Introduction
Solitary fibrous tumor (SFT) and hemangiopericytoma
(HPC) are rare tumors that derive from mesenchyme. SFT and HPC were
formerly considered to be different diseases, however closely
related due to similarities observed in immunohistochemical
positive staining in CD34, CD99, vimentin, BCL2, and epithelial
membrane antigen (1-5).
This lack of specificity in SFT/HPC occasionally caused problems in
differentiating them from other tumors that are
immunohistologically alike them. In 2013, three groups reported
that SFT and HPC have a common gene fusion between NGFI-A-binding
protein 2 (NAB2) and signal transducer and activator of
transcription 6 (STAT6) (1,6,7).
Thereafter, STAT6, which has dual functions as a signal transducer
and as transcription activator in SFT and HPC, was recognized as
the highly sensitive and specific immunohistochemical marker for
SFT/HPC (2-5,8-11).
Clinical progression of SFT/HPC is different in each
case. For example, we reported one case where it was diagnosed
accidentally and treated completely by a successful surgery,
whereas in other cases relapse occurred with local recurrence
and/or multiple metastases after many years of surgical treatment
followed by radiotherapy and/or intensive chemotherapy (1,3,9,10,12-18).
Regardless of the many attempts that have been made to classify
NAB2-STAT6 fusion variants to prognose clinical characteristics of
SFT/HPC, any absolute fusion variant related to malignancy has not
yet been detected (11,19,20).
However, recently classical grading of SFT/HPC with
histopathological backgrounds, such as mitosis and necrosis, have
been reevaluated to detect factors that may be associated with a
malignant prognosis (15,16,21).
Furthermore, several groups reported that Ki-67, a protein widely
known to associate with a poor prognosis in various cancers
(2,22-25),
is related to recurrence in SFT/HPC derived from the pleura and
central nervous system (2,14,15).
We also detected a significant relationship to recurrence between
necrosis, mitosis ≥1/10 HPF (magnification, x400), and Ki-67 >5%
in SFT/HPC regardless of its origin (26).
In this study, we added evaluation of Fascin-1
immunostaining as a potential factor that may predict recurrence of
SFT/HPC. Fascin-1, an actin-bundling protein, plays an important
role in the regulation of cell adhesion, migration and invasion
(27,28). It is known that Fascin-1 has a
strong upregulation in various human carcinomas. However, regarding
sarcomas, there are only a few earlier reports (29). Our study showed that Fascin-1 was
strongly associated with recurrence of SFT/SFT and it suggested
that Fascin-1 could be used as a predictive factor for malignancy
of SFT/HPC.
Materials and methods
Materials
A total of 20 Japanese patients, previously
diagnosed with SFT/HPC at Kochi University Hospital from January
2000 to December 2019, were included in this study. Table I shows the backgrounds of these
patients. All patients underwent one or more tumor resection
surgery. Tissues obtained during surgery were embedded in paraffin
blocks after formalin fixation and preserved. All patients were
observed at Kochi University Hospital following surgery. Seven
cases had one or more recurrence and two patients (cases III and
VII) died due to the disease.
 | Table IClinical background of patients. |
Table I
Clinical background of patients.
| Case | Sex | Age of onset
(years) | Tumor location | Tumor size (cm) | STAT6 | Ki-67 >5% | Mitosis ≥1/10
HPF | Necrosis | Fascin-1 | Recurrence | Recurrence free
months |
|---|
| I | F | 80-89 | Bone and soft
tissue | 3.5 | + | - | - | - | + | - | 205 |
| II | F | 50-59 | Bone and soft
tissue | 10.5 | + | - | - | - | - | - | 198 |
| III | M | 50-59 | Bone and soft
tissue | 12.5 | + | + | + | - | Strongly
positive | + | 89 |
| IV | M | 70-79 | Bone and soft
tissue | 10.0 | + | + | + | + | Strongly
positive | + | 0 |
| V | M | 60-69 | Bone and soft
tissue | 15.0 | + | - | - | - | + | - | 97 |
| VI | M | 30-39 | Bone and soft
tissue | 11.0 | + | - | - | - | - | - | 91 |
| VII | F | 70-79 | Bone and soft
tissue | 10.0 | + | + | + | + | Strongly
positive | + | 4 |
| VIII | F | 50-59 | Bone and soft
tissue | 12.5 | + | - | - | - | + | - | 60 |
| IX | F | 60-69 | Bone and soft
tissue | 9.0 | + | - | - | - | + | - | 6 |
| X | F | 50-59 | Head and neck | 1.7 | + | + | - | - | + | - | 175 |
| XI | F | 60-69 | Lung | 4.0 | + | - | - | - | - | - | 170 |
| XII | M | 70-79 | Lung | 6.0 | + | + | + | + | - | + | 2 |
| XIII | M | 30-39 | Lung | 14.0 | + | - | - | - | + | - | 114 |
| XIV | F | 50-59 | Lung | 8.0 | + | - | - | - | - | - | 111 |
| XV | F | 30-39 | CNS | 1.0 | + | - | + | - | Strongly
positive | + | 136 |
| XVI | M | 60-69 | CNS | 5.0 | + | - | - | - | - | - | 195 |
| XVII | F | 50-59 | CNS | 2.0 | + | - | - | - | - | - | 188 |
| XVIII | F | 60-69 | CNS | 1.0 | + | + | + | - | - | + | 2 |
| XIX | F | 30-39 | CNS | 5.5 | + | + | + | - | Strongly
positive | + | 49 |
| XX | F | 60-69 | CNS | 1.5 | + | - | + | - | - | - | 49 |
Immunohistochemical examination and
evaluation
For the present study, formalin-fixed
paraffin-embedded tissue samples were freshly cut into 4 µm thick
slices and heat-treated with ULTRA cell conditioning 1 retrieval
solution (CC1; Ventana Automated Systems). Immunohistochemical
examination was performed using a Ventana automated system with the
following antibodies: STAT6 (D-1, sc-374021, dilution 1:50; Santa
Cruz Biotechnology, Inc.), Ki-67 (MIB-1, dilution 1:50; Dako;
Agilent Technologies, Inc.), and anti-Fascin-1 mouse monoclonal
antibody (55k-2, dilution 1:50; Dako; Agilent Technologies, Inc.).
Immunohistochemical expression of STAT6, Ki-67, and Fascin-1 was
evaluated in the density of the nuclear staining and graded as
‘negative’, ‘weak’, ‘moderate’, or ‘strong’. Grades ‘moderate’ and
‘strong’, were then defined as ‘positive’ in terms of diagnosis. To
investigate the proportion of positive Ki-67, a total of 100 tumor
cells were counted at five different hot spots. Then, the mean
value of positive cells was calculated and input as a percentage
for statistical analysis. As for the evaluation for Fascin-1, the
intensity and extent of staining were examined (28,29).
The intensity of staining was scored as 0 (negative), 1 (weak), 2
(moderate), or 3 (strong). The extent of staining was scored as 0
(0%), 1 (1-20%), 2 (21-70%), and 3 (71-100%). When the sum of
staining intensity and extent scores was 2-4 and 5-6, it was
defined as ‘positive’ and ‘strongly positive’, respectively.
Independent evaluation of immunostaining was performed by two
different expert pathologists who were blinded to the clinical
data.
Statistical analysis
Statistical relationship was examined between
recurrence and the following variables: Sex, onset age, tumor
origin, tumor size, mitosis ≥1/10 HPF (magnification, x400),
necrosis, Ki-67>5% and Fascin-1. Pearson's correlation
coefficient analysis was applied to detect relationship between
recurrence and sex, tumor origin, mitosis ≥1/10 HPF (magnification,
x400), necrosis, or Ki-67>5%. Logistic regression analysis and
Wilcoxon rank test were applied to compare recurrence with tumor
size and onset age, respectively. Regarding Fascin-1, after the
determination of its cut-off point for recurrence as between
‘positive’ and ‘strongly positive’, Wilcoxon rank test was applied
to detect the relationship between recurrence and Fascin ≥‘strongly
positive’. Kaplan-Meier analysis was conducted to analyze the
recurrence-free survival distributions between patients with
Fascin-1 ‘strongly positive’ or not.
This study was reviewed and approved by the Ethics
Committee for Clinical Research of the School of Medicine, Kochi
University (ERB-105384). All procedures were carried out with the
adequate understanding and written consent of each patient.
Results
Clinical background of patients
Table I includes the
results of histopathological and immunochemical evaluation of all
cases.
Fascin-1 staining
Table II shows
sensitivity, false positive rate, and concordance rate in two
proposed groups with different cut-off points of Fascin-1
immunostaining. When the cut-off point was set between ‘positive’
and ‘strongly positive’, the sensitivity, false positive rate, and
concordance were 0.71, 0.00, and 0.90, respectively. On the other
hand, when the cut-off point was set between ‘negative’ and
‘positive’, its sensitivity, false positive rate and concordance
were 0.71, 0.46 and 0.6, respectively. By these findings, the most
effective cut-off point of Fascin-1 was determined to be between
‘positive’ and ‘strongly positive’.
 | Table IIExamination of Fascin-1 staining cut
off points. |
Table II
Examination of Fascin-1 staining cut
off points.
| Cut-off point for
Fascin-1 staining | Sensitivity | False positive
rate | Concordance
rate |
|---|
|
‘negative’-‘positive’,‘strongly
positive’ | 0.71 | 0.46 | 0.60 |
|
‘negative’,‘positive’-‘strongly
positive’ | 0.71 | 0.00 | 0.90 |
Statistical analyses
Table III shows
the results of relationship between recurrence and each variable. A
significant relationship to reference was detected with necrosis
(P<0.05), mitosis ≥1/10 HPF (magnification, x400) (P<0.01),
Ki-67 >5% (P<0.01), and Fascin-1 ≥‘strongly positive’
(P<0.01). Sex, onset age, tumor size, or tumor origin did not
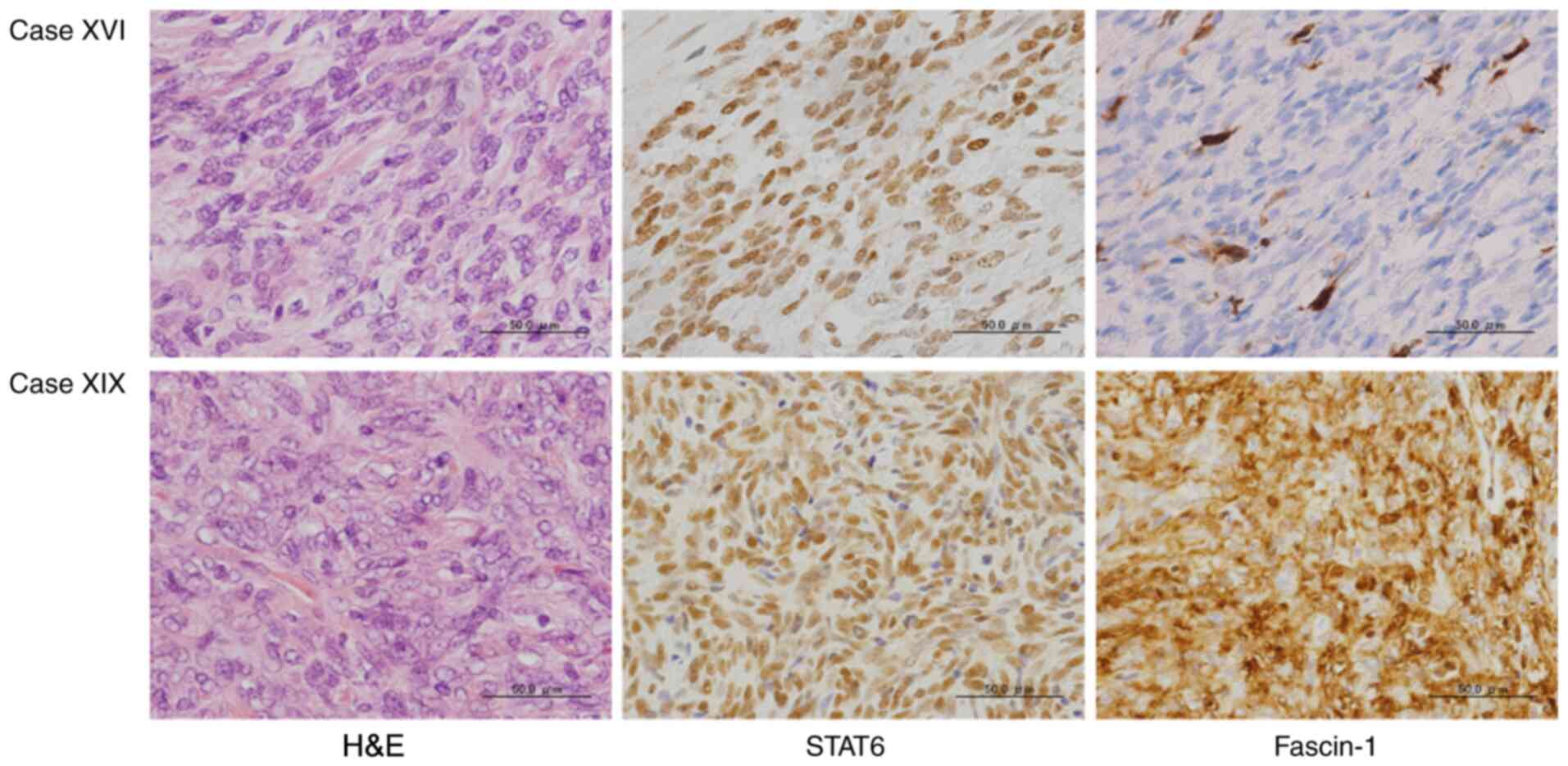
relate to recurrence. Fig. 1 shows
representative images of the microscopic and immunohistochemical
findings, specifically from Case XVI and Case XIX, where Fascin-1
was ‘negative’ in Case XVI and was ‘strongly positive’ in Case XIX.
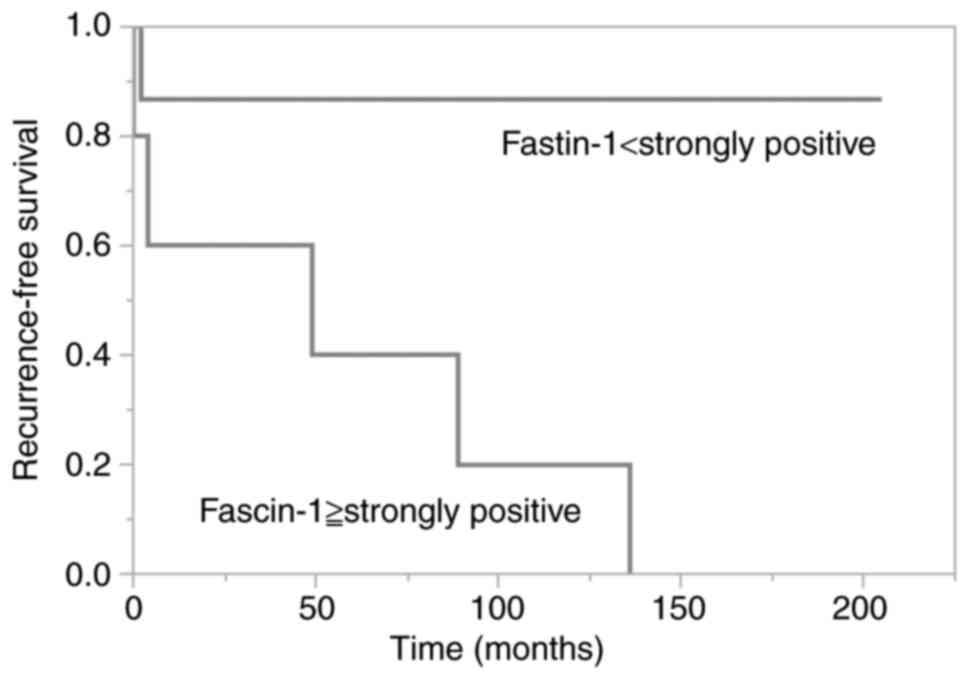
Fig. 2 shows Kaplan-Meier curve of
patients with Fascin-1 ‘strongly positive’ or not.
 | Table IIIAnalyses of the relationship between
recurrence and each variable. |
Table III
Analyses of the relationship between
recurrence and each variable.
| Variables | P-value |
|---|
| Male vs.
female | NS |
| Onset age | NS |
| Tumor origin | NS |
| Tumor size
(cm) | NS |
| Necrosis | <0.05 |
| Ki-67 >5% | <0.01 |
| Mitosis >1/10
HPF | <0.01 |
| Fascin-1 ≥strongly
positive | <0.01 |
Discussion
SFT and HPC had long been regarded as different
tumors since 1931, when Klemperer and Coleman first reported on
primary mesenchymal tumors of the pleura (29). However, after the year 2000,
controversial discussions about the classification of SFT/HPC
prompted a unification of these tumors into a single disease
entity. As a result, the 2013 WHO Classification of Tumors of Soft
Tissue and Bone removed the term ‘hemangiopericytoma’ as a synonym
for SFT, joining these tumors together as SFT under the category of
fibroblastic/myofibroblastic tumors (5,8,30-32).
In the same year, the common gene fusion between NAB2 and STAT6 was
discovered in SFT/HPC. The 2016 WHO Classification of Tumors of the
Central Nervous System designated these tumors, characterized by
the NAB2-STAT6 gene fusion, as SFT/HPC in
mesenchymal/non-mesenchymal tumors (2,9,33,34).
The discovery of the NAB2-STAT6 gene fusion, has
resulted in both quicker and more accurate diagnosis of SFT/HPC.
Cases difficult to diagnose with classical immunostainings, such as
CD34, CD99, and vimentin, can easily be definitively diagnosed as
SFT/HPC through the evaluation of STAT6 immunostaining. The
prognosis of SFT/HPC is generally favorable, however, fatalities
are possible with repeated recurrence and distant metastasis. In
this study, seven of 20 cases had recurrence and two patients
passed away due to the disease. The advancement of diagnostic
techniques for SFT/HPC by STAT6 immunostaining may result in more
cases being properly diagnosed in the future. Therefore, it is
crucial for clinicians to identify patients with high risk of
recurrence to adequately carry out their follow-ups.
Previously, we reported that recurrence of SFT/HPC
was significantly related to necrosis, mitosis ≥1/10 HPF
(magnification, x400), and Ki-67 >5% (26). Here, an additional factor
potentially related to recurrence has been added, specifically,
Fascin-1 immunostaining. Fascin-1, an actin-bundling protein, plays
an important role in the regulation of cell adhesion, migration,
and invasion (27-29,35).
Fascin-1 widely exists in different tissues of the human body, such
as mesenchyme and nervous tissue, however it is not present in most
normal epithelia. Fascin-1 has been commonly observed to be highly
upregulated in various human carcinomas (27,28).
Furthermore, the overexpression of Fascin-1 is positively
correlated with poor prognosis of carcinomas, because it increases
the chance of metastasis. Regarding sarcomas, few reports had been
made about any relationship with Fascin-1. However, after 2019,
Arlt et al reported Fascin-1 expression also correlates with
progression and metastasis in osteosarcoma and chondrosarcoma
(29). Additionally, Richmond et
al reported that Fascin-1 is a mediator of invasion in uterine
carcinosarcoma as a component of epithelial-mesenchymal transition
(35).
Since 2012, pazopanib hydrochloride, a
broad-spectrum tyrosine kinase inhibitor, has been approved for the
treatment of soft tissue sarcoma in Japan, and its effectiveness
has been reported in several papers (36,37).
In this study, three patients (Case VII, XV and XI) had been
treated with pazopanib hydrochloride, however, Case VII patient
passed away within six months following its administration. The
development of new medicines that may directly target the
NAB2-STAT6 gene fusion is desired for patients with SFT/HPC.
Furthermore, additional therapeutics that may target and inhibit
Fascin-1 will also greatly benefit patients with malignant
tumors.
We attempted to detect the relationship between
Fascin-1 immunostaining and recurrence of SFT/HPC. Presently,
classical histological findings, such as mitosis and necrosis, are
generally accepted to be useful to prognose its recurrence. As
shown in Table I, sensitivity of
mitosis was excellent, but there was a false-positive case. As for
necrosis, its specificity was excellent, but there were four
false-negative cases, which implied necrosis was not a clinically
favorable factor to predict its recurrence. Regarding Fascin-1, as
shown in Tables I and II, sensitivity was 0.71 and two cases had
false-negative, although, its false-positive rate was 0.0 and it
was negative in Case XX, where mitosis had a false-positive. By
using two factors, Fascin-1 and mitosis, recurrence in SFT/HPC
could be prognosed more accurately. To confirm the benefit of
Fascin-1 as a factor to predict recurrence in SFT/HPC, further
studies with more cases should be performed in the future. However,
in this study, we observed Fascin-1 immunostaining to be one of the
most effective and useful evaluation factors to predict poor
prognosis in patients with SFT/HPC. Through a meticulous
histological and immunochemical observation of these factors after
the initial surgery, clinicians should be better informed during
follow-ups with patients most at risk for recurrence and
subsequently able to treat them at early stages of recurrence.
The evaluation of Fascin-1 immunostaining is useful
for recurrence prediction of SFT/HPC. These data indicate that
Fascin-1 may play an important role in the recurrence of SFT/HPC,
one of sarcomas.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY and YH conceived the current study. YY and YH
performed the histological examination. YY and HS performed
statistical analysis. YY and IM made substantial contributions to
study conception and design. IM critically revised the manuscript
and gave final approval for the manuscript to be published. All
authors read and approved the final manuscript. YY and YH confirmed
the authenticity of all the raw data.
Ethics approval and consent to
participate
The current study was reviewed and approved by the
Ethics Committee for Clinical Research of the School of Medicine,
Kochi University (approval no. ERB-105384). All procedures were
carried out with adequate understanding and the written consent of
each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Robinson DR, Wu YM, Kalyana-Sundaram S,
Cao X, Lonigro RJ, Sung YS, Chen CL, Zhang L, Wang R, Su F, et al:
Identification of recurrent NAB2-STAT6 gene fusions in solitary
fibrous tumor by integrative sequencing. Nat Genet. 45:180–185.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Shukla P, Gulwani HV, Kaur S and
Shanmugasundaram D: Reappraisal of morphological and
immunohistochemical spectrum of intracranial and spinal solitary
fibrous tumors/hemangiopericytomas with impact on long-term
follow-up. Indian J Cancer. 55:214–221. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Davanzo B, Emerson RE, Lisy M, Koniaris LG
and Kays JK: Solitary fibrous tumor. Transl Gastroenterol Hepatol.
3(94)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang Q, Qin J, Li Y and Wu T: Primary
solitary fibrous tumor of kidney: A case report and literature
review. Urol Case Rep. 23:92–94. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Doyle LA, Vivero M, Fletcher CD, Mertens F
and Hornick JL: Nuclear expression of STAT6 distinguishes solitary
fibrous tumor from histologic mimics. Mod Pathol. 27:390–395.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chmielecki J, Crago AM, Rosenberg M,
O'Connor R, Walker SR, Ambrogio L, Auclair D, McKenna A, Heinrich
MC, Frank DA and Meyerson M: Whole-exome sequencing identifies a
recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet.
45:131–132. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Mohajeri A, Tayebwa J, Collin A, Nilsson
J, Magnusson L, von Steyern FV, Brosjö O, Domanski HA, Larsson O,
Sciot R, et al: Comprehensive genetic analysis identifies a
pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic
imbalances, and a characteristic gene expression profile in
solitary fibrous tumor. Genes Chromosomes Cancer. 52:873–886.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schweizer L, Koelsche C, Sahm F, Piro RM,
Capper D, Reuss DE, Pusch S, Habel A, Meyer J, Göck T, et al:
Meningeal hemangiopericytoma and solitary fibrous tumors carry the
NAB2-STAT6 fusion and can be diagnosed by nuclear expression of
STAT6 protein. Acta Neuropathol. 125:651–658. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rinaldo L, Xu SCY, Eggers SD, Salomão DR,
Chen JJ and Raghunathan A: Rare occurrence of an intraocular
choroidal solitary fibrous tumor/hemangiopericytoma. Ocul Oncol
Pathol. 4:213–219. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tai HC, Chuang IC, Chen TC, Li CF, Huang
SC, Kao YC, Lin PC, Tsai JW, Lan J, Yu SC, et al: NAB2-STAT6 fusion
types account for clinicopathological variations in solitary
fibrous tumors. Mod Pathol. 28:1324–1335. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kakkar A, Sakthivel P, Rajeshwari M, Kairo
A and Sharma MC: Recurrent sinonasal CD34-negative malignant
solitary fibrous tumor diagnosed on STAT6 immunohistochemistry and
NAB2-STAT6 fusion. Head Neck Pathol. 14:250–256. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Penel N, Amela EY, Decanter G, Robin YM
and Marec-Berard P: Solitary fibrous tumors and so-called
hemangiopericytoma. Sarcoma. 2012(690251)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Thway K, Ng W, Noujaim J, Jones RL and
Fisher C: The current status of solitary fibrous tumor: Diagnostic
features, variants, and genetics. Int J Surg Pathol. 24:281–292.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schmid S, Csanadi A, Kaifi JT, Kübler M,
Haager B, Kayser G, Passlick B and Wiesemann S: Prognostic factors
in solitary fibrous tumors of the pleura. J Surg Res. 195:580–587.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Diebold M, Soltermann A, Hottinger S,
Haile SR, Bubendorf L, Komminoth P, Jochum W, Grobholz R,
Theegarten D, Berezowska S, et al: Prognostic value of MIB-1
proliferation index in solitary fibrous tumors of the pleura
implemented in a new score-a multicenter study. Respir Res.
18(210)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fountas KN, Kapsalaki E, Kassam M, Feltes
CH, Dimopoulos VG, Robinson JS and Smith JR: Management of
intracranial meningeal hemangiopericytomas: Outcome and experience.
Neurosurg Rev. 29:145–153. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Robinson LA: Solitary fibrous tumor of the
pleura. Cancer Control. 13:264–269. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ghose A, Guha G, Kundu R, Tew J and
Chaudhary R: CNS hemangiopericytoma: A systematic review of 523
patients. Am J Clin Oncol. 40:223–227. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yuzawa S, Nishihara H, Wang L, Tsuda M,
Kimura T, Tanino M and Tanaka S: Analysis of NAB2-STAT6 gene fusion
in 17 cases of meningeal solitary fibrous tumor/hemangiopericytoma:
Review of the literature. Am J Surg Pathol. 40:1031–1040.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vogels R, Macagno N, Griewank K, Groenen
P, Verdijk M, Fonville J and Kusters B: French CNS SFT/HPC
Consortium; Dutch CNS SFT/HPC Consortium. Figarella-Branger D, et
al: Prognostic significance of NAB2-STAT6 fusion variants and TERT
promotor mutations in solitary fibrous tumors/hemangiopericytomas
of the CNS: Not (yet) clear. Acta Neuropathol. 137:679–682.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
England DM, Hochholzer L and McCarthy MJ:
Localized benign and malignant fibrous tumors of the pleura. A
clinicopathologic review of 223 cases. Am J Surg Pathol.
13:640–658. 1989.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Piri R, Ghaffari A, Azami-Aghdash S,
Ali-Akbar YP, Saleh P and Naghavi-Behzad M: Ki-67/MIB-1 as a
prognostic marker in cervical cancer-a systematic review with
meta-analysis. Asian Pac J Cancer Prev. 16:6997–7002.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Prueter J, Norvell D and Backous D: Ki-67
index as a predictor of vestibular schwannoma regrowth or
recurrence. J Laryngol Otol. 133:205–207. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kammerer-Jacquet SF, Ahmad A, Møller H,
Sandu H, Scardino P, Soosay G, Beltran L, Guzick J and Berney DM:
Ki-67 is an independent predictor of prostate cancer death in
routine needle biopsy samples: Proving utility for routine
assessments. Mod Pathol. 32:1303–1309. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Go SI, Ko GH, Lee WS, Lee JH, Jeong SH,
Lee YJ, Hong SC and Ha WS: The use of CD44 variant 9 and Ki-67
combination can predicts prognosis better than their single use in
early gastric cancer. Cancer Res Treat. 51:1411–1419.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yamamoto Y, Hayashi Y and Murakami I:
Recurrence of solitary fibrous tumor/hemangiopericytoma could be
predicted by Ki-67 regardless of its origin. Acta Med Okayama.
74:335–343. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hayashi Y, Osanai M and Lee GH: Fascin-1
expression correlates with repression of E-cadherin expression in
hepatocellular carcinoma cells and augments their invasiveness in
combination with matrix metalloproteinases. Cancer Sci.
102:1228–1235. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang CQ, Tang CH, Chang HT, Li XN, Zhao
YM, Su CM, Hu GN, Zhang T, Sun XX, Zen Y, et al: Fascin-1 as a
novel diagnostic marker of triple-negative breast cancer. Cancer
Med. 5:1983–1988. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Arlt MJ, Kuzmanov A, Snedeker JG, Fuchs B,
Silvan U and Sabile AA: Fascin-1 enhances experimental osteosarcoma
tumor formation and metastasis and is related to poor patient
outcome. BMC Cancer. 19(83)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Barthelmeß S, Geddert H, Boltze C,
Moskalev EA, Bieg M, Sirbu H, Brors B, Wiemann S, Hartmann A,
Agaimy A and Haller F: Solitary fibrous tumors/hemangiopericytomas
with different variants of the NAB2-STAT6 gene fusion are
characterized by specific histomorphology and distinct
clinicopathological features. Am J Pathol. 184:1209–1218.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Doyle LA: Sarcoma classification: An
update based on the 2013 World Health Organization classification
of tumors of soft tissue and bone. Cancer. 120:1763–1774.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jo VY and Fletcher CD: WHO classification
of soft tissue tumours: An update based on the 2013 (4th) edition.
Pathology. 46:95–104. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Macagno N, Vogels R, Appay R, Colin C and
Mokhtari K: French CNS SFT/HPC Consortium; Dutch CNS SFT/HPC
Consortium. Küsters B, Wesseling P, Figarella-Branger D, et al:
Grading of meningeal solitary fibrous tumors/hemangiopericytomas:
Analysis of the prognostic value of the marseille grading system in
a cohort of 132 patients. Brain Pathol. 29:18–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Richmond AM, Blake EA, Torkko K, Smith EE,
Spillman MA and Post MD: Fascin is associated with aggressive
behavior and poor outcome in uterine carcinosarcoma. Int J Gynecol
Cancer. 27:1895–1903. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Apra C, Alentorn A, Mokhtari K,
Kalamarides M and Sanson M: Pazopanib efficacy in recurrent central
nervous system hemangiopericytomas. J Neurooncol. 139:369–372.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Martin-Broto J, Stacchiotti S, Lopez-Pousa
A, Redondo A, Bernabeu D, de Alava E, Casali PG, Italiano A,
Gutierrez A, Moura DS, et al: Pazopanib for treatment of advanced
malignant and dedifferentiated solitary fibrous tumour: A
multicentre, single-arm, phase 2 trial. Lancet Oncol. 20:134–144.
2019.PubMed/NCBI View Article : Google Scholar
|