Introduction
Pulmonary metastasis of colorectal cancer (CRC) is
the second-most frequent type of metastasis after liver metastasis
and has been reported to occur in 10-29% patients of CRC (1-7).
Thus, the establishment of a treatment strategy for pulmonary
metastasis from CRC is important. The resection of metastasis,
radiofrequency ablation, systemic drug therapy and radiation
treatments have been applied in the treatment of pulmonary
metastasis from CRC. However, unlike other cancers, in CRC, there
is a great deal of evidence to support that complete resection of
metastasis leads to an improved prognosis in comparison to other
treatments, including chemotherapy (7-10);
thus, resection of pulmonary metastasis is considered the most
effective treatment for metastasis from CRC and has been generally
accepted as the only potentially curative treatment (9-14).
However, it is difficult to say that surgery alone is a sufficient
treatment, because the 5-year survival rate after complete
resection of pulmonary metastasis from CRC is only 30-60% (15,16).
In clinical practice, adjuvant chemotherapy is often
performed after resection of pulmonary metastasis from CRC and its
effectiveness has been reported a number of times (17,18).
However, the efficacy and safety of adjuvant chemotherapy after
resection of pulmonary metastasis remain controversial, because its
effectiveness has not been reported in any large clinical trials
and the optimal regimen has not been described. The purpose of this
study was to evaluate the efficacy or safety of single-agent
adjuvant chemotherapy after complete resection of pulmonary
metastasis from CRC.
Materials and methods
Patients and therapy
We retrospectively reviewed the medical records of
16 patients who underwent complete macroscopic and microscopic (R0)
resection of pulmonary metastasis from CRC at Osaka City University
Hospital between April 1998 and October 2019. All patients enrolled
in this study had undergone resection of pulmonary metastasis for
the first time. In all patients, the primary tumors were resected
before surgical treatment for pulmonary metastasis. After the
operation for primary tumor, contrast-enhanced computed tomography
(CT) was performed routinely every six months. The details of
pulmonary metastases and the presence or absence of other distant
metastases were evaluated by contrast-enhanced CT. Patients who had
extrapulmonary metastasis were excluded from this study. It was
defined that the pulmonary metastasis found at the resection of
primary tumor as synchronous metastasis and the pulmonary
metastasis found after the resection of primary tumor as
metachronous metastasis.
Eight patients were treated with single-agent
adjuvant chemotherapy after resection of pulmonary metastasis and
oral fluoropyrimidines was selected in all regimens. The other 8
patients were treated with the resection of pulmonary metastasis
alone. The administration period of postoperative single-agent
adjuvant chemotherapy was 6 months. The decision as to whether or
not postoperative adjuvant chemotherapy should be administered, and
the selection of the regimen was at the discretion of the treating
physician. We evaluated the correlation between relapse-free
survival (RFS) rate after the first complete resection of pulmonary
metastasis from CRC and postoperative adjuvant chemotherapy.
The feasibility of postoperative adjuvant
chemotherapy was evaluated to calculate completion rate and
relative dose intensity (RDI) and to investigate adverse events.
Adverse events were graded using the Common Terminology Criteria
for Adverse Events version 5.0(19).
Classification of patients according
to risk factors
In addition, a subgroup analysis of patients who
were divided according to each clinicopathological factor was
performed. We divided the patients into 2 groups according to sex,
age and the risk (high or low) of some clinicopathological factors
that were reported to be associated with the prognosis after
resection of pulmonary metastasis of CRC. The clinicopathological
factors included the primary T/N classification, as defined by the
Union for International Cancer Control 8th edition (20,21),
the timing of metastasis (metachronous or synchronous) (20,22),
number of metastases (13,23), diameter of the metastatic tumors
(23) and the carcinoembryonic
antigen (CEA) level before the resection of pulmonary metastases
(13,14,24,25).
We also included the immunological prognostic factors for CRC that
we have reported (26). These
factors were the neutrophil-to-lymphocyte ratio (NLR), as a
systemic inflammatory marker (27-29),
and the density of tumor-infiltrating lymphocytes (TILs), which
reflects the immunological status in the tumor microenvironment
(26,30,31).
According to previous reports, patients with a CEA
level of <5.0 mg/dl were classified into the CEA Low-group and
those with a CEA level of ≥5.0 mg/dl were classified into the CEA
High-group (14,23-25).
The NLR was calculated from a blood sample obtained
within 2 weeks before pulmonary surgery by dividing the absolute
neutrophil count by the absolute lymphocyte count. Based on the
median value, patients were classified into the NLR-High or NLR-Low
groups.
We set cluster of differentiation (CD)3 as the pan-T
cell marker and CD8 as the cytotoxic T cell marker.
Regarding the immunohistochemistry protocol of
CD3/CD8, all of the resected specimens of the pulmonary metastases
from CRC were fixed in 10% buffered formalin and embedded in
paraffin. Immunohistochemical staining was performed on 4-µm-thick
sections. All of the sections were deparaffinized in xylene and
dehydrated in decreasing concentrations of ethanol. The sections
were then subjected to endogenous peroxidase blocking in 1%
H2O2 solution in methanol for 15 min. Antigen
retrieval was performed by autoclaving the sections at 105˚C for 15
min in Dako Target Retrieval Solution (Dako; Agilent Technologies,
Inc.). The serum blocking was performed with 10% normal rabbit
serum for 10 min. All of the sections were incubated at 4 C
overnight with the primary mouse monoclonal antibody CD3/CD8 (clone
F7.2.38; Dako; dilution 1:400;/colon C8/144B, Dako; dilution
1:200). The secondary antibody was a biotin-labeled rabbit
anti-mouse IgG, IgA, IgM for 10 min. All of the sections were
labeled with the peroxidase-labeled streptavidin for 5 min. The 10%
normal rabbit serum, biotin-labeled rabbit anti-mouse antibody and
peroxidase-labeled streptavidin were included in the Histofine
SAB-PO(M) kit (Nichirei Biosciences, Inc.) and used according to
the protocol of the manufacturer. Detection was performed with the
3,3'-diaminobenzidine tetrahydrochloride kit (Histofine Simple
Stain kit; Nichirei Biosciences, Inc.). All of the sections were
counterstained with hematoxylin, dehydrated, cleared and mounted on
glass coverslips.
Based on the approach in a previous report, the
number of CD3+TILs/CD8+TILs at the invasive
margin of the pulmonary metastases was counted in 5 randomly
selected fields at a magnification of x400. The mean of the 5
values obtained was then used for the analysis (26).
To determine the appropriate cut-off value, we
performed a receiver operating characteristic curve analysis. Based
on the cut-off values, patients were classified into the TIL-high
or TIL-low groups.
The association between RFS rate after resection of
pulmonary metastasis and postoperative adjuvant chemotherapy was
evaluated according to each risk factor.
Statistical analysis
Fisher's exact test and Mann-Whitney U test were
used to analyze the significance of the association among the
background factors between surgery alone and adjuvant chemotherapy.
RFS was defined as the interval between the date of resection of
pulmonary metastasis and the date of the diagnosis of the first
recurrence, death from any cause or last follow-up. Survival curves
were made using the Kaplan-Meier method. Differences in survival
curves were assessed using the log-rank test. A multivariate Cox
proportional hazards model was used to evaluate the prognostic
factors associated with the RFS. The factors with P-values of
<0.10 on the univariate analysis were included in the
multivariate analysis.
All statistical analyses were performed using JMP
14.2.0 (SAS Institute). P-values of <0.05 were considered to
indicate statistical significance.
Ethical considerations
This retrospective study was approved by the Ethics
Committee of Osaka City University (approval no. 2020-026) and
conducted in accordance with the Declaration of Helsinki. All
patients provided their written informed consent.
Results
Patient characteristics
The patient characteristics are summarized in
Table I. The study cohort included
10 males and 6 females, with a median age of 69.5 years
(range=57-84 years). The primary tumor site was located on the
right in 2 patients (from the cecum to transverse colon) on the
left in 14 patients (from descending colon to rectum). The T factor
of the primary tumor was T1-T3 in 11 patients and T4 in 5 patients.
The N factor of the primary tumor was N0 in 7 patients and N1-N3 in
9 patients. The histological type of 16 patients was well or
moderately differentiated adenocarcinoma. Six patients had
synchronous pulmonary metastasis and 10 patients had metachronous
pulmonary metastasis. Regarding distant metastases other than
pulmonary metastases, six patients had a history of liver
metastases, all of whom had undergone curative resection of liver
metastases before resection of pulmonary metastases. Three patients
had received preoperative chemotherapy for pulmonary metastases,
and two of them had undergone hepatectomy followed by pulmonary
resection after chemotherapy for simultaneous liver and pulmonary
metastases. Three patients had K-RAS wild-type, two patients had
K-RAS mutant-type, and the other patients had no data. BRAF
mutations were not evaluated in any patients. One patient had
undergone chemotherapy with the addition of a molecular-targeted
drug before resection of pulmonary metastasis. Fifteen patients had
pulmonary metastasis in one lung field and one patient had
pulmonary metastasis in both lung fields. Ten patients had only one
lesion of pulmonary metastasis and 6 patients had 2 or more
lesions. The maximum diameter of pulmonary metastasis was <20 mm
in 11 patients and ≥20 mm in 5 patients. The CEA level within 2
weeks before the resection of pulmonary metastasis was ≤5 ng/ml in
11 patients and >5 ng/ml in 5 patients. The median NLR was 1.89,
and the value was ≤18.9 in 8 patients and >18.9 in 8
patients.
 | Table IAssociation of backgrounds between
surgery alone and adjuvant chemotherapy. |
Table I
Association of backgrounds between
surgery alone and adjuvant chemotherapy.
| Preoperative
patient characteristics (n=16) | Surgery alone (n=8)
(%) | Adjuvant
chemotherapy (n=8) (%) | P-value |
|---|
| Sex, n (%) | | | |
|
Male | 3 (37.5) | 7 (87.5) | 0.039 |
|
Female | 5 (62.5) | 1 (12.5) | |
| Median age, years
(range) | 74 (61-84) | 66.5 (57-77) | 0.247 |
| Location of primary
tumor, n (%) | | | |
|
Right
colon | 0 (0) | 2 (25.0) | 0.131 |
|
Left colon
and rectum | 8(100) | 6 (75.0) | |
| Primary T status, n
(%) | | | |
|
≤T3 | 5 (62.5) | 6 (75.0) | 0.59 |
|
T4 | 3 (37.5) | 2 (25.0) | |
| Lymph node
metastasis of primary tumor, n (%) | | | |
|
N0 | 3 (37.5) | 4 (50.0) | 0.614 |
|
≥N1 | 5 (62.5) | 4 (50.0) | |
| Histological type
of primary tumor, n (%) | | | |
|
Well/Moderately
differentiated adenocarcinoma | 8(100) | 8(100) | >0.999 |
|
Poorly
differentiated/mucinous adenocarcinoma | 0 (0) | 0 (0) | |
| Detection of
pulmonary metastases, n (%) | | | |
|
Metachronous | 5 (62.5) | 5 (62.5) | >0.999 |
|
Synchronous | 3 (37.5) | 3 (37.5) | |
| History of
resection of liver metastases before the resection pulmonary
metastases, n (%) | | | |
|
No | 4 (50.0) | 6 (75.0) | 0.302 |
|
Yes | 4 (50.0) | 2 (25.0) | |
| History of
chemotherapy for the pulmonary metastases before the resection
pulmonary metastases, n (%) | | | |
|
No | 7 (87.5) | 6 (75.0) | 0.521 |
|
Yes | 1 (12.5) | 2 (25.0) | |
| Location of
pulmonary metastases, n (%) | | | |
|
One lung
field | 7 (87.5) | 8(100) | 0.302 |
|
Both lung
fields | 1 (12.5) | 0 (0) | |
| Number of pulmonary
metastases, n (%) | | | |
|
1 | 4 (50.0) | 6 (75.0) | 0.302 |
|
≥2 | 4 (50.0) | 2 (25.0) | |
| Maximum diameter of
pulmonary metastases, n (%) | | | |
|
<20
mm | 4 (50.0) | 7 (87.5) | 0.106 |
|
≥20 mm | 4 (50.0) | 1 (12.5) | |
| Method of resection
of pulmonary metastases, n (%) | | | |
|
Segmentectomy | 7 (87.5) | 8(100) | 0.302 |
|
Lobectomy | 1 (12.5) | 0 (0) | |
| CEA level before
resection of pulmonary metastases, n (%) | | | |
|
≤5
ng/ml | 5 (62.5) | 6 (75.0) | 0.59 |
|
>5
ng/ml | 3 (37.5) | 2 (25.0) | |
|
Neutrophil-to-lymphocyte ratio before the
resection of pulmonary metastases, n (%) | | | |
|
≤1.89 | 2 (25.0) | 6 (75.0) | 0.046 |
|
>1.89 | 6 (75.0) | 2 (25.0) | |
Eight patients were treated with surgery alone and 8
were treated with surgery and postoperative single-agent adjuvant
chemotherapy. The regimens were oral agents with fluoropyrimidines
as follows: tegafur-uracil and leucovorin (n=4); capecitabine
(n=2); and S-1 (n=2). As no particular complications were observed
after lung resection, no patients had delayed initiation of
adjuvant chemotherapy due to complications. Three patients had
undergone chemotherapy with the addition of a molecular-targeted
drug after recurrence that occurred after resection of pulmonary
metastases.
There was a significant male and low-NLR
predominance among patients who received postoperative adjuvant
chemotherapy (P=0.039, P=0.046, respectively). The other patient
characteristics of the surgery alone and postoperative adjuvant
chemotherapy groups did not differ to a statistically significant
extent (Table I).
Eleven patients (68.8%) had recurrence, and the
5-year RFS rate after complete resection of pulmonary metastasis
from CRC was 22.6%. In the 11 patients, the types of recurrence
after resection of pulmonary metastasis were as follows: pulmonary
metastasis (n=6); peritoneal metastasis (n=2); lymph node
metastasis (n=2); and hepatic metastasis (n=1).
The correlation between RFS rate and
postoperative adjuvant chemotherapy
In the group that received postoperative adjuvant
chemotherapy RFS rate was significantly improved in comparison to
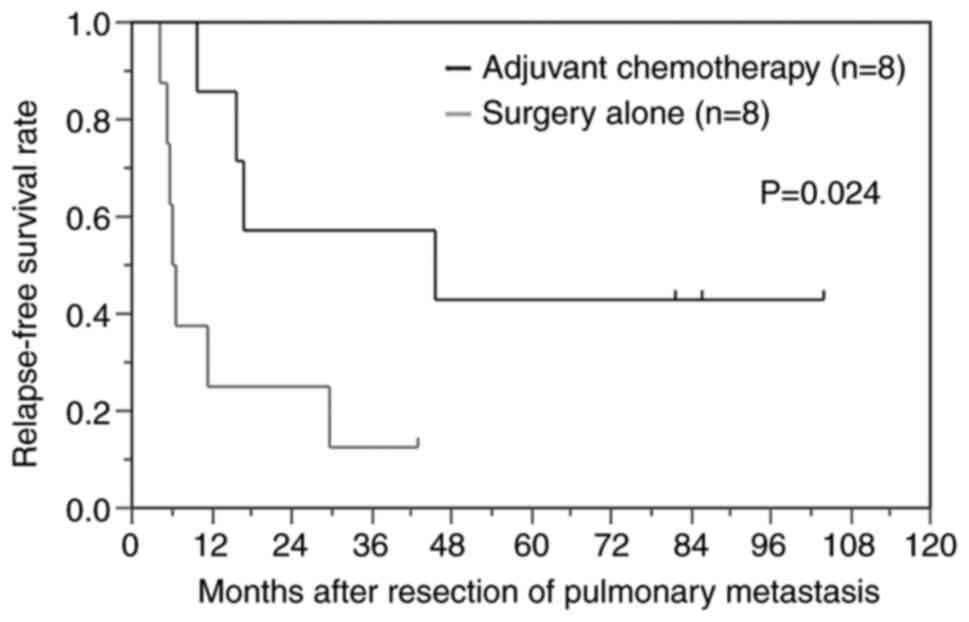
the surgery alone group (P=0.024) (Fig.
1).
Cut-off value for the number of
positive TILs
The number of positive TILs, which was a continuous
variable, was used as the test variable and the occurrence of
recurrence after the complete resection of pulmonary metastases was
used as the state variable. When the cut-off value for the number
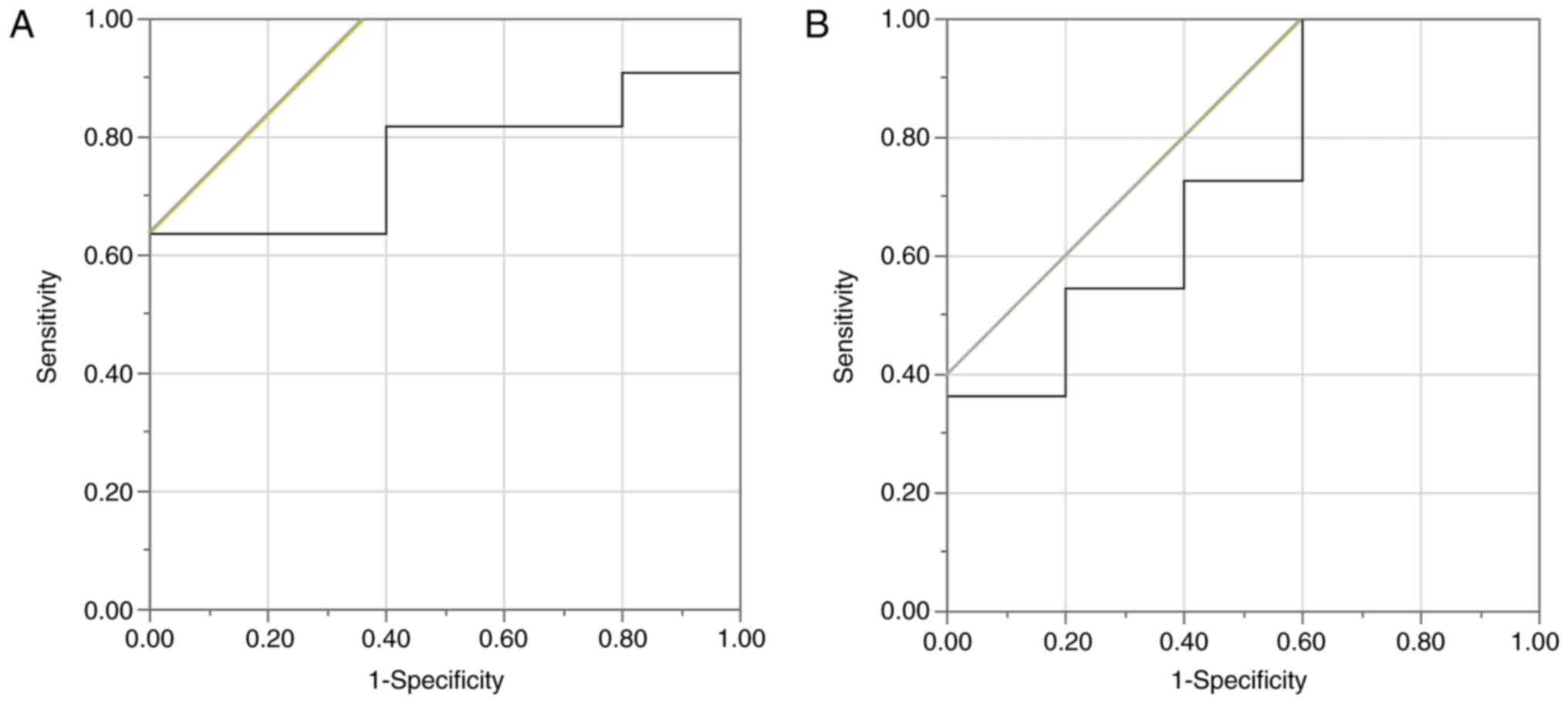
of positive TILs was investigated using the ROC curve, the
appropriate cut-off value for the CD3+TILs was 13.6
(sensitivity of 63.6%; specificity of 100.0%) (Fig. 2A). Using the ROC curve in the same
manner, the cut-off value for the CD8+TILs was set at
21.0 (sensitivity of 100.0%; specificity of 40.0%) (Fig. 2B). With each of these values set as
the cut-off value, the patients were classified into respective
high and low groups.
Evaluation of prognostic factors for
RFS
The correlation was evaluated between RFS and the
prognostic factors, such as sex, the timing of the detection of
metastasis, the number of pulmonary metastases, the location of
pulmonary metastasis, the size of pulmonary metastasis, the CEA
value before resection of pulmonary metastasis, whether or not
postoperative adjuvant chemotherapy had been performed, the NLR and
the CD3/CD8+ TIL density. According to a univariate
analysis, the RFS after resection of pulmonary metastasis in the
male group, the ≥2 pulmonary metastases group, the surgery alone
group and the low-CD3+TIL group was significantly
shorter than in the female group, the single pulmonary metastasis
group, the adjuvant chemotherapy group and the
high-CD3+TIL group (P=0.044, P=0.007, P=0.028, P=0.019,
respectively). The multivariate analysis indicated that the number
of pulmonary metastases, and the postoperative adjuvant
chemotherapy and the density of CD3+ TILs were
independent prognostic factors for RFS (P=0.001, P=0.002, P=0.010,
respectively) (Table II).
 | Table IICorrelations between relapse-free
survival and clinicopathological factors. |
Table II
Correlations between relapse-free
survival and clinicopathological factors.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Clinicopathological
factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex (Male vs.
Female) | 0.268 | 0.067-0.964 | 0.044 | 1.477 | 0.271-10.88 | 0.655 |
| Detection of
pulmonary metastases | 0.490 | 0.107-1.703 | 0.272 | | | |
|
Synchronous
vs. Metachronous | | | | | | |
| Location of
pulmonary metastases | 6.984 | 0.325-72.95 | 0.172 | | | |
|
One lung
field vs. both lung fields | | | | | | |
| Number of pulmonary
metastases | 6.559 | 1.690-31.63 | 0.007 | 30.93 | 3.193-1205.6 | 0.001 |
|
1 vs.
≥2 | | | | | | |
| Maximum diameter of
pulmonary metastases | 1.328 | 0.347-4.418 | 0.656 | | | |
|
<20.0 mm
vs. ≥20.0 mm | | | | | | |
| CEA level before
resection of pulmonary metastases | 1.434 | 0.373-4.796 | 0.575 | | | |
|
≤5.0 ng/ml
vs. >5.0 ng/ml | | | | | | |
| Treatment performed
to pulmonary metastases | 0.234 | 0.050-0.855 | 0.028 | 0.026 | 0.001-0.285 | 0.002 |
|
Surgery
alone vs. single-agent adjuvant chemotherapy after resection | | | | | | |
| NLR before
resection of pulmonary metastases | 1.448 | 0.434-5.043 | 0.542 | | | |
|
≤18.9 vs.
>18.9 | | | | | | |
| Density of
CD3+ TILs of pulmonary metastasis | 4.402 | 1.282-17.32 | 0.019 | 16.08 | 1.830-415.9 | 0.010 |
|
>13.6 vs.
≤13.6 | | | | | | |
| Density of
CD8+ TILs of pulmonary metastasis | 3.509 | 0.667-64.54 | 0.158 | | | |
|
>21.0 vs.
≤21.0 | | | | | | |
Feasibility and adverse events in
patients who received postoperative adjuvant chemotherapy
The completion rate of the 8 patients who received
adjuvant chemotherapy was 100.0% and the median RDI of adjuvant
chemotherapy in these 8 patients was 100.0%. Some adverse events
were occurred, including fatigue (n=2), hand and foot syndrome
(n=1) and oral mucositis (n=1). However, no patients experienced
grade 3-5 adverse events (Table
III).
 | Table IIIFeasibility and adverse event of
postoperative single-agent adjuvant chemotherapy in eight
patients. |
Table III
Feasibility and adverse event of
postoperative single-agent adjuvant chemotherapy in eight
patients.
| Parameter | Patients |
|---|
| Feasibility | |
|
Completion
rate, % | 100% |
|
Median
relative dose intensity, % (range) | 100%
(75.0-100.0%) |
| Adverse event,
grade 1-2, n (%) | |
|
Fatigue | 2 (25.0%) |
|
Oral
mucositis | 1 (12.5%) |
|
Hand and
foot syndrome | 1 (12.5%) |
The effectiveness of postoperative
adjuvant chemotherapy for suppressing recurrence according to each
risk factor
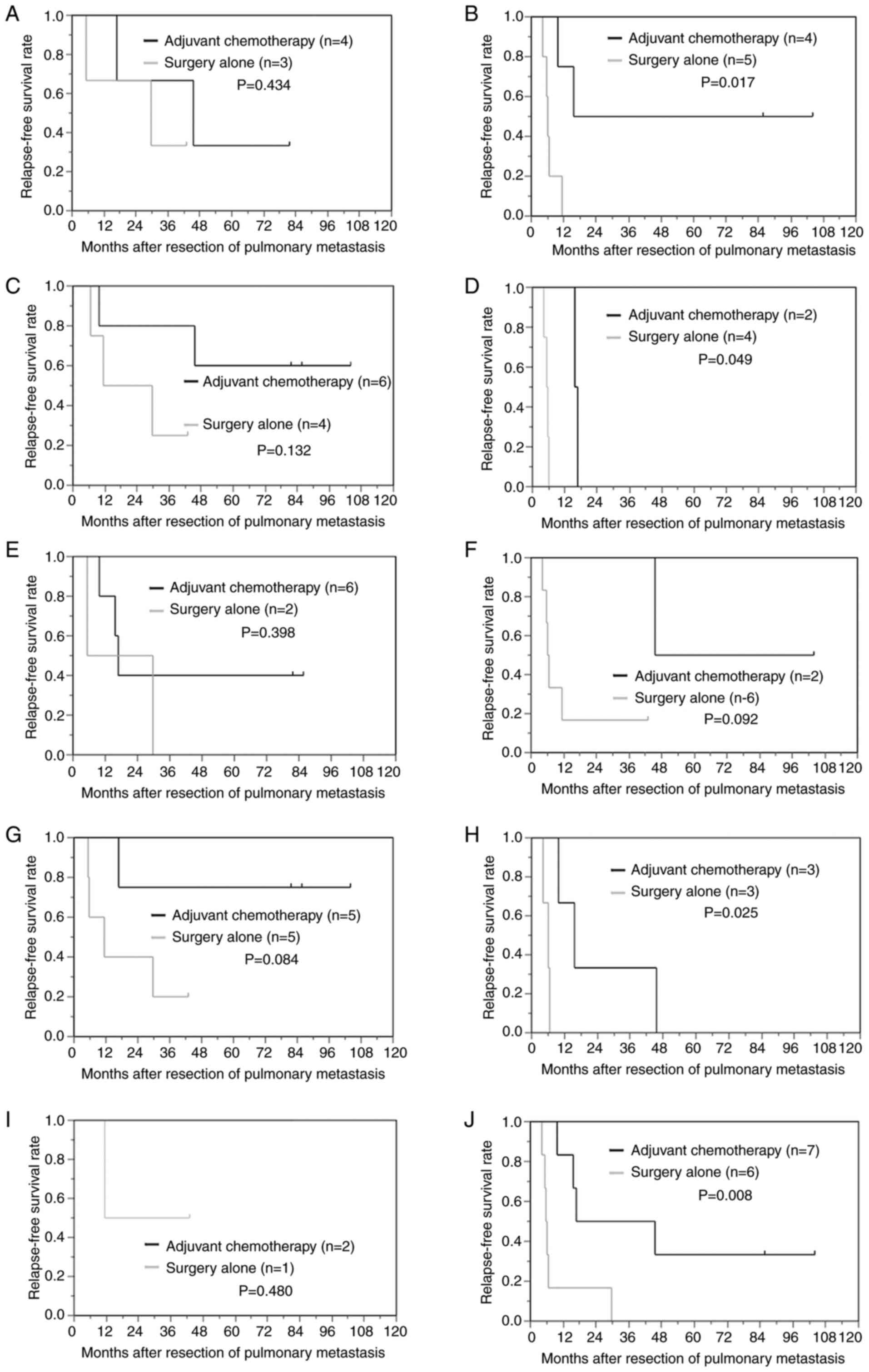
In the subgroups of patients who were positive for
lymph node metastasis of the primary tumor, patients with 2 or more
pulmonary metastases and patients with a low density of
CD3+TILs or a low density of CD8+TILs at the
site of pulmonary metastasis, postoperative adjuvant chemotherapy
significantly reduced the risk of recurrence in comparison to
surgery alone (P=0.017; 0.049; 0.025; 0.008, respectively). In the
subgroups of patients with a high NLR(>1.89), postoperative
adjuvant chemotherapy tended to reduce the risk of recurrence in
comparison to surgery alone (P=0.092) (Fig. 3).
Discussion
In cases of distant metastasis from CRC, unlike
other cancers, the complete resection of distant metastasis is
reported to improve the prognosis in comparison to chemotherapy.
Thus, the resection of metastasis from CRC has been performed
positively (7-10).
However, the recurrence rate after complete resection of distant
metastasis from CRC was comparatively high, thus, postoperative
adjuvant chemotherapy has been often performed in clinical
practice.
Regarding adjuvant chemotherapy after complete
resection of pulmonary metastasis from CRC, some studies reported
that it was effective for suppressing recurrence and prolonging the
prognosis (17,18). On the other hand, other studies
reported that it was not effective (32,33).
The reason why the results can be different between cohorts for the
adjuvant chemotherapy after resection of pulmonary metastases of
colorectal cancer was considered to be the difference of regimen.
In the existing reports, the single-agent adjuvant chemotherapy
regimen and the combination adjuvant chemotherapy regimen were
evaluated simultaneously, and the percentage of these regimen were
different in each cohort. In addition, there have been no reports
based on the results of large clinical trials; thus, the efficacy
of adjuvant chemotherapy after resection of pulmonary metastasis
from CRC has remained controversial. Therefore, in clinical
practice, the indication and regimen of adjuvant chemotherapy
largely depends on the judgment of the individual physician.
In this study, RFS rate was significantly improved
in the group that received single-agent fluoropyrimidine adjuvant
chemotherapy after resection of pulmonary metastasis. With the
exception of sex and the NLR, the patient characteristics of the
adjuvant chemotherapy and surgery alone groups did not differ to a
statistically significant extent; thus, the postoperative adjuvant
chemotherapy was correlated with the extension of the prognosis. In
addition, the rate of incidences of adverse events was not high and
no Grade 3-5 adverse events were occurred. Thus, the tolerability
of single-agent adjuvant chemotherapy after resection of pulmonary
metastasis from CRC was considered acceptable.
It has been reported that single-agent adjuvant
chemotherapy was effective for suppressing recurrence not only
after complete resection of primary CRC (34) but also after complete resection of
hepatic metastasis from CRC (35).
Similarly, in this study, in patients with pulmonary metastasis of
CRC, the RFS rate of patients who received single-agent adjuvant
chemotherapy with fluoropyrimidines was significantly improved.
Thus, it was suggested that even single-agent adjuvant chemotherapy
reduced the risk of recurrence. Additionally, single-agent adjuvant
chemotherapy was associated with few adverse events and was safe to
administer to patients with decreased physical strength after
resection of pulmonary metastasis. Therefore, it was suggested that
single-agent adjuvant chemotherapy was one of the treatment options
after complete resection of pulmonary metastasis from CRC.
Okumura et al reported that the efficacy of
adjuvant chemotherapy after resection of pulmonary metastasis
tended to be recognized by the risk classification and limitation
of the patients who are likely to benefit from postoperative
adjuvant chemotherapy (36). We
hypothesize that the fact that postoperative adjuvant chemotherapy
was performed without considering the risk classifications and
limitations of patients is one of the reasons why the prognosis was
not effectively prolonged by postoperative adjuvant chemotherapy in
the previous reports. Even in our small study population, we found
that some subgroups of patients with risk factors of recurrence
based on patient clinicopathological or immunological factors may
receive more benefit from adjuvant chemotherapy after resection of
pulmonary metastasis. As systemic inflammation or a poor local
immune status provide a favorable environment for the development
of micrometastases (37-40),
the risk of recurrence after the resection of pulmonary metastases
increases in high NLR patients or in low density TILs patients
(26). Therefore, the risk
classification according to the status of immunological biomarkers
as well as the risk factors of recurrence, which have been
described in previous reports, may help in the patient selection
process to identify those who will most benefit from postoperative
adjuvant chemotherapy.
The present study was associated with some
limitations. First, this was a retrospective study with a
relatively small population of patients who were treated at a
single institution. A prospective study in multiple institutions
with an increased number of patients is considered necessary.
Second, the prognosis was only evaluated by RFS rate. This was
because the chemotherapies used to treat recurrence after complete
resection of pulmonary metastasis have changed over the past 20
years with the development of oxaliplatin, irinotecan and molecular
targeted drugs; thus, overall survival rate was difficult to
evaluate. Third, the treatment methods were not unified because the
decision of whether to perform postoperative adjuvant chemotherapy
was made by the attending physician. Fourth, in this study, no
patients received combination adjuvant chemotherapy and the
difference in the effectiveness or safety between single-agent
adjuvant chemotherapy and combination adjuvant chemotherapy was not
compared.
A large prospective study is necessary to identify
patients who will be benefit from postoperative adjuvant
chemotherapy and to determine the most effective regimen.
In conclusion, single-agent adjuvant chemotherapy
after the resection of pulmonary metastasis from CRC was effective
for reducing the risk of recurrence and was safe to administer. In
addition, certain risk classifications based on clinicopathological
factors, including immunological markers, may be useful for
identifying patients who would receive more benefit from adjuvant
chemotherapy after resection of pulmonary metastasis from CRC.
Acknowledgements
The authors thank Mr Brian Quinn (Japan Medical
Communication, Fukuoka, Japan) for providing medical writing
services.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YO designed the study, performed the statistical
analysis and drafted the manuscript. MS designed the study and
assisted in writing the manuscript. EW, HN, TF and YI collected the
clinical data and revised the manuscript critically. YO, MS and EW
confirm the authenticity of all the raw data. KM, KH and MO
assisted with designing the study and critically reviewed the
manuscript. All the authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Osaka City University (approval no. 2020-026) and all patients
provided their written informed consent. All procedures performed
in studies involving human participants were in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang C, Tan Y and Xu H: Does adjuvant
chemotherapy improve the prognosis of patients after resection of
pulmonary metastasis from colorectal cancer? A systematic review
and meta-analysis. Int J Colorectal Dis. 34:1661–1671.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mitry E, Guiu B, Cosconea S, Jooste V,
Faivre J and Bouvier AM: Epidemiology, management and prognosis of
colorectal cancer with lung metastases: A 30-year population-based
study. Gut. 59:1383–1388. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Labianca R, Beretta GD, Kildani B, Milesi
L, Merlin F, Mosconi S, Pessi MA, Prochilo T, Quadri A, Gatta G, et
al: Colon cancer. Crit Rev Oncol Hematol. 74:106–133.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tampellini M, Ottone A, Bellini E, Alabiso
I, Baratelli C, Bitossi R, Brizzi MP, Ferrero A, Sperti E, Leone F,
et al: The role of lung metastasis resection in improving outcome
of colorectal cancer patients: Results from a large retrospective
study. Oncologist. 17:1430–1438. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang Z, Wang X, Yuan J, Zhang X, Zhou J,
Lu M, Liu D, Li J and Shen L: Survival benefit of palliative local
treatments and efficacy of different pharmacotherapies in
colorectal cancer with lung metastasis: Results from a large
retrospective study. Clin Colorectal Cancer. 17:e233–e255.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kato T, Yasui K, Hirai T, Kanemitsu Y,
Mori T, Sugihara K, Mochizuki H and Yamamoto J: Therapeutic results
for hepatic metastasis of colorectal cancer with special reference
to effectiveness of hepatectomy: Analysis of prognostic factors for
763 cases recorded at 18 institutions. Dis Colon Rectum. 46 (Suppl
10):S22–S31. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chua TC, Saxena A, Liauw W, Chu F and
Morris DL: Hepatectomy and resection of concomitant extrahepatic
disease for colorectal liver metastases-a systematic review. Eur J
Cancer. 48:1757–1765. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Koga R, Yamamoto J, Saiura A, Yamaguchi T,
Hata E and Sakamoto M: Surgical resection of pulmonary metastases
from colorectal cancer: Four favourable prognostic factors. Jpn J
Clin Oncol. 36:643–648. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Iizasa T, Suzuki M, Yoshida S, Motohashi
S, Yasufuku K, Iyoda A, Shibuya K, Hiroshima K, Nakatani Y and
Fujisawa T: Prediction of prognosis and surgical indications for
pulmonary metastasectomy from colorectal cancer. Ann Thorac Surg.
82:254–260. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kandioler D, Krömer E, Tüchler H, End A,
Müller MR, Wolner E and Eckersberger F: Long-term results after
repeated surgical removal of pulmonary metastases. Ann Thorac Surg.
65:909–912. 1998.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Regnard JF, Grunenwald D, Spaggiari L,
Girard P, Elias D, Ducreux M, Baldeyrou P and Levasseur P: Surgical
treatment of hepatic and pulmonary metastases from colorectal
cancers. Ann Thorac Surg. 66:214–218. 1998.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Okumura S, Kondo H, Tsuboi M, Nakayama H,
Asamura H, Tsuchiya R and Naruke T: Pulmonary resection for
metastatic colorectal cancer: Experiences with 159 patients. J
Thorac Cardiovasc Surg. 112:867–874. 1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Saito Y, Omiya H, Kohno K, Kobayashi T,
Itoi K, Teramachi M, Sasaki M, Suzuki H, Takao H and Nakade M:
Pulmonary metastasectomy for 165 patients with colorectal
carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg.
124:1007–1013. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pfannschmidt J, Dienemann H and Hoffmann
H: Surgical resection of pulmonary metastases from colorectal
cancer: A systematic review of published series. Ann Thorac Surg.
84:324–338. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nakajima J: Pulmonary metastasis:
Rationale for local treatments and techniques. Gen Thorac
Cardiovasc Surg. 58:445–451. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shiomi K, Naito M, Sato T, Nakamura T,
Nakashima H, Naito M, Mikubo M, Matsui Y, Watanabe M and Satoh Y:
Effect of adjuvant chemotherapy after pulmonary metastasectomy on
the prognosis of colorectal cancer. Ann Med Surg (Lond). 20:19–25.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Menna C, Berardi G, Tierno SM, Andreetti
C, Maurizi G, Ciccone AM, D'Andrilli A, Cassiano F, Poggi C, Diso
D, et al: Do repeated operations for recurrent colorectal lung
metastases result in improved survival? Ann Thorac Surg.
106:421–427. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Department of Health and Human Services,
National Institutes of Health, National Cancer Institute. Common
terminology criteria for adverse events (CTCAE) v5.0 2017.
Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
|
|
20
|
Hirosawa T, Itabashi M, Ohnuki T,
Yamaguchi N, Sugihara K and Kameoka S: Prognostic factors in
patients undergoing complete resection of pulmonary metastases of
colorectal cancer: A multi-institutional cumulative follow-up
study. Surg Today. 43:494–499. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
TNM Classification of Malignant Tumours.
8th edition. Brierley JD, Gospodarowicz MK and Wittekind C (eds.).
John Wiley & Sons, Ltd., New Jersey, 2017.
|
|
22
|
Lin BR, Chang TC, Lee YC, Lee PH, Chang KJ
and Liang JT: Pulmonary resection for colorectal cancer metastases:
Duration between cancer onset and lung metastasis as an important
prognostic factor. Ann Surg Oncol. 16:1026–1032. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Iida T, Nomori H, Shiba M, Nakajima J,
Okumura S, Horio H, Matsuguma H, Ikeda N, Yoshino I, Ozeki Y, et
al: Prognostic factors after pulmonary metastasectomy for
colorectal cancer and rationale for determining surgical
indications: A retrospective analysis. Ann Surg. 257:1059–1064.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gonzalez M and Gervaz P: Risk factors for
survival after lung metastasectomy in colorectal cancer patients:
Systematic review and meta-analysis. Future Oncol. 11 (2
Suppl):S31–S33. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Watanabe K, Nagai K, Kobayashi A, Sugito M
and Saito N: Factors influencing survival after complete resection
of pulmonary metastases from colorectal cancer. Br J Surg.
96:1058–1065. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Okazaki Y, Shibutani M, Wang EN, Nagahara
H, Fukuoka T, Iseki Y, Kashiwagi S, Tanaka H, Maeda K, Hirakawa K
and Ohira M: Prognostic significance of the immunological indices
in patients who underwent complete resection of pulmonary
metastases of colorectal cancer. In vivo. 35:1091–1100.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
An X, Ding PR, Li YH, Wang FH, Shi YX,
Wang ZQ, He YJ, Xu RH and Jiang WQ: Elevated neutrophil to
lymphocyte ratio predicts survival in advanced pancreatic cancer.
Biomarkers. 15:516–522. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chiang SF, Hung HY, Tang R, Changchien CR,
Chen JS, You YT, Chiang JM and Lin JR: Can neutrophil-to-lymphocyte
ratio predict the survival of colorectal cancer patients who have
received curative surgery electively? Int J Colorectal Dis.
27:1347–1357. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mallappa S, Sinha A, Gupta S and Chadwick
SJ: Preoperative neutrophil to lymphocyte ratio >5 is a
prognostic factor for recurrent colorectal cancer. Colorectal Dis.
15:323–328. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hendry S, Salgado R, Gevaert T, Russell
PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV,
Gonzalez-Ericsson PI, et al: Assessing tumor-infiltrating
lymphocytes in solid tumors: A practical review for pathologists
and proposal for a standardized method from the International
immunooncology biomarkers working group: Part 1: Assessing the host
immune response, TILs in invasive breast carcinoma and ductal
carcinoma in situ, metastatic tumor deposits and areas for further
research. Adv Anat Pathol. 24:235–251. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hendry S, Salgado R, Gevaert T, Russell
PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV,
Gonzalez-Ericsson PI, et al: Assessing tumor-infiltrating
lymphocytes in solid tumors: A practical review for pathologists
and proposal for a standardized method from the International
immuno-oncology biomarkers working group: Part 2: TILs in melanoma,
gastrointestinal tract carcinomas, non-small cell lung carcinoma
and mesothelioma, endometrial and ovarian carcinomas, squamous cell
carcinoma of the head and neck, genitourinary carcinomas, and
primary brain tumors. Adv Anat Pathol. 24:311–335. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Imanishi M, Yamamoto Y, Hamano Y, Yamada
T, Moriwaki T, Gosho M, Okumura T, Boku N, Kondo H and Hyodo I:
Efficacy of adjuvant chemotherapy after resection of pulmonary
metastasis from colorectal cancer: A propensity score-matched
analysis. Eur J Cancer. 106:69–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Park S, Kang BW, Lee SJ, Yoon S, Chae YS,
Kim JG, Lee KH, Koh SA, Song HS, Park KU, et al: Clinical
significance of systemic chemotherapy after curative resection of
metachronous pulmonary metastases from colorectal cancer. Cancer
Chemother Pharmacol. 80:187–193. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sakamoto J, Kodaira S, Hamada C, Ito K,
Maehara Y, Takagi H, Sugimachi K, Nakazato H and Ohashi Y:
Meta-analysis group of the Japanese society of strategies for
Cancer Research and Therapy. An individual patient data
meta-analysis of long supported adjuvant chemotherapy with oral
carmofur in patients with curatively resected colorectal cancer.
Oncol Rep. 8:697–703. 2001.PubMed/NCBI
|
|
35
|
Hasegawa K, Saiura A, Takayama T, Miyagawa
S, Yamamoto J, Ijichi M, Teruya M, Yoshimi F, Kawasaki S, Koyama H,
et al: Adjuvant oral uracil-tegafur with leucovorin for colorectal
cancer liver metastases: A randomized controlled trial. PLoS One.
11(e0162400)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Okumura T, Boku N, Hishida T, Ohde Y,
Sakao Y, Yoshiya K, Higashiyama M, Hyodo I, Mori K and Kondo H:
Surgical Outcome and prognostic stratification for pulmonary
metastasis from colorectal cancer. Ann Thorac Surg. 104:979–987.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shibutani M, Nagahara H, Fukuoka T, Iseki
Y, Hirakawa K and Ohira M: Efficacy of adjuvant chemotherapy
according to the classification of recurrence risk based on
systemic inflammatory markers in patients with liver metastases of
colorectal cancer. Anticancer Res. 39:5039–5045. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mantovani C, Levra NG, Filippi AR, Novello
S, Buffoni L, Ragona R and Ricardi U: Postoperative radiotherapy
for patients with completely resected pathologic n2 non-small-cell
lung cancer: A retrospective analysis. Clin Lung Cancer.
14:194–199. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chiang AC and Massagué J: Molecular basis
of metastasis. N Engl J Med. 359:2814–2823. 2008.PubMed/NCBI View Article : Google Scholar
|