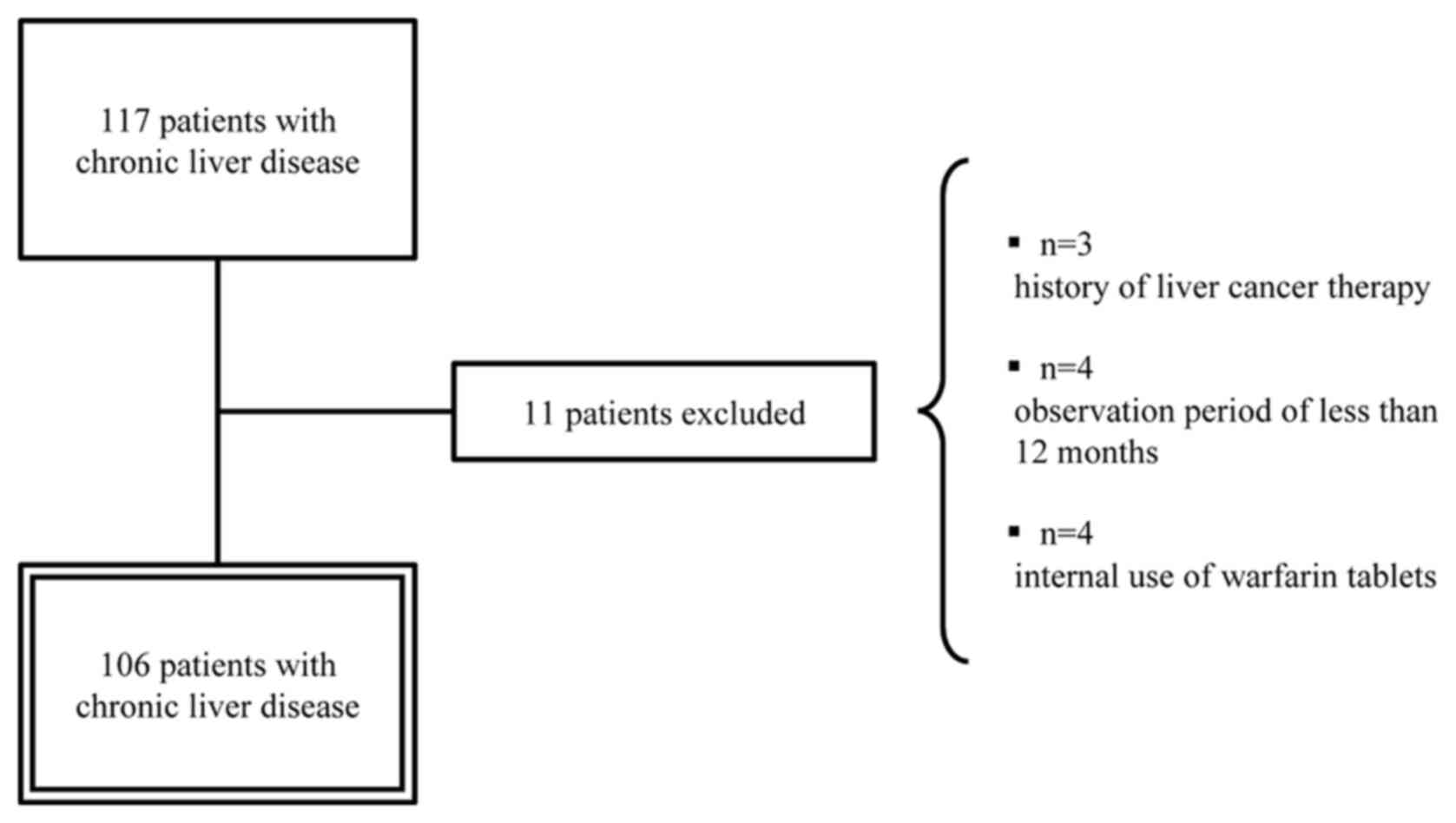
was performed on 106 patients, excluding those who had been treated
for HCC, those who had been under observation for less than 12
months, and those who were taking warfarin tablets (Fig. 1). In most patients with chronic
hepatitis, liver imaging with US was performed every 6 to 12
months, and in patients with cirrhosis, CT, MRI, or US was
performed every 3 to 6 months. The definitive diagnosis of HCC that
occurred during follow-up was made by interventional radiology CT
(IVR-CT). HBV was defined as Hepatitis B surface antigen (HBsAg)
positivity. HCV was defined as anti-HCV antibody positivity.
Measurement of serum AFP and
hs-AFP-L3
AFP and hs-AFP-L3 levels were measured using the
cryopreserved serum. Hs-AFP-L3 was measured by microchip capillary
electrophoresis and a liquid-phase binding assay on a µ-TASWako i30
automatic analyzer (Wako Pure Chemical Industries, Ltd.) (13). When hs-AFP-L3 was not detectable,
the percentage of hs-AFP-L3 was defined as 0%.
Statistical analysis
First, we considered the correlation between the
hs-AFP-L3 level and other clinical data. In addition, the presence
of HCC as of March 31, 2018, was confirmed, and the ability to
predict hepatocarcinogenesis using liver tumor markers was compared
using a receiver operating characteristic (ROC) curve. Finally, we
investigated the factors contributing to hepatocarcinogenesis using
univariate and multivariate analyses. The cut-off value was set to
an optimal value using the Youden index (sensitivity +
specificity-1) (14).
Statistical analyses were performed using the SPSS
statistical software program, version 25 (IBM Corp.). Categorical
data were compared using the chi-squared test and Fisher's exact
test, as appropriate. Continuous variables were analyzed using the
Mann-Whitney U test. The correlation coefficient was tested using
Spearman's rank correlation coefficient or Pearson's correlation
coefficient. The Kaplan-Meier method was used to estimate
cumulative incidence rate of HCC, and its distribution curves were
compared using the log-rank test. P-values of <0.05 were
considered to indicate statistical significance. Factors
contributing to hepatocarcinogenesis were determined using Cox's
proportional hazards model with forward selection using P<0.10
as a cut-off for inclusion in the model.
Results
Clinical feature of patients
The clinical characteristics of the population
analyzed are shown (Table I). The
causes of chronic liver disease were HBV in 23 cases, HCV in 60
cases, and non-HBV and non-HCV in 23 cases, of which 17 were liver
cirrhosis. The median observation period was 88 months (15-132
months). The AFP value in the analysis subject population was
27.1±119.3 ng/ml, and the hs-AFP-L3 value was 2.9±5.3%.
 | Table IClinical features of patients with
benign liver disease (n=106). |
Table I
Clinical features of patients with
benign liver disease (n=106).
| Variable | Value |
|---|
| Agea, years | 57.5 (11-82) |
| Sex (male/female),
n | 38/68 |
| CH/LC, n | 89/17 |
| Etiology
(HBV/HCV/NBNC), n | 23/60/23 |
| Child-Pugh class
(A/B/C/unknown), n | 83/3/2/18 |
| AFPb, ng/ml | 27.1±119.3 |
|
hs-AFP-L3b,
% | 2.9±5.3 |
| DCPb, mAU/ml | 18±8 |
| Platelet
countb,
x104/µl | 16.4±7.2 |
| ALTb, U/l | 79±121 |
| Total
bilirubinb, mg/dl | 1.0±0.6 |
| Albuminb, g/dl | 4.2±0.5 |
| Hyaluronic
acidb, ng/ml | 175.7±444.6 |
| Observation
perioda, months | 88 (15-132) |
Correlation between hs-AFP-L3 and
clinical data
We confirmed the correlation between hs-AFP-L3 and
other clinical data. Hs-AFP-L3 showed a positive correlation with
the age, alanine transaminase (ALT), hyaluronic acid, and AFP and a
negative correlation with the platelet count and albumin (Table IIA). In a study of 90 patients with
AFP <20 ng/ml, hs-AFP-L3 showed a positive correlation with the
age, hyaluronic acid, and AFP and a negative correlation with the
platelet count (Table IIB).
 | Table IIAssociation between highly sensitive
Lens culinaris agglutinin-reactive fraction of AFP and
clinical data. |
Table II
Association between highly sensitive
Lens culinaris agglutinin-reactive fraction of AFP and
clinical data.
| A, Correlation with
highly sensitive Lens culinaris agglutinin-reactive fraction
of AFP (n=106) |
|---|
| Variable | Agea | ALTa | Hyaluronic
acida | AFPa | DCPa | Pltb |
Albumina | T-Bila |
|---|
| Correlation
coefficient | 0.232 | 0.262 | 0.479 | 0.724 | -0.080 | -0.256 | -0.354 | 0.163 |
| P-value | 0.017 | 0.007 | <0.001 | <0.001 | 0.418 | 0.008 | <0.001 | 0.096 |
| n | 106 | 106 | 106 | 106 | 106 | 106 | 101 | 105 |
| B, Correlation with
highly sensitive Lens culinaris agglutinin-reactive fraction
of AFP (n=90; AFP <20 ng/ml) |
| Variable | Agea | ALTa | Hyaluronic
acida | AFPa | DCPa | Pltb |
Albumina | T-Bila |
| Correlation
coefficient | 0.269 | 0.164 | 0.352 | 0.666 | -0.159 | -0.282 | -0.211 | -0.011 |
| P-value | 0.010 | 0.123 | 0.001 | <0.001 | 0.134 | 0.007 | 0.052 | 0.922 |
| n | 90 | 90 | 90 | 90 | 90 | 90 | 85 | 89 |
Cumulative incidence of HCC
The presence of HCC as of March 31, 2018, was
confirmed, and 17 out of 106 patients (16.0%) were found to have
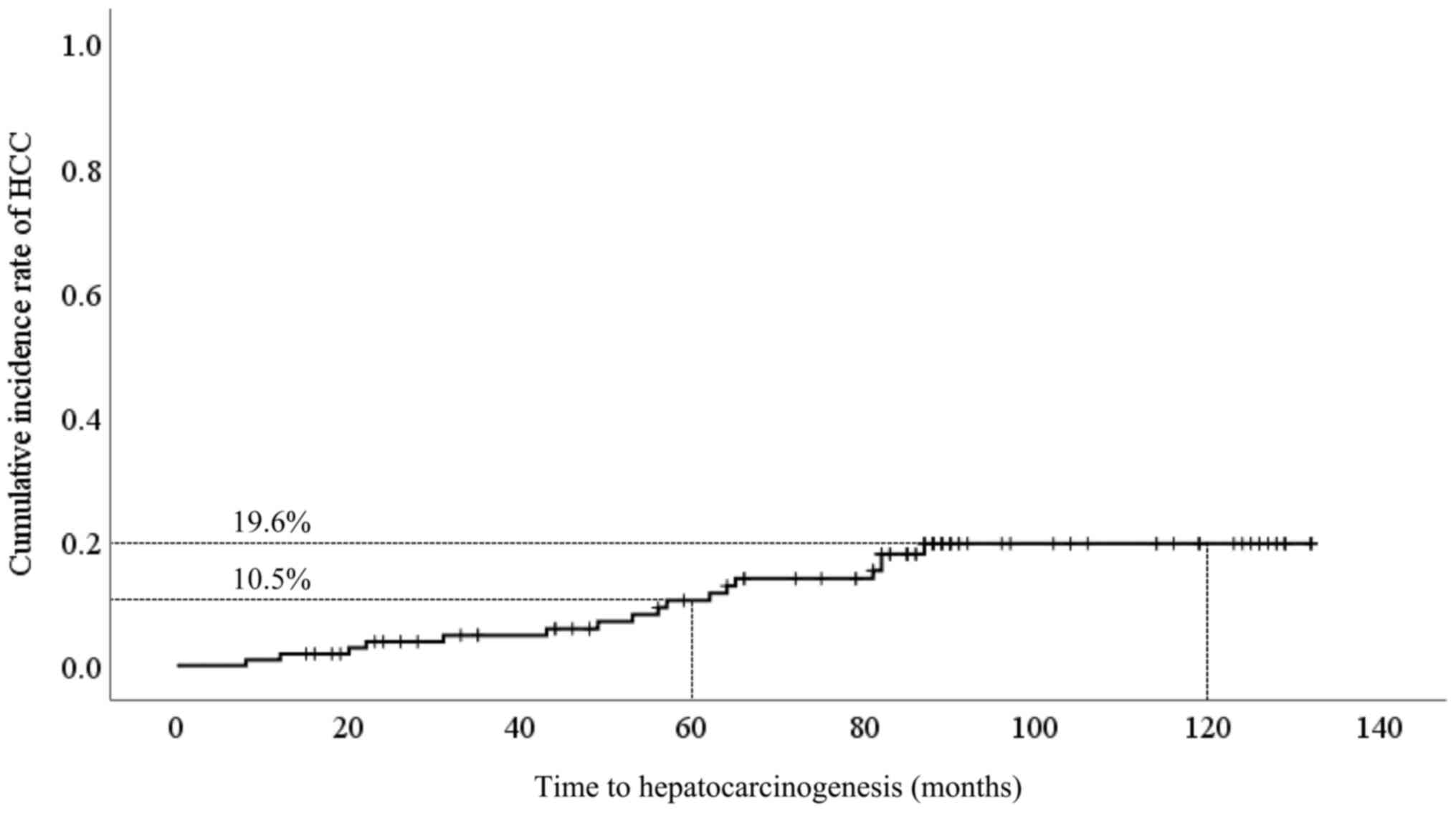
developed HCC. Cumulative incidence of HCC development was 10.5% at
5 years and 19.6% at 10 years (Fig.
2). The clinical characteristics of hepatocarcinogenesis cases
are shown in Tables III and
IV. In the background comparison
between the non-carcinogenic group and the carcinogenic group, the
age (P=0.009), AFP (P<0.001), hs-AFP-L3 (P<0.001), platelet
count (P<0.001), ALT (P=0.018), albumin (P=0.023), and
hyaluronic acid (P<0.001) differed significantly (Table IV).
 | Table IIICharacterization of 17 patients with
benign liver disease who developed HCC. |
Table III
Characterization of 17 patients with
benign liver disease who developed HCC.
| Case no. | Age, years | Sex | CH/LC | Etiology | AFP, ng/ml | hs-AFP-L3, % | DCP, mAU/ml | ALT, U/l | Months until HCC
detection |
|---|
| 1 | 60 | Male | LC | HCV | 28.5 | 9.6 | 32 | 65 | 8 |
| 2 | 70 | Female | LC | NBNC | 10.9 | 8.4 | 15 | 39 | 12 |
| 3 | 65 | Female | CH | HCV | 3.3 | <0.5 | 26 | 82 | 20 |
| 4 | 69 | Male | CH | HCV | 4.7 | 6.4 | 13 | 52 | 22 |
| 5 | 70 | Female | CH | HCV | 8.3 | 7.0 | 15 | 48 | 31 |
| 6 | 56 | Female | CH | HCV | 46.9 | 4.5 | 5 | 98 | 43 |
| 7 | 73 | Female | CH | HCV | 11.4 | 7.3 | 12 | 54 | 49 |
| 8 | 59 | Female | CH | HCV | 32.0 | 3.7 | 13 | 116 | 53 |
| 9 | 52 | Male | CH | HBV | 23.6 | 7.1 | 10 | 489 | 56 |
| 10 | 73 | Female | LC | HCV | 9.6 | 6.6 | 13 | 71 | 57 |
| 11 | 70 | Female | CH | HBV | 24.3 | 8.4 | 13 | 30 | 62 |
| 12 | 53 | Male | CH | HCV | 6.4 | 5.2 | 29 | 145 | 64 |
| 13 | 66 | Female | CH | HCV | 10.0 | 5.8 | 19 | 122 | 65 |
| 14 | 57 | Female | CH | HCV | 7.8 | 7.9 | 45 | 52 | 81 |
| 15 | 53 | Male | LC | NBNC | 3.7 | <0.5 | 53 | 22 | 82 |
| 16 | 62 | Male | CH | HCV | 23.0 | 7.5 | 35 | 72 | 82 |
| 17 | 66 | Female | CH | HCV | 576.0 | 3.1 | 8 | 126 | 87 |
 | Table IVClinical features of patients in the
non-carcinogenic and carcinogenic groups. |
Table IV
Clinical features of patients in the
non-carcinogenic and carcinogenic groups.
| Variable | Non-carcinogenic
group (n=89) | Carcinogenic group
(n=17) | P-value |
|---|
| Agea, years | 54±15 | 63±7 | 0.009b |
| Sex (male/female),
n | 32/57 | 6/11 | 0.958c |
| CH/LC, n | 76/13 | 13/4 | 0.468d |
| Etiology
(HBV/HCV/NBNC), n | 21/47/21 | 2/13/2 | 0.197c |
| Child-Pugh class
(A/B/C/unknown), n | 68/2/2/17 | 15/1/0/1 | 0.428c |
| AFPa, ng/ml | 22.9±116.1 | 48.8±136.4 |
<0.001b |
|
hs-AFP-L3a, % | 2.4±5.5 | 5.8±2.8 |
<0.001b |
| DCPa, mAU/ml | 17±6 | 21±14 | 0.928b |
| Platelet
counta,
x104/µl | 17.5±7.1 | 10.7±4.9 |
<0.001b |
| ALTa, U/l | 75±123 | 99±107 | 0.018b |
| Total
bilirubina,
mg/dl | 1.0±0.6 | 0.9±0.4 | 0.585b |
|
Albumina,
g/dl | 4.2±0.5 | 4.0±0.5 | 0.023b |
| Hyaluronic
acida, ng/ml | 172.6±483.2 | 191.6±110.9 |
<0.001b |
| Observation
perioda, months | 84±32 | 98±18 | 0.060b |
Predictive ability for
hepatocarcinogenesis
On comparing the predictive ability for
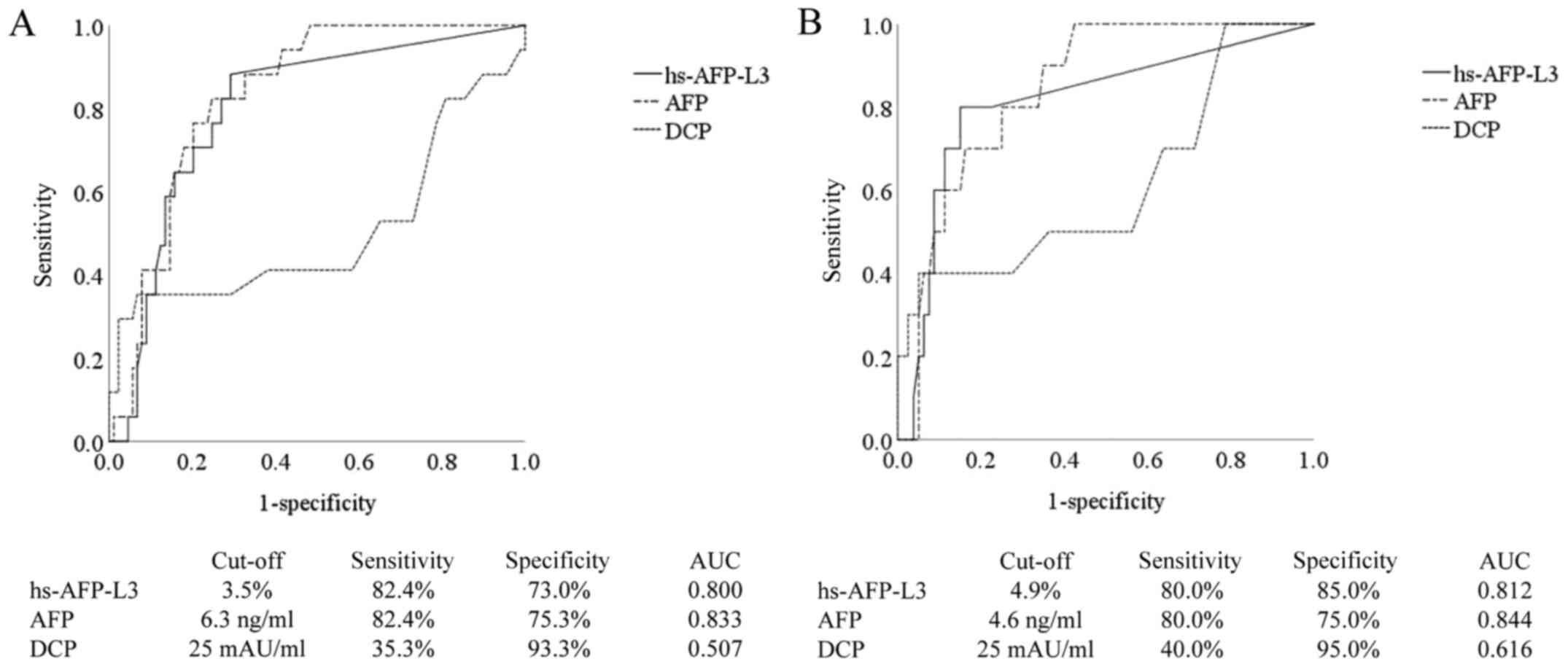
hepatocarcinogenesis using an ROC curve, the cut-off value of
hs-AFP-L3 was 3.5%, and the sensitivity, specificity, and the area
under the ROC curve (AUC) were 82.4, 73.0%, and 0.800,
respectively. Similarly, the cut-off value of AFP was 6.3 ng/ml,
and the sensitivity, specificity, and AUC were 82.4, 75.3%, and
0.833, respectively. The cut-off value of DCP was 25 mAU/ml, and
the sensitivity, specificity, and AUC were 35.3, 93.3%, and 0.507,
respectively (Fig. 3A). The ability
of hs-AFP-L3 and AFP to predict HCC development was higher than
that of DCP. In the analysis of 90 patients with AFP <20 ng/ml,
the cut-off value of hs-AFP-L3 was 4.9%, and the sensitivity,
specificity, and AUC were 80.0, 85.0%, and 0.812, respectively.
Similarly, the cut-off value of AFP was 4.6 ng/ml, and the
sensitivity, specificity, and AUC were 80.0, 75.0%, and 0.844,
respectively. The cut-off value of DCP was 25 mAU/ml, and the
sensitivity, specificity, and AUC were 40.0%, 95.0%, and 0.616,
respectively (Fig. 3B). In patients
with AFP <20 ng/ml, the ability of hs-AFP-L3 and AFP to predict
HCC development was higher than that of DCP.
Factors contributing to
hepatocarcinogenesis
An examination of the factors contributing to
hepatocarcinogenesis according to a univariate analysis showed that
age ≥55 years old (P=0.016), platelet count ≤13.1x104/µl
(P=0.001), hyaluronic acid ≥80.8 ng/ml (P<0.001), ALT ≥47 U/l
(P=0.008), AFP ≥6.3 ng/ml (P<0.001), hs-AFP-L3 ≥3.5%
(P<0.001), DCP ≥25 mAU/ml (P=0.002) were significant factors. In
the multivariate analysis, the platelet count
≤13.1x104/µl (hazard ratio [HR]=4.966, 95% confidence
interval [CI] 1.597-15.437, P=0.006) and hs-AFP-L3 ≥3.5% (HR=5.450,
95% CI 1.522-19.512, P=0.009) were extracted as significant factors
contributing to hepatocarcinogenesis (Table V). In addition, in patients with AFP
<20 ng/ml, the univariate analysis showed that age ≥64 years old
(P=0.005), liver cirrhosis (P=0.047), platelet count
≤13.1x104/µl (P=0.002), hyaluronic acid ≥67.7 ng/ml
(P=0.010), ALT ≥47 U/l (P=0.037), AFP ≥4.6 ng/ml (P=0.002),
hs-AFP-L3 ≥4.9% (P<0.001), DCP ≥25 mAU/ml (P=0.003) were
significant factors. In the multivariate analysis, hs-AFP-L3 ≥4.9%
(HR=11.608, 95% CI 2.422-55.629, P=0.002) and DCP ≥25 mAU/ml
(HR=3.936, 95% CI 1.088-14.231, P=0.037) were extracted as
significant factors contributing to hepatocarcinogenesis (Table VI).
 | Table VFactors contributing to
hepatocarcinogenesis (n=106). |
Table V
Factors contributing to
hepatocarcinogenesis (n=106).
| | Univariate
analysisa | Multivariate
analysisb |
|---|
| Variable | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age (<55 vs. ≥55
years) | 0.016 | | | |
| Sex (female vs.
male) | 0.978 | | | |
| Background liver
(CH vs. LC) | 0.128 | | | |
| Total bilirubin
(<0.6 vs. ≥0.6 mg/dl) | 0.212 | | | |
| Albumin (>4.4
vs. ≤4.4 g/dl) | 0.113 | | | |
| Platelet count
(>13.1 vs. ≤13.1x104/µl) | 0.001 | 4.966 | 1.597-15.437 | 0.006 |
| Hyaluronic acid
(<80.8 vs. ≥80.8 ng/ml) | <0.001 | | | |
| ALT (<47 vs. ≥47
U/l) | 0.008 | 3.019 | 0.841-10.836 | 0.090 |
| AFP (<6.3 vs.
≥6.3 ng/ml) | <0.001 | | | |
| hs-AFP-L3 (<3.5
vs. ≥3.5%) | <0.001 | 5.450 | 1.522-19.512 | 0.009 |
| DCP (<25 vs. ≥25
mAU/ml) | 0.002 | | | |
 | Table VIFactors contributing to
hepatocarcinogenesis (n=90; AFP <20 ng/ml). |
Table VI
Factors contributing to
hepatocarcinogenesis (n=90; AFP <20 ng/ml).
| | Univariate
analysisa | Multivariate
analysisb |
|---|
| Variable | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age (<64 vs. ≥64
years) | 0.005 | | | |
| Sex (female vs.
male) | 0.844 | | | |
| Background liver
(CH vs. LC) | 0.047 | | | |
| Total bilirubin
(<1.2 vs. ≥1.2 mg/dl) | 0.218 | | | |
| Albumin (>4.4
vs. ≤4.4 g/dl) | 0.119 | | | |
| Platelet count
(>13.1 vs. ≤13.1x104/µl) | 0.002 | | | |
| Hyaluronic acid
(<67.7 vs. ≥67.7 ng/ml) | 0.010 | | | |
| ALT (<47 vs. ≥47
U/l) | 0.037 | | | |
| AFP (<4.6 vs.
≥4.6 ng/ml) | 0.002 | | | |
| hs-AFP-L3 (<4.9
vs. ≥4.9%) | <0.001 | 11.608 | 2.422-55.629 | 0.002 |
| DCP (<25 vs. ≥25
mAU/ml) | 0.003 | 3.936 | 1.088-14.231 | 0.037 |
Comparison of cumulative incidence of
HCC by hs-AFP-L3
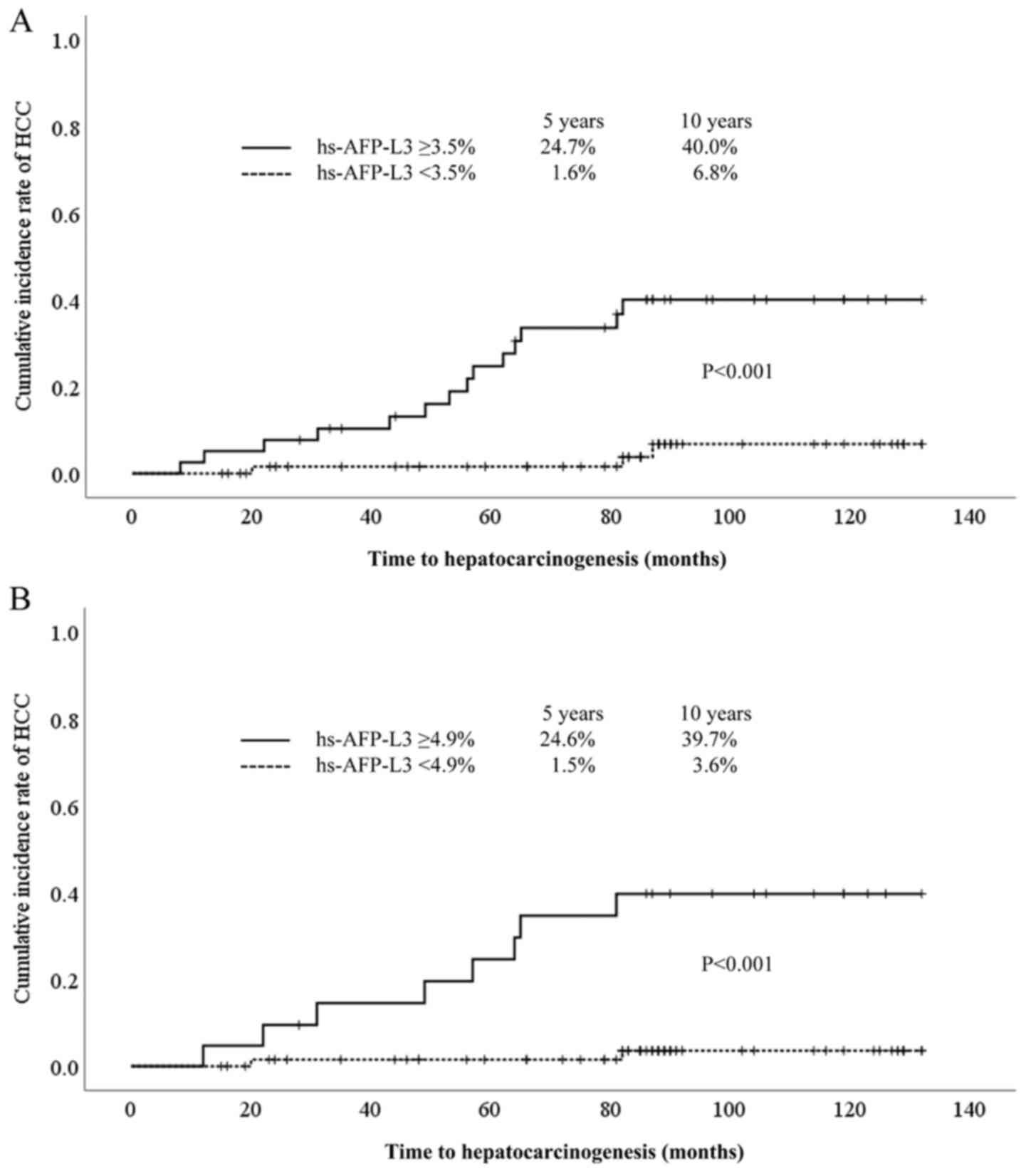
The cumulative incidence of HCC was significantly
higher in patients with hs-AFP-L3 ≥3.5% than in those with
hs-AFP-L3 <3.5% (24.7% at 5 years, 40.0% at 10 years vs. 1.6% at
5 years, 6.8% at 10 years, P<0.001) (Fig. 4A). In addition, in cases of AFP
<20 ng/ml, the cumulative incidence of HCC was significantly
higher in patients with hs-AFP-L3 ≥4.9% than in those with
hs-AFP-L3 <4.9% (24.6% at 5 years, 39.7% at 10 years vs. 1.5% at
5 years, 3.6% at 10 years, P<0.001) (Fig. 4B).
Discussion
Recent advances in imaging technology have enabled
the early detection of HCC (15-17),
so low AFP cases often result in a diagnosis of
hepatocarcinogenesis. In the present study, it was found that 10 of
17 patients with AFP <20 ng/ml (58.8%) had hepatocarcinogenic on
long-term follow-up. In our previous study, we found that hs-AFP-L3
was a useful marker for predicting hepatocarcinogenesis, but the
observation period averaged 32.8 months, and the examination was
made by a univariate analysis (9).
In the present study, after a long-term observation, we performed a
multivariate analysis of factors associated with the development of
HCC and revealed that hs-AFP-L3 was the best predictive marker for
hepatocarcinogenesis. It was found to be particularly useful in
cases with AFP <20 ng/ml.
AFP is a glycoprotein with a molecular weight of 67
kDa that was first reported in human fetal serum by Bergstrand and
Czar (18) in 1956. AFP is elevated
in patients with HCC but also in the active phases of chronic
hepatitis and cirrhosis as well as in AFP-producing tumors other
than liver cancer (19). The sugar
chains of AFP differ depending on the producing cell, and the L3
fraction is specific for HCC in terms of its affinity for lentil
lectin (20-24).
However, measurement of AFP-L3 has not always been reliable for
serum samples with a low total AFP concentration, as determined by
conventional lectin affinity system (LiBASys) (25). The highly sensitive AFP-L3
measurement method uses an on-chip electrokinetic reaction and
separation by affinity electrophoresis (micro-total analysis
system; µ-TAS) (26). This system
has enabled the accurate measurement of AFP-L3 at very low AFP
concentrations.
In a previous report on the prediction of
hepatocarcinogenesis, Kumada et al (22) conducted a study of 104 patients with
hepatocarcinogenesis and 104 controls matched by propensity scores
in HCC surveillance involving 2,830 patients with chronic liver
disease. One year before the diagnosis of HCC, the cut-off value of
hs-AFP-L3 was 7%, and the sensitivity and specificity were 34.3 and
74.7%, respectively. Similarly, with cut-off values of AFP 20 ng/ml
and DCP 40 mAU/ml, the respective sensitivity was 35.0 and 12.1%,
and the respective specificity was 86.4 and 93.9% (22). In the present study, the best
cut-off values of hs-AFP-L3, AFP, and DCP for predicting HCC were
3.5%, 6.3 ng/ml, and 25 mAU/ml, respectively, with respective
sensitivities of 82.4, 82.4 and 35.3% and respective specificities
of 73.0, 75.3, and 93.3%. Similarly, in patients with AFP <20
ng/ml, the best cut-off values of hs-AFP-L3, AFP, and DCP were
4.9%, 4.6 ng/ml and 25 mAU/ml, respective, with respective
sensitivities of 80.0, 80.0 and 40.0% and respective specificities
of 85.0, 75.0 and 95.0%. In this way, our findings differed from
those of previous reports. This discrepancy is attributed to
differences in the study design, as the previous report used stored
sera collected annually for three years before the diagnosis of
HCC. In addition, the median observation period in our study was 88
months (15 to 132 months), which was longer than in the previous
study.
In this study, 11 out of 17 cases of
hepatocarcinogenesis developed HCC more than 4 years after the
test, and 9 out of 11 cases (81.8%) had hs-AFP-L3 ≥3.5%. The
doubling time of HCC is reported to be 100 days, and it
theoretically takes about 9 years for a 10-µm HCC to become a 10-mm
lesion, which can be detected by diagnostic imaging (27). In other words, the involvement of
hs-AFP-L3 in hepatocarcinogenesis several years later may indicate
the presence of minute HCC.
However, in hepatitis C patients treated with
interferon, the hepatocarcinogenesis rate decreases after achieving
a sustained virologic response (SVR), and AFP values after
antiviral therapy are known to be independent predictors of
hepatocarcinogenesis (28,29). In addition, it has been reported
that serum Wisteria floribunda agglutinin positive Mac-2
binding protein (WFA+M2BP), a liver fibrosis marker that
has recently been clinically applied, becomes a risk factor for
hepatocarcinogenesis after achieving an SVR of hepatitis C
(30,31). Direct-acting antivirals (DAAs) have
been developed for HCV, and virus elimination by DAA reportedly
suppresses hepatocarcinogenesis (32,33).
The usefulness of measuring the hs-AFP-L3 value before and after
DAA therapy is unclear at present and needs to be clarified in the
future.
The study is limited by its retrospective nature and
the small number of cases.
In conclusion, hs-AFP-L3 is a useful marker for
predicting hepatocarcinogenesis in the long-term observation of
patients with chronic liver disease.
Acknowledgements
The authors would like to thank Ms. Hiromi Eguchi,
Ms. Yuko Nakamura and Ms. Eriko Koreeda (all Digestive and
Lifestyle Diseases, Department of Human and Environmental Sciences,
Kagoshima University Graduate School of Medical and Dental
Sciences, Kagoshima, Japan) for their technical assistance and data
management.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KT, SM, KO, KK and AI conceived the study. KT, SM,
KO, OT, AT, SI, HS, KK and SK contributed the acquisition of data.
KT and SM confirm the authenticity of all the raw data. KT and SM
analyzed the data and prepared the manuscript. KT, SM and AI
reviewed the manuscript. KT, SM, KO, OT, AT, SI, HS, KK, SK and AI
have been involved in revising the manuscript critically for
important intellectual content and agreed to be accountable for all
aspects of the work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol conformed to the ethical
guidelines of the Declaration of Helsinki and was approved by the
Kagoshima University Ethics Committee on Epidemiological Studies
(approval no. 180162; Kagoshima, Japan). Written informed consent
was obtained from all patients for the use of stored serum
samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
European Association For The Study Of The
Liver; European Organisation For Research And Treatment Of Cancer.
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Global Burden of Disease Liver Cancer
Collaboration. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu
MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, et al: The
burden of primary liver cancer and underlying etiologies from 1990
to 2015 at the global, regional, and national level: Results from
the global burden of disease study 2015. JAMA Oncol. 3:1683–1691.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Llovet JM, Zucman-Rossi J, Pikarsky E,
Sangro B, Schwartz M, Sherman M and Gores G: Hepatocellular
carcinoma. Nat Rev Dis Primers. 2(16018)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang BH, Yang BH and Tang ZY: Randomized
controlled trial of screening for hepatocellular carcinoma. J
Cancer Res Clin Oncol. 130:417–422. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Grazi GL, Mazziotti A, Legnani C, Jovine
E, Miniero R, Gallucci A, Palareti G and Gozzetti G: The role of
tumor markers in the diagnosis of hepatocellular carcinoma, with
special reference to the des-gamma-carboxy prothrombin. Liver
Transpl Surg. 1:249–255. 1995.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ishii M, Gama H, Chida N, Ueno Y, Shinzawa
H, Takagi T, Toyota T, Takahashi T and Kasukawa R: Simultaneous
measurements of serum alpha-fetoprotein and protein induced by
vitamin K absence for detecting hepatocellular carcinoma. South
Tohoku District study group. Am J Gastroenterol. 95:1036–1040.
2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Marrero JA, Su GL, Wei W, Emick D,
Conjeevaram HS, Fontana RJ and Lok AS: Des-gamma carboxyprothrombin
can differentiate hepatocellular carcinoma from nonmalignant
chronic liver disease in american patients. Hepatology.
37:1114–1121. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Oda K, Ido A, Tamai T, Matsushita M,
Kumagai K, Mawatari S, Saishoji A, Kure T, Ohno K, Toyokura E, et
al: Highly sensitive Lens culinaris agglutinin-reactive
α-fetoprotein is useful for early detection of hepatocellular
carcinoma in patients with chronic liver disease. Oncol Rep.
26:1227–1233. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Oka H, Tamori A, Kuroki T, Kobayashi K and
Yamamoto S: Prospective study of alpha-fetoprotein in cirrhotic
patients monitored for development of hepatocellular carcinoma.
Hepatology. 19:61–66. 1994.PubMed/NCBI
|
|
11
|
Okuda H, Nakanishi T, Takatsu K, Saito A,
Hayashi N, Takasaki K, Takenami K, Yamamoto M and Nakano M: Serum
levels of des-gamma-carboxy prothrombin measured using the revised
enzyme immunoassay kit with increased sensitivity in relation to
clinicopathologic features of solitary hepatocellular carcinoma.
Cancer. 88:544–549. 2000.PubMed/NCBI
|
|
12
|
Wang CS, Lin CL, Lee HC, Chen KY, Chiang
MF, Chen HS, Lin TJ and Liao LY: Usefulness of serum
des-gamma-carboxy prothrombin in detection of hepatocellular
carcinoma. World J Gastroenterol. 11:6115–6119. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kagebayashi C, Yamaguchi I, Akinaga A,
Kitano H, Yokoyama K, Satomura M, Kurosawa T, Watanabe M, Kawabata
T, Chang W, et al: Automated immunoassay system for AFP-L3% using
on-chip electrokinetic reaction and separation by affinity
electrophoresis. Anal Biochem. 388:306–311. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Youden WJ: Index for rating diagnostic
tests. Cancer. 3:32–35. 1950.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kierans AS, Kang SK and Rosenkrantz AB:
The diagnostic performance of dynamic contrast-enhanced MR imaging
for detection of small hepatocellular carcinoma measuring Up to 2
cm: A meta-analysis. Radiology. 278:82–94. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Onishi H, Kim T, Imai Y, Hori M, Nagano H,
Nakaya Y, Tsuboyama T, Nakamoto A, Tatsumi M, Kumano S, et al:
Hypervascular hepatocellular carcinomas: Detection with gadoxetate
disodium-enhanced MR imaging and multiphasic multidetector CT. Eur
Radiol. 22:845–854. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sangiovanni A, Manini MA, Iavarone M,
Romeo R, Forzenigo LV, Fraquelli M, Massironi S, Della Corte C,
Ronchi G, Rumi MG, et al: The diagnostic and economic impact of
contrast imaging techniques in the diagnosis of small
hepatocellular carcinoma in cirrhosis. Gut. 59:638–644.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bergstrand CG and Czar B: Demonstration of
a new protein fraction in serum from the human fetus. Scand J Clin
Lab Invest. 8(174)1956.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Taketa K: Alpha-fetoprotein: Reevaluation
in hepatology. Hepatology. 12:1420–1432. 1990.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Aoyagi Y, Isemura M, Suzuki Y, Sekine C,
Soga K, Ozaki T and Ichida F: Fucosylated alpha-fetoprotein as
marker of early hepatocellular carcinoma. Lancet. 2:1353–1354.
1985.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Korekane H, Hasegawa T, Matsumoto A,
Kinoshita N, Miyoshi E and Taniguchi N: Development of an
antibody-lectin enzyme immunoassay for fucosylated
alpha-fetoprotein. Biochim Biophys Acta. 1820:1405–1411.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kumada T, Toyoda H, Tada T, Kiriyama S,
Tanikawa M, Hisanaga Y, Kanamori A, Tanaka J, Kagebayashi C and
Satomura S: High-sensitivity Lens culinaris
agglutinin-reactive alpha-fetoprotein assay predicts early
detection of hepatocellular carcinoma. J Gastroenterol. 49:555–563.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Oka H, Saito A, Ito K, Kumada T, Satomura
S, Kasugai H, Osaki Y, Seki T, Kudo M and Tanaka M: Collaborative
Hepato-Oncology Study Group of Japan. Multicenter prospective
analysis of newly diagnosed hepatocellular carcinoma with respect
to the percentage of Lens culinaris agglutinin-reactive
alpha-fetoprotein. J Gastroenterol Hepatol. 16:1378–1383.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Taketa K, Sekiya C, Namiki M, Akamatsu K,
Ohta Y, Endo Y and Kosaka K: Lectin-reactive profiles of
alpha-fetoprotein characterizing hepatocellular carcinoma and
related conditions. Gastroenterology. 99:508–518. 1990.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nakamura K, Imajo N, Yamagata Y, Katoh H,
Fujio K, Tanaka T, Satomura S and Matsuura S: Liquid-phase binding
assay of alpha-fetoprotein using a sulfated antibody for bound/free
separation. Anal Chem. 70:954–957. 1998.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kawabata T, Wada HG, Watanabe M and
Satomura S: Electrokinetic analyte transport assay for
alpha-fetoprotein immunoassay integrates mixing, reaction and
separation on-chip. Electrophoresis. 29:1399–1406. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cucchetti A, Garuti F, Pinna AD and
Trevisani F: Italian Liver Cancer (ITA.LI.CA) group. Length time
bias in surveillance for hepatocellular carcinoma and how to avoid
it. Hepatol Res. 46:1275–1280. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hiramatsu N, Oze T and Takehara T:
Suppression of hepatocellular carcinoma development in hepatitis C
patients given interferon-based antiviral therapy. Hepatol Res.
45:152–161. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Oze T, Hiramatsu N, Yakushijin T, Miyazaki
M, Yamada A, Oshita M, Hagiwara H, Mita E, Ito T, Fukui H, et al:
Post-treatment levels of α-fetoprotein predict incidence of
hepatocellular carcinoma after interferon therapy. Clin
Gastroenterol Hepatol. 12:1186–1195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nagata H, Nakagawa M, Asahina Y, Sato A,
Asano Y, Tsunoda T, Miyoshi M, Kaneko S, Otani S, Kawai-Kitahata F,
et al: Effect of interferon-based and -free therapy on early
occurrence and recurrence of hepatocellular carcinoma in chronic
hepatitis C. J Hepatol. 67:933–939. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sasaki R, Yamasaki K, Abiru S, Komori A,
Nagaoka S, Saeki A, Hashimoto S, Bekki S, Kugiyama Y, Kuno A, et
al: Serum Wisteria floribunda agglutinin-positive Mac-2
binding protein values predict the development of hepatocellular
carcinoma among patients with chronic hepatitis C after sustained
virological response. PLoS One. 10(e0129053)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ioannou GN, Green PK and Berry K: HCV
eradication induced by direct-acting antiviral agents reduces the
risk of hepatocellular carcinoma. J Hepatol. 68:25–32.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kanwal F, Kramer J, Asch SM, Chayanupatkul
M, Cao Y and El-Serag HB: Risk of hepatocellular cancer in HCV
patients treated with direct-acting antiviral agents.
Gastroenterology. 153:996–1005.e1. 2017.PubMed/NCBI View Article : Google Scholar
|