Introduction
Brain metastases (BMs) are the most prevalent
malignant tumors of the CNS. More than 200,000 patients are
diagnosed with harboring BMs in the United States each year, and
the number is more than 10-fold of the primary CNS tumors (1). Breast cancer is the second most
frequent cause of BMs after lung cancer, and BMs occur in 24.6% of
patients with metastatic lesions (2). The reported median survival time from
diagnosis of BM ranges from six to 23 months (3). BMs are one of the major causes of
systemic cancer-related mortality. Lately, the frequency of BMs is
increasing due to advancements in treatment for primary cancers and
in imaging techniques (4,5). On the other hand, the heterogeneity of
BMs has been consistently reported since the 1950s. While such
studies were performed postmortem in early years, image analysis
with CT or MRI has recently become the primary research modality
for this kind of study. Preferential involvement of the anatomic
‘watershed areas,’ the gray-white matter junction and the
cerebellum, has been demonstrated in the development of BMs, and
the relationship between the spatial distribution of BMs and
primary cancer type has been discussed (6-9).
Few reports, however, have discussed the relationship between the
spatial distribution of BMs and biological subtypes of breast
cancer.
In this report, the authors hypothesized that the
spatial distribution of BMs could be affected by the biological
subtype of tumors. To test this hypothesis, the authors compared
the spatial distributions of breast cancer BMs and biological
subtypes.
Materials and methods
Patients and data collection
This study was conducted in accordance with the
Declaration of Helsinki, and the internal review board of the Osaka
International Cancer Institute approved the clinical data used in
this research. The authors retrospectively reviewed CT or MRI of
radiation- and operation-naive breast cancer BMs treated at Osaka
International Cancer Institute from 2008 to 2017, totaling 87
patients. Patients who underwent systemic chemotherapy, hormone
therapy, molecular targeted therapy with agents such as
trastuzumab, pertuzumab, or lapatinib, or radiation therapy were
included. However, patients who underwent previous neurosurgical
resection or brain radiation therapy of the lesion were excluded.
Furthermore, the following patients were excluded from analysis:
Five patients due to preexisting malignant diseases, three patients
lacking a sufficient immunohistochemical profile of their breast
cancer subtype, and 12 patients due to CNS lesions confined to the
meninges, the dura, or the skull. As a result, BMs of 67 patients
using contrast-enhanced CT, gadolinium-enhanced MRI T1-weighted
images (1.5 or 3.0T), or plain T2-weighted images were included for
analysis (1 by CT, 39 by 1.5T MRI, 27 by 3.0T MRI; 26 by thin slice
with <1.5 mm thickness, 41 with 2-7 mm thickness).
Biological subtypes of breast
cancer
Patients were divided into four groups according to
the biological subtype of their breast cancer. Selection criteria
were based on the expression of hormone receptors (HR) such as
estrogen receptor (ER) and progesterone receptor (PgR), as well as
human epidermal growth factor receptor 2 (HER2). Classified
subtypes were as follows: HR-positive and HER2-negative (i.e.,
ER+ and/or PgR+, HER2-; luminal
A), HR-positive and HER2-positive (ER+ and/or
PgR+, HER2+; luminal B), HR-negative and
HER2-positive (ER- and PgR-, and HER2+;
HER2), and HR-negative and HER2-negative (ER- and
PgR-, and HER2-; triple-negative breast
cancer; TNBC). Positivity of ER, PgR, and HER2 was evaluated via
immunohistochemistry of the primary breast cancer or the BM (66 by
primary cancer and one by BM). The threshold for a positive test
was set at 1% or greater of cells being positive for ER or PgR.
Fluorescence in situ hybridization analysis of HER2
amplification was carried out for cases where immunohistochemistry
showed higher than 2+ for HER2. The authors also reviewed medical
records to identify the initial symptoms of breast cancer BMs that
led to performing brain imaging and compared these symptoms and
breast cancer subtypes.
Image registration and frequency map
reconstruction
All Digital Imaging and Communications in Medicine
(DICOM) images were converted to Neuroimaging Informatics
Technology Initiative (NIfTI) format using dcm2nii software
(http://www.cabiatl.com/mricro/mricron/dcm2nii.html).
These NIfTI data were registered to a 1.0-mm isotropic,
high-resolution, T1-weighted brain atlas provided by the Montreal
Neurological Institute (MNI152 database) using a mutual information
algorithm with a 12-degree of freedom transformation using the
Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software
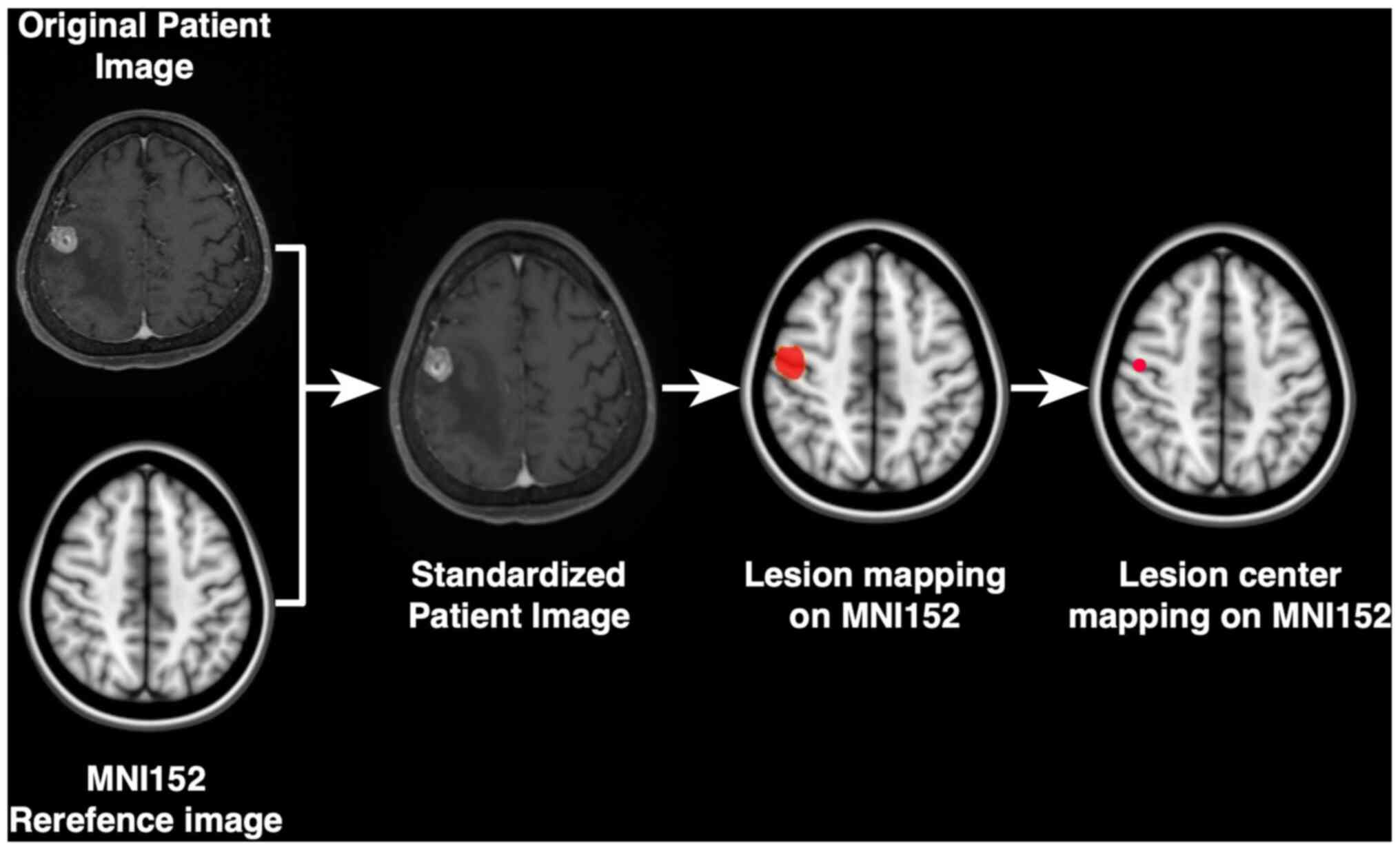
Library/FMRIB Linear Image Registration Tool (FSL-FLIRT; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL) (Fig. 1). Image registrations were visually
confirmed in all cases before using FSL-FLIRT (10,11).
All datasets were exported to in-house software
written in MatLab 7.14 (MathWorks) for further analysis. The
lesions were manually identified. Thereafter, the center of gravity
of each lesion was calculated automatically, and that voxel was
defined as the primary site of occurrence. For frequency map
reconstruction, all lesions were reconstructed to a site-centered
spherical shape with a diameter of 20 mm. A heat map for the lesion
occurrence frequency was reconstructed and superimposed on the
reference MNI152 (Fig. 2). The
voxel corresponding to the center of gravity of the lesion
registered to the MNI structural atlas (12) was assigned to 11 segments as
follows: Brainstem, caudate, cerebellum, frontal lobe, occipital
lobe, parietal lobe, temporal lobe, pineal body, pituitary gland,
putamen, and thalamus. As a result, each lesion was assigned to a
specific anatomical region on the MNI152 structural atlas.
Statistical analysis
Statistical analysis was performed by JMP 14 (SAS
Institute Inc.) and R version 3.5.2 (The R Foundation, Vienna,
Austria). All P-values were two-sided, and P-values <0.05 were
considered statistically significant. Data were compared between
groups using the Chi-square test with residual analysis and the
Kruskal-Wallis test for categorical and continuous variables. If
not otherwise indicated, data are shown as medians with an
interquartile range.
Results
Frequency map of metastatic brain
lesions from breast cancer
In total, the authors analyzed 437 lesions from 67
patients as follows: 162 lesions from 28 patients with luminal A
subtype, 30 lesions from 9 patients with luminal B subtype, 196
lesions from 14 patients with HER2 subtype, and 49 lesions from 16
patients with TNBC. Detailed characteristics of the patients are
shown in Table I. There were no
significant differences in the median numbers of BMs, the interval
time from initial diagnosis of the primary lesion to development of
BMs, and the ratio of patients with multiple BMs among biological
subtypes.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | All | Luminal A | Luminal B | HER2 | TNBC | P-value |
|---|
| Patients (n) | 67 | 28 | 9 | 14 | 16 | - |
| Female (n) | 67 | 28 | 9 | 14 | 16 | - |
| Age at diagnosis | | | | | | 0.59 |
|
Median
(years) | 56 | 57.5 | 59 | 52.5 | 56.5 | |
|
IQR | (44.5-65.5) | (43.3-67.3) | (51.0-64.0) | (38.3-61.0) | (48.0-70.3) | |
| Interval time from
initial diagnosisto BMs | | | | | | 0.17 |
|
Median
(months) | 46 | 70 | 24 | 33 | 47 | |
|
IQR | (23.0-93.0) | (28.8-112.3) | (23.0-57.0) | (18.5-59.8) | (29.5-68.8) | |
| Total number of BMs
(n) | 437 | 162 | 30 | 196 | 49 | - |
| Number of BMs, n,
median (IQR) | | | | | | 0.26 |
|
Median
(n) | 2 | 3.5 | 1 | 2 | 2 | |
|
IQR | (1-5.3) | (1.8-6.0) | (1.0-4.0) | (1.0-11.0) | (1.0-3.3) | |
| Patients with
multiple BMs, n (%) | 43(64) | 21(75) | 6(33) | 9(64) | 10(63) | 0.81 |
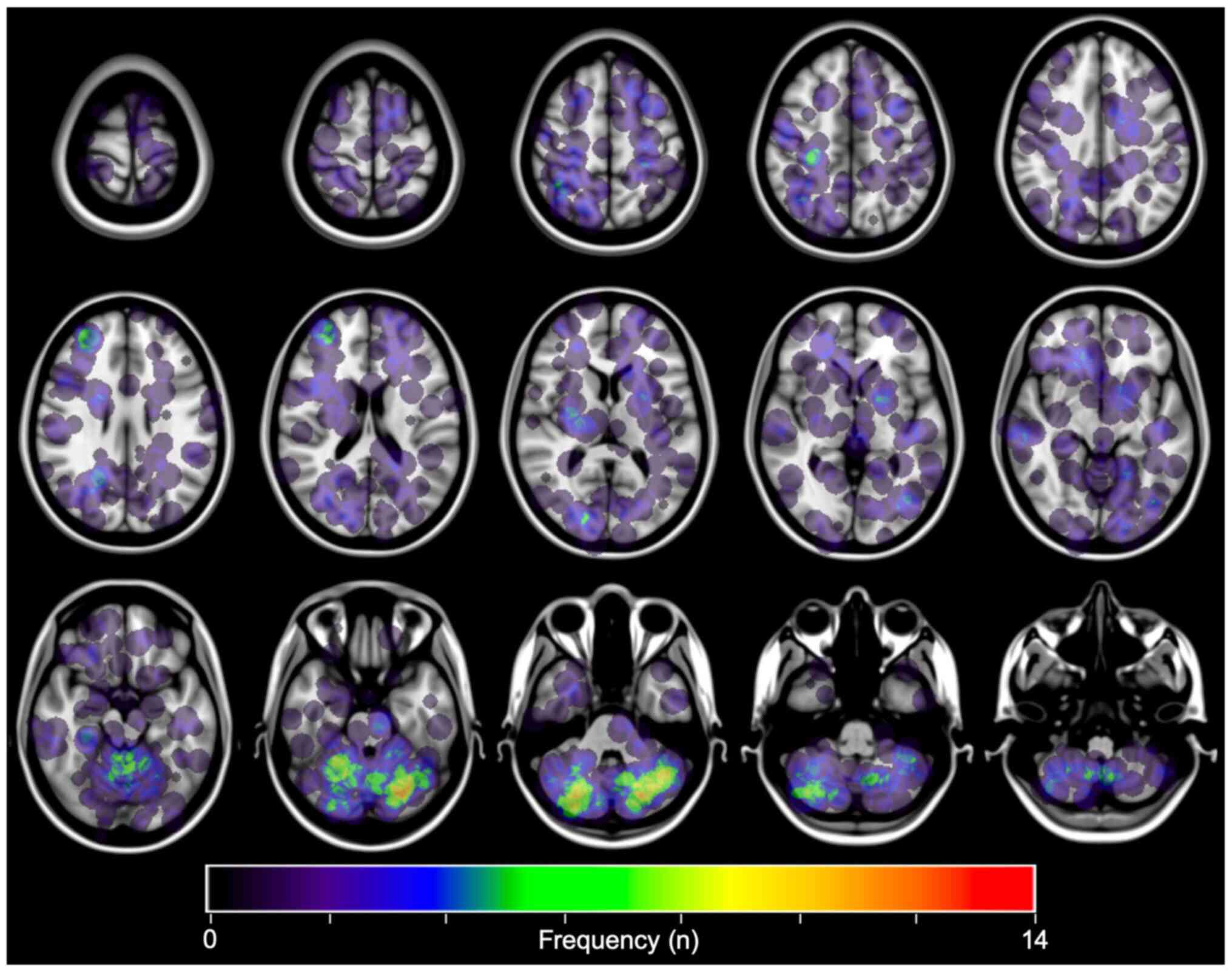
MRI from 66 patients and CT from one patient were
successfully transformed and registered on MNI152. The coordinates
corresponding to each lesion's cancer were identified, with all
lesions converted to spheres with a 20 mm diameter on MNI152. As
can be appreciated in Fig. 2,
breast cancer BMs most commonly involved the posterior fossa. This
observation was consistent with past studies (6,9,13,14).
Different intracerebral distribution
of breast cancer BMs by biological subtype
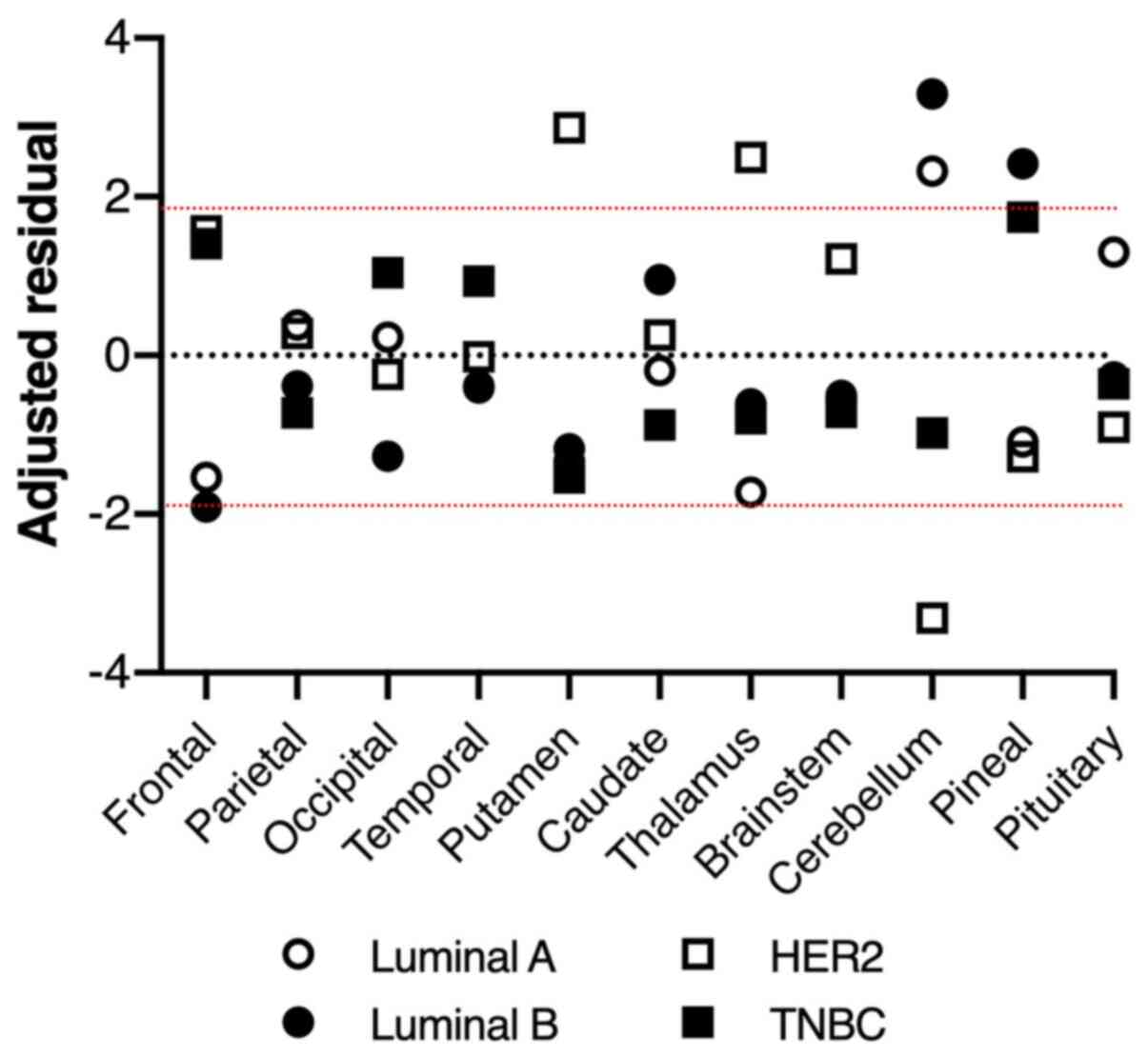
There was a significant difference in BMs'
intracerebral distribution among subtypes using the Chi-square test
(P=0.0057, Table II). Furthermore,
the residual analysis revealed that, while BMs from luminal A and B
type breast cancer predominantly occurred in the cerebellum with
higher proportions than those from the other subtypes, BMs from
HER2 breast cancer occurred in the cerebellum with a lower
proportion and in the putamen and thalamus with higher proportions
than those from the others (Fig.
3). Regarding the TNBC subtype, there were no significant
characteristics in common with the other subtypes.
 | Table IIDistribution of brain metastases. |
Table II
Distribution of brain metastases.
| Location | All n=437 (%) | LuminalA n=162
(%) | LuminalB n=30
(%) | HER2 n=196 (%) | TNBC n=49 (%) |
|---|
| Frontal lobe | 107 | (24.5) | 33 | (20.4) | 3 | (10.0) | 55 | (28.1) | 16 | (32.7) |
| Parietal lobe | 69 | (15.8) | 27 | (16.7) | 4 | (13.3) | 32 | (16.3) | 6 | (12.2) |
| Occipital lobe | 44 | (10.1) | 17 | (10.5) | 1 | (3.3) | 19 | (9.7) | 7 | (14.3) |
| Temporal lobe | 38 | (8.7) | 13 | (8.0) | 2 | (6.7) | 17 | (8.7) | 6 | (12.2) |
| Putamen | 18 | (4.1) | 4 | (2.5) | 0 | (0.0) | 14 | (7.1) | 0 | (0.0) |
| Caudate | 6 | (1.4) | 2 | (1.2) | 1 | (3.3) | 3 | (1.5) | 0 | (0.0) |
| Thalamus | 5 | (1.1) | 0 | (0.0) | 0 | (0.0) | 5 | (2.6) | 0 | (0.0) |
| Brain stem | 4 | (0.9) | 1 | (0.6) | 0 | (0.0) | 3 | (1.5) | 0 | (0.0) |
| Cerebellum | 143 | (32.7) | 64 | (39.5) | 18 | (60.0) | 48 | (24.5) | 13 | (26.5) |
| Pineal body | 2 | (0.5) | 0 | (0.0) | 1 | (3.3) | 0 | (0.0) | 1 | (2.0) |
| Pituitary
gland | 1 | (0.2) | 1 | (0.6) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
Anticancer drug therapies before BM
occurrence
Most of the patients underwent multiple treatment
regimens and various anticancer drugs were administered to each
patient before their BMs were discovered. Three patients did not
undergo any anticancer drugs because BMs had already occurred at
the initial diagnosis of primary breast cancer. Detailed past
treatment histories were not available for another three patients
from outside institutions. We compared the distributions of BMs
between two groups with and without chemotherapies, hormone
therapies, and molecular targeted therapies for 431 BMs from 64
patients. There was no statistical significance in these
comparisons with P-values being 0.43, 0.13, and 0.09, respectively
(Chi-square test).
Symptoms at brain metastasis
identification
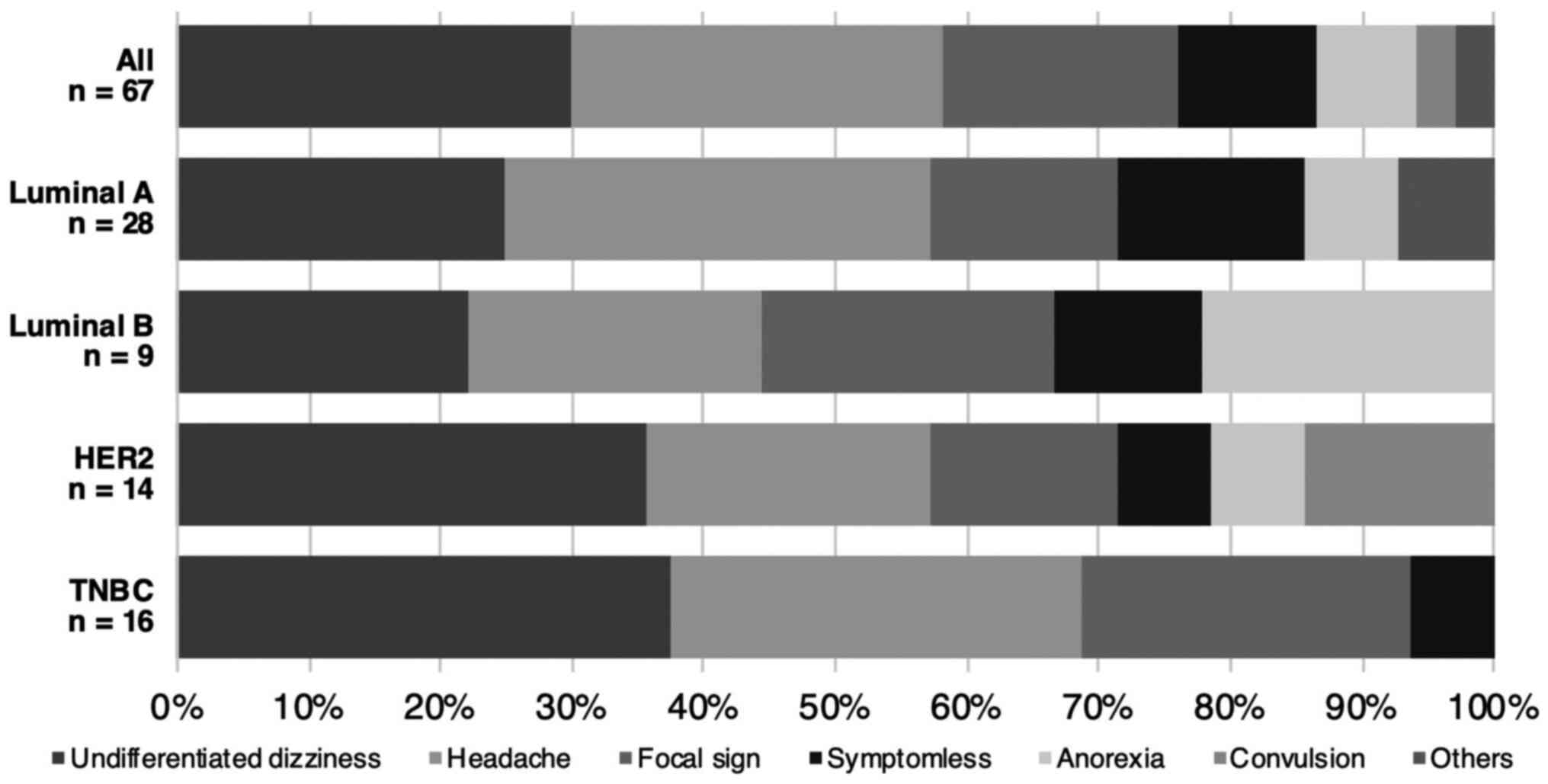
The most frequently encountered clinical symptom was
undifferentiated dizziness (29.9%), followed by headache (28.4%),
and focal signs (17.9%) such as motor aphasia, hemiparesis, and
visual disturbances. Seven patients did not present any
neurological symptoms (10.4%). There was no significant difference
in symptomatology among different breast cancer subtypes (P=0.51,
Fig. 4).
Discussion
Several classifications of biological subtypes based
on their clinical, histopathological, and molecular characteristics
are used for breast cancers. Furthermore, breast cancers can be
grouped by their molecular expression states into luminal A or B,
HER2, and triple-negative, and each subtype has different prognoses
(15,16). TNBC and HER2 breast cancers are
liable to develop BMs (2,3,17-19).
Of note, the onset of BMs in TNBC is earlier, and overall survival
is notably shorter than other subtypes (2,3).
Anatomical watershed areas such as the gray-white matter junction
are known as common sites for BMs (7). It is known that the spatial
distribution of BMs are affected by the biological features of
tumors. For example, the authors have recently reported that
epidermal growth factor receptor (EGFR) mutated lung cancer BMs
occurred more frequently in the caudate nucleus, cerebellum, and
temporal lobe than those with an EGFR exon 19 deletion (8).
The primary purpose of the current study was to
evaluate the spatial distribution of BMs from breast cancer in a
more objective manner than past reports by using a voxel-based
lesion mapping technique. In line with previous reports, the
predominance of breast cancer BMs in the posterior fossa was
visually confirmed (6,9,13,14).
It is speculated that this tendency is due to the difference in
blood flow between the cerebrum and the cerebellum (20). This study investigated the
correlation between the spatial distributions of breast cancer BMs
and their subtypes. The authors ascertained that BMs from luminal A
and B type breast cancers concentrated in the cerebellum and these
proportions were higher than those of the other subtypes, while
HER2 breast cancer BMs occurred more frequently around the basal
ganglia and less frequently in the cerebellum than the others. This
tendency was inconsistent with previous reports as it was reported
that BMs from HER2 positive and luminal type occurred dominantly in
occipital lobe and cerebellum (21).
Kyeong et al reported that BMs from luminal
type breast cancers were less frequently encountered in the
cerebellum and that BMs from the TNBC subtype were more frequently
encountered in the frontal lobe. This inconsistency with our
findings may have been caused by differences in the segmentation of
brain regions and the classification of biological subtypes. In the
report by Kyeong et al, the whole brain was divided into 116
segments using the standardized automated anatomical labeling (AAL)
template (21,22). Thereafter, those segments were
rearranged into seven regions before analysis. This is a different
segmentation scheme than employed in this study. Furthermore,
Kyeong et al assigned patients with BMs from breast cancer
to three groups: Luminal (HR-positive and HER2-negative), HER2
(HER2-positive and HR-positive or HR-negative), and TNBC
(HR-negative and HER2-negative). This is different from the
classification scheme of the current report. Further study is
required to evaluate these differences.
Different metastatic patterns among breast cancer
subtypes or among primary cancer entities were reported (23,24).
Although the mechanisms causing differences of metastatic patters
are not completely clarified, the ‘seed and soil hypothesis’
proposed by Paget is well accepted (25). The hypothesis assumes that
compatibility between the biological characteristics of the cancer
cells (‘seeds’) and the microenvironment of the metastatic site
(‘soil’) plays an important role in cancer metastasis. Some key
molecules for this phenomenon in breast cancer metastasis have been
reported (26,27).
Regarding symptoms at the time of BM diagnoses,
dizziness was the most frequently encountered neurological deficit.
This finding could be related to the cerebellar distribution of
BMs. On the other hand, screening for BMs was conducted only in 10%
of patients as screening for BMs from asymptomatic breast cancer is
not yet recommended. As the occurrence of BMs continues to increase
in breast cancer patients (4,5), some
reports propose clinical trials focusing on the value of BM
screening in patients with breast cancer, particularly for
subgroups at high risk of BMs (i.e., HER2 or TNBC with extracranial
metastatic lesions) (28).
Regarding the treatment of breast cancer BMs,
following treatment options are offered to the patients, which are
surgical resection, whole-brain radiotherapy, stereotactic
radiosurgery, chemotherapy, and targeted therapy (29,30).
In addition, a treatment concept of prophylactic cranial
irradiation (PCI) targeting breast cancer patients with high BMs
risk has been proposed (31)
similar to lung cancer. A randomized controlled trial evaluating
the efficacy of PCI for high-risk breast cancer patients was
performed, which revealed that patients receiving PCI did not
develop BMs, while 6.4% of patients not receiving PCI developed BMs
(32). In this context,
understanding the characteristics of BMs due to breast cancer
subtype may add insight into improving treatment strategies against
this diseased condition. This includes the possibility of
prophylactic irradiation with dose modulation to sites where BMs
preferentially occur. For example, our research suggested that HER2
breast cancer occurred less often in the cerebellum but slightly
more often in the putamen and thalamus. This localized treatment
would aim for a preventive effect while reducing side effects.
Further research of this kind could suggest different radiation
dose modulation strategies according to the different subtypes of
breast cancer.
This study has some limitations. Firstly, most of
the brain lesions were not resected. Therefore, pathohistological
diagnoses of brain lesions and concordances of biological subtypes
between brain lesions and primary lesions were not confirmed.
Secondarily, because of the retrospective analysis and small sample
number, we could not analyze the genetic mutations which occurring
frequently in breast cancers or associated with cancer metastasis.
Likewise, some patients were referred to our department form
outside institutions requesting treatment of brain lesions.
Therefore, we could not calculate the true incidence of metastasis
analyze the correlation between the frequency of metastasis by
subtype and the brain region.
As a conclusion, BMs from luminal A and B types
occurred more often in the cerebellum, while brain metastases from
HER2-positive type breast cancer occurred more often in the putamen
and the thalamus, and less frequently in the cerebellum than other
types. These findings suggest that spatial distributions of BMs
from breast cancers are affected by their biological subtypes.
Acknowledgements
Not applicable.
Funding
This research was funded by the Japan Society for the Promotion
of Science (grant no. 19K09526) and the Takeda Science Foundation,
and MSD Life Science Foundation.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NI confirmed the authenticity of the data, analyzed
the data, and wrote the manuscript. MK confirmed the authenticity
of the data, analyzed the data, supervised the study, and wrote the
manuscript. TO analyzed the data. MS and KN collected MRI data. TN
collected clinical data. YT collected clinical data and supervised
the study. HK analyzed the data, wrote the manuscript and
supervised the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was conducted following the Declaration
of Helsinki, and the internal review board of the Osaka
International Cancer Institute approved the clinical data used in
this research (Approval number: 1707109126). The local institution
waivered obtaining written informed consent from the patients due
to the study's retrospective and non-invasive nature.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maher EA, Mietz J, Arteaga CL, DePinho RA
and Mohla S: Brain metastasis: Opportunities in basic and
translational research. Cancer Res. 69:6015–6020. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Darlix A, Louvel G, Fraisse J, Jacot W,
Brain E, Debled M, Mouret-Reynier MA, Goncalves A, Dalenc F,
Delaloge S, et al: Impact of breast cancer molecular subtypes on
the incidence, kinetics and prognosis of central nervous system
metastases in a large multicentre real-life cohort. Br J Cancer.
121:991–1000. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kim YJ, Kim JS and Kim IA: Molecular
subtype predicts incidence and prognosis of brain metastasis from
breast cancer in SEER database. J Cancer Res Clin. 144:1803–1816.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nagao E, Yoshiura T, Hiwatashi A, Obara M,
Yamashita K, Kamano H, Takayama Y, Kobayashi K and Honda H: 3D
turbo spin-echo sequence with motion-sensitized driven-equilibrium
preparation for detection of brain metastases on 3T MR imaging.
AJNR Am J Neuroradiol. 32:664–670. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lin NU and Winer EP: Brain metastases: The
HER2 paradigm. Clin Cancer Res. 13:1648–1655. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chason JL, Walker FB and Landers JW:
Metastatic carcinoma in the central nervous system and dorsal root
ganglia. A prospective autopsy study. Cancer. 16:781–787.
1963.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Delattre JY, Krol G, Thaler HT and Posner
JB: Distribution of brain metastases. Arch Neurol. 45:741–744.
1988.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Takano K, Kinoshita M, Takagaki M, Sakai
M, Tateishi S, Achiha T, Hirayama R, Nishio K, Uchida J, Kumagai T,
et al: Different spatial distributions of brain metastases from
lung cancer by histological subtype and mutation status of
epidermal growth factor receptor. Neuro Oncol. 18:716–724.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Meyer PC and Reah TG: Secondary Neoplasms
of the central nervous system and meninges. Br J Cancer. 7:438–448.
1953.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ellingson BM, Cloughesy TF, Pope WB, Zaw
TM, Phillips H, Lalezari S, Nghiemphu PL, Ibranim H, Naeini KM,
Harris RJ and Lai A: Anatomic localization of O6-methylguanine DNA
methyltransferase (MGMT) promoter methylated and unmethylated
tumors: A radiographic study in 358 de novo human glioblastomas.
Neuroimage. 59:908–916. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kinoshita M, Sasayama T, Narita Y,
Yamashita F, Kawaguchi A, Chiba Y, Kagawa N, Tanaka K, Kohmura E,
Arita H, et al: Different spatial distribution between germinal
center B and non-germinal center B primary central nervous system
lymphoma revealed by magnetic resonance group analysis. Neuro
Oncol. 16:728–734. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mazziotta J, Toga A, Evans A, Fow P,
Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, et al: A
probabilistic atlas and reference system for the human brain:
International Consortium for Brain Mapping (ICBM). Philos Trans R
Soc Lond B Biol Sci. 356:1293–1322. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bender ET and Tomé WA: Distribution of
brain metastases: Implications for non-uniform dose prescriptions.
Br J Radiol. 84:649–658. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Quattrocchi CC, Errante Y, Gaudino C,
Mallio CA, Giona A, Santini D, Tonini G and Zobel BB: Spatial brain
distribution of intra-axial metastatic lesions in breast and lung
cancer patients. J Neurooncol. 110:79–87. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Blows FM, Driver KE, Schmidt MK, Broeks A,
van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO,
Blomqvist C, et al: Subtyping of breast cancer by
immunohistochemistry to investigate a relationship between subtype
and short and long term survival: A collaborative analysis of data
for 10,159 cases from 12 studies. PLoS Med.
7(e1000279)2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Weigelt B, Baehner FL and Reis-Filho JS:
The contribution of gene expression profiling to breast cancer
classification, prognostication and prediction: A retrospective of
the last decade. J Pathol. 220:263–280. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gabos Z, Sinha R, Hanson J, Chauhan N,
Hugh J, Mackey JR and Abdulkarim B: Prognostic significance of
human epidermal growth factor receptor positivity for the
development of brain metastasis after newly diagnosed breast
cancer. J Clin Oncol. 24:5658–5663. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nam BH, Kim SY, Han HS, Kwon Y, Lee KS,
Kim TH and Ro J: Breast cancer subtypes and survival in patients
with brain metastases. Breast Cancer Res. 10(R20)2008.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Tham YL, Sexton K, Kramer R, Hilsenbeck S
and Elledge R: Primary breast cancer phenotypes associated with
propensity for central nervous system metastases. Cancer.
107:696–704. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pakneshan S, Safarpour D, Tavassoli F and
Jabbari B: Brain metastasis from ovarian cancer: A systematic
review. J Neurooncol. 119:1–6. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kyeong S, Cha YJ, Ahn SG, Suh SH, Son EJ
and Ahn SJ: Subtypes of breast cancer show different spatial
distributions of brain metastases. PLoS One.
12(e0188542)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tzourio-Mazoyer N, Landeau B,
Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B and
Joliot M: Automated anatomical labeling of activations in SPM using
a macroscopic anatomical parcellation of the MNI MRI single-subject
brain. Neuroimage. 15:273–289. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo Y, Arciero CA, Jiang R, Behera M, Peng
L and Li X: Different breast cancer subtypes show different
metastatic patterns: A study from a large public database. Asian
Pac J Cancer Prev. 21:3587–3593. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schroeder T, Bittrich P, Kuhne JF, Noebel
C, Leischner H, Fiehler J, Schroeder J, Schoen G and Gellißen S:
Mapping distribution of brain metastases: Does the primary tumor
matter? J Neurooncol. 147:229–235. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Paget S: The distribution of secondary
growths in cancer of the breast 1889. Cancer Metastasis Rev.
8:98–101. 1989.PubMed/NCBI
|
|
26
|
Morgan AJ, Giannoudis A and Palmieri C:
The genomic landscape of breast cancer brain metastases: A
systematic review. Lancet Oncol. 22:e7–e17. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu
J, Klijn JG, Foekens JA and Martens JW: Subtypes of breast cancer
show preferential site of relapse. Cancer Res. 68:3108–3114.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Komorowski AS, Warner E, MacKay HJ, Sahgal
A, Pritchard KI and Jerzak KJ: Incidence of brain metastases in
non-metastatic and metastatic breast cancer: Is there a role for
screening? Clin Breast Cancer. 20:e54–e64. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gil-Gil MJ, Martinez-Garcia M, Sierra A,
Conesa G, Del Barco S, González-Jimenez S and Villà S: Breast
cancer brain metastases: A review of the literature and a current
multidisciplinary management guideline. Clin Transl Oncol.
16:436–446. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Takahashi H and Isogawa M: Management of
breast cancer brain metastases. Chin Clin Oncol.
7(30)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gandhi AK, Sharma DN and Rath GK:
Prophylactic cranial irradiation in breast cancer: A new way
forward. Indian J Medical Paediatr Oncol. 36:77–78. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hashem T, Eldin KK, Metwaly H and Elkholy
E: Prophylactic cranial irradiation (PCI) in high-risk breast
cancer patients: Preliminary data. J Clin Oncol. 26(11501)2008.
|