Introduction
Oral cancer is the most common malignant tumor in
the oral cavity and its survival rate has not improved, despite
innovations in diagnostic techniques and treatments (1). In general practice, various serum
tumor markers have been found to be useful for the screening of
cancer and for determining the degree of progression, evaluating
therapeutic efficacy, and predicting the prognosis, recurrence and
metastasis, and they are currently employed for prostate, lung and
liver cancer. However, although the squamous cell carcinoma antigen
(SCC Ag) is used as a tumor marker in general practice (2), there are currently no suitable
diagnostic biomarkers for oral squamous cell carcinoma (OSCC) to
the best of our knowledge. Therefore, it is necessary to
investigate reliable serum tumor markers for routine OSCC
diagnosis.
The serum antip53 antibody (Ap53Ab) is an IgG
antibody that reacts with the p53 protein that accumulates in the
nucleus as a result of mutations in the TP53 tumor
suppressor gene, which exhibits the highest mutation frequency
among malignant tumors (3). Unlike
other conventional tumor markers that detect tumor cell-derived
proteins, Ap53Ab has been considered as an innovative tumor marker,
in the sense that it detects serum antibodies that emerge in
response to tumor cell-derived proteins (4). Ap53Ab triggers an antigen-antibody
reaction, which is positive even in the early stages of cancer, and
can detect micro-residual tumor cells after treatment (5,6). As a
result of the remarkable results of a study by Shimada et al
(7), the measurement of Ap53Ab
levels has been covered from 2007 onwards by medical insurance in
Japan for esophageal, colorectal and breast cancer, and can be
applied to daily clinical practice. Although several studies have
investigated the expression of Ap53Ab in OSCC (2,8,9), to
the best of our knowledge, there have been no reports on measuring
Ap53Ab in daily clinical practice, despite the need to re-evaluate
the clinicopathological significance of Ap53Ab in oral cancer.
The prognostic significance of Ap53Ab (as measured
by an ELISA kit approved by the Japanese Health Insurance System in
2007) has been revalidated and reported for several malignancies,
such as esophageal, liver, gastric and colorectal cancer (10-12).
Although a relatively high positive rate was reported by Shimada
et al (7), no studies have
yet evaluated the utility of Ap53Ab measurement in daily clinical
practice as a prognostic marker for head and neck cancer, including
OSCC. Given that TP53 mutations occur frequently and that
these mutations adversely affect the malignant phenotype (13), it is important to re-evaluate the
utility of Ap53Ab as a prognostic factor for Japanese patients with
OSCC in clinical practice.
The present study was undertaken to revalidate the
detection rate of Ap53Ab and examine the correlation between Ap53Ab
status and p53 expression in primary OSCC, and to re-evaluate the
clinical significance of Ap53Ab in OSCC.
Materials and methods
Patients
The study population comprised 94 patients with OSCC
who underwent radical resection as primary treatment at the
Kumamoto University Hospital (Kumamoto, Japan) between April 2015
and March 2018. The median age of the study population was 66.8
years (range, 24-88 years). Of the 94 patients, 57 were male and 37
were female. The primary tumors were located in the tongue (n=51),
maxilla (n=5), mandible (n=21), hard palate (n=1), oral floor (n=9)
and buccal mucosa (n=7). The clinical T-category (cT-category) was
recorded as cT1, cT2, cT3 and cT4a in 21, 50, 13 and 10 patients,
respectively. The clinical N-category (cN-category) was recorded as
cN0, cN1, cN2b, cN2c and cN3b in 73, 7, 12, 1 and 1 patients,
respectively. The clinical stage (cStage) was recorded as I-III and
IV in 21, 42, 14 and 17 patients, respectively. The pathological
T-category (pT-category) was recorded as pT1, pT2, pT3 and pT4a in
19, 55, 14 and 6 patients, respectively. The pathological
N-category (pN-category) was recorded as pN0, pN1, pN2b, pN2c and
pN3b in 84, 4, 2, 2 and 2 patients, respectively. Finally, the
pathological stage (pStage) was recorded as I-III and IV in 19, 52,
12 and 11 patients, respectively. Patients who had distant
metastases prior to treatment initiation were excluded from the
present study. The patients were diagnosed based on histological
and radiological findings, including computed tomography, magnetic
resonance imaging, ultrasonography and positron emission
tomography-computed tomography findings. All tumors were staged
according to the 8th edition of the TNM classification of the
American Joint Committee on Cancer (14), and the differentiation was
determined according to the World Health Organization
classification (15). The worst
pattern of invasion was recorded as follows: Non-aggressive
patterns included broad pushing margin (WPOI-1), broad finger-like
projections or separate large islands (WPOI-2), and those
exhibiting invasive islands (>15 cells; WPOI-3). Aggressive
patterns included WPOI-4, with islands of <5 cells, strands of
tumor cells or single-cell infiltration, and WPOI-5, exhibiting
tumor satellites separated from the main tumor interface by >1
mm. After undergoing curative surgery, the patients were followed
up until March 2020. The study was conducted with the approval of
the Ethics Committee of Kumamoto University (approval no.
RINRI:1427), in accordance with the guidelines for Good Clinical
Practice and the Declaration of Helsinki. The present study was a
retrospective analysis, which does not require individual consent;
however, it guarantees inclusion in the study and offers the
opportunity to refuse participation in an opt-out format
(RINRI1427).
Blood samples and Ap53Ab
measurement
Total blood samples (5 µl) were routinely collected
from the patients with OSCC prior to surgery. The titers of Ap53Ab
were assessed using an ELISA kit (MESACUP anti-p53 Test; cat. no.
7640E, Medical & Biological Laboratories, Co., Ltd.). Briefly,
samples were added to microtiter plate wells coated with either
wild-type human p53 or control protein and incubated for 1 h.
Peroxidase-conjugated goat anti-human immunoglobulin G-binding
Ap53Ab was then added, followed by incubation for 1 h. Thereafter,
substrate solution was added, followed by incubation for 30 min. A
calibration curve was constructed from specific signals of
standards and from the levels of antibodies indicated on the vials
containing the standards. To ensure the accuracy of the test,
researchers blinded to the clinical data of the patient performed
the measurements together with a laboratory specialist. Considering
that the cutoff value of Ap53Ab has generally been set to 1.3 U/ml
for other malignant tumors in daily clinical practices across
Japan, the present study adopted the same cutoff value.
Immunohistochemical (IHC) staining and
assessment
Formalin-fixed, paraffin-embedded specimens were cut
into 4-µm sections and mounted on MAS-GP-coated slides (Matsunami
Glass Ind., Ltd.). To retrieve the p53 antigen, the sections were
subjected to deparaffinization and rehydration, and then heated in
an autoclave in 0.01 mol/l citrate buffer (pH 7.0) for 15 min at
121˚C. The sections were subsequently incubated with 3% hydrogen
peroxide in absolute methanol for 15 min to block endogenous
peroxidase activity. The sections were also incubated with Protein
Block Serum-Free Reagent (Dako; Agilent Technologies, Inc.) at room
temperature for 10 min to block non-specific staining. After the
blocking step, the sections were incubated at 4˚C overnight with an
anti-p53 antibody (1:100; clone DO-1, cat. no. sc-126, Santa Cruz
Biotechnology, Inc.; 1:100; clone PAb240, cat. no. ab26, Abcam),
followed by sequential 60-min incubations with the secondary
antibody (EnVision + System-HRP Labelled Polymer, cat. no. K4000 or
4002, Dako; Agilent Technologies, Inc.) and the Liquid DAB+
Substrate Chromogen System (Dako; Agilent Technologies, Inc.). All
slides were counterstained using hematoxylin at room temperature
for 60 sec before the dehydration and mounting steps. Two
independent observers, who were blinded to the patients' clinical
status, interpreted the IHC data. Based on previous reports
(16-18),
tumor samples with >10% of tumor cells exhibiting positive
nuclear staining were considered to be positive for p53.
Disagreements regarding the IHC staining scores were resolved
through discussion and consensus.
Statistical analysis
Fisher's exact test was employed for categorical
factors to calculate the P-values indicating the significance of
the associations between the Ap53Ab status and clinicopathological
factors. The pre-treatment values of Ap53Ab were not normally
distributed according to the χ2 goodness-of-fit test.
Therefore, the Kruskal-Wallis and Bonferroni-Dunn tests were used
to evaluate differences between the pre-treatment values of Ap53Ab
for each clinical stage. Survival analysis was performed using the
Kaplan-Meier method and the log-rank test and multivariate survival
analyses were performed using Cox regression models to evaluate the
association between Ap53Ab status and overall survival (OS) and
disease-free survival (DFS). All P-values were based on two-tailed
tests, and P<0.05 were considered to indicate statistically
significant differences. All statistical analyses were performed
using JMP software version 9 (SAS Institute Inc.).
Results
Rate of Ap53Ab positivity in the study
population
To determine the Ap53Ab positivity rate in patients
with OSCC, the Ap53Ab values in the study population were reviewed.
The cutoff value was set at 1.3 U/ml, which is employed in daily
clinical practice in Japan. Of the 94 patients with OSCC, 23
(23.4%) were Ap53Ab-positive. The mean Ap53Ab level (± SD) in
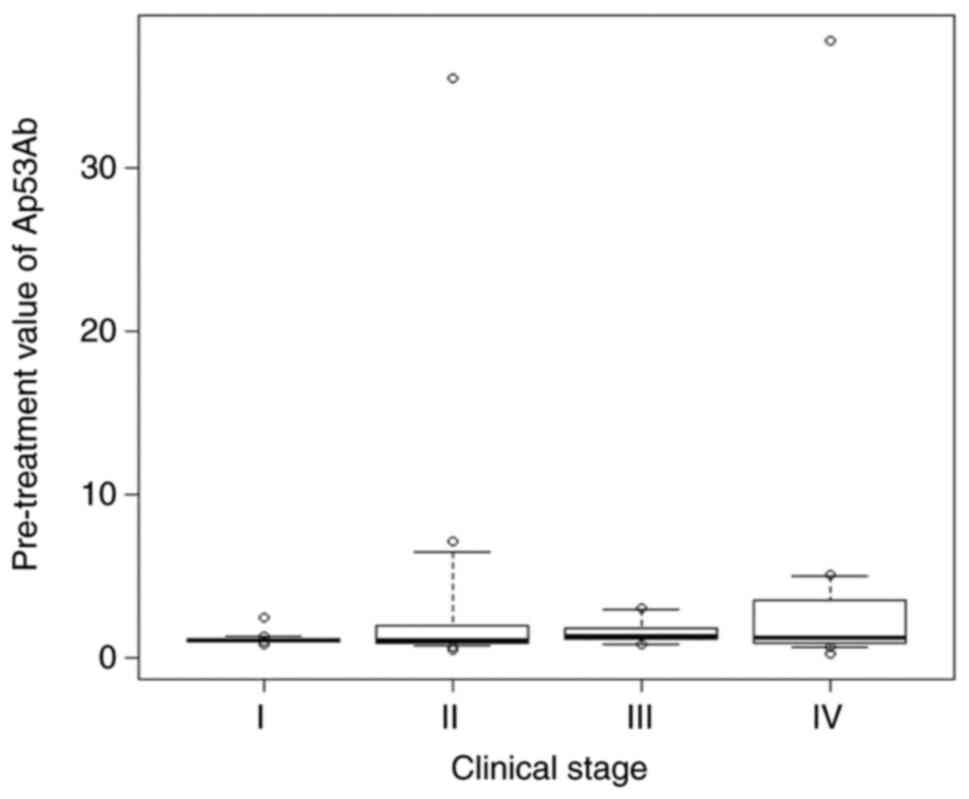
patients with OSCC was 1.08±6.75 U/ml (range, 0.2-37.8 U/ml). The
Ap53Ab levels tended to increase with advancing clinical stage,
although the differences among different stages were not
statistically significant (Fig. 1).
Moreover, a decrease in Ap53Ab level was observed in 13 of the 23
patients (56.5%) whose Ap53Ab levels were measurable prior to
surgery. In particular, among the 11 patients positive for Ap53Ab
(>1.3 U/ml) prior to surgery, 8 exhibited a decrease in the
Ap53Ab titer postoperatively (72.7%; Table I).
 | Table IChanges in serum antip53 antibody
titer among the 23 patients with oral squamous cell carcinoma after
surgery. |
Table I
Changes in serum antip53 antibody
titer among the 23 patients with oral squamous cell carcinoma after
surgery.
| Case no. | Before surgery | After surgery | Type of change |
|---|
| 4 | 5.05 | 4.61 | Decrease |
| 6 | 3.00 | 3.49 | Increase |
| 13 | 2.62 | 2.49 | Decrease |
| 21 | 2.05 | 1.48 | Decrease |
| 22 | 2.20 | 1.83 | Decrease |
| 26 | 0.84 | 1.19 | Increase |
| 31 | 0.23 | 0.76 | Increase |
| 32 | 0.53 | 0.56 | Increase |
| 37 | 0.87 | 0.34 | Decrease |
| 39 | 1.43 | 1.56 | Increase |
| 40 | 6.28 | 0.79 | Decrease |
| 53 | 1.01 | 1.22 | Increase |
| 64 | 3.56 | 3.98 | Increase |
| 65 | 2.79 | 2.34 | Decrease |
| 66 | 1.02 | 1.00 | Decrease |
| 67 | 35.50 | 33.20 | Decrease |
| 68 | 0.86 | 0.71 | Decrease |
| 71 | 0.62 | 0.88 | Increase |
| 73 | 0.63 | 1.02 | Increase |
| 74 | 0.79 | 1.25 | Increase |
| 77 | 1.32 | 1.01 | Decrease |
| 93 | 1.26 | 1.20 | Decrease |
| 94 | 0.95 | 0.94 | Decrease |
Associations between Ap53Ab status and
IHC staining results of p53
To examine the association between Ap53Ab status and
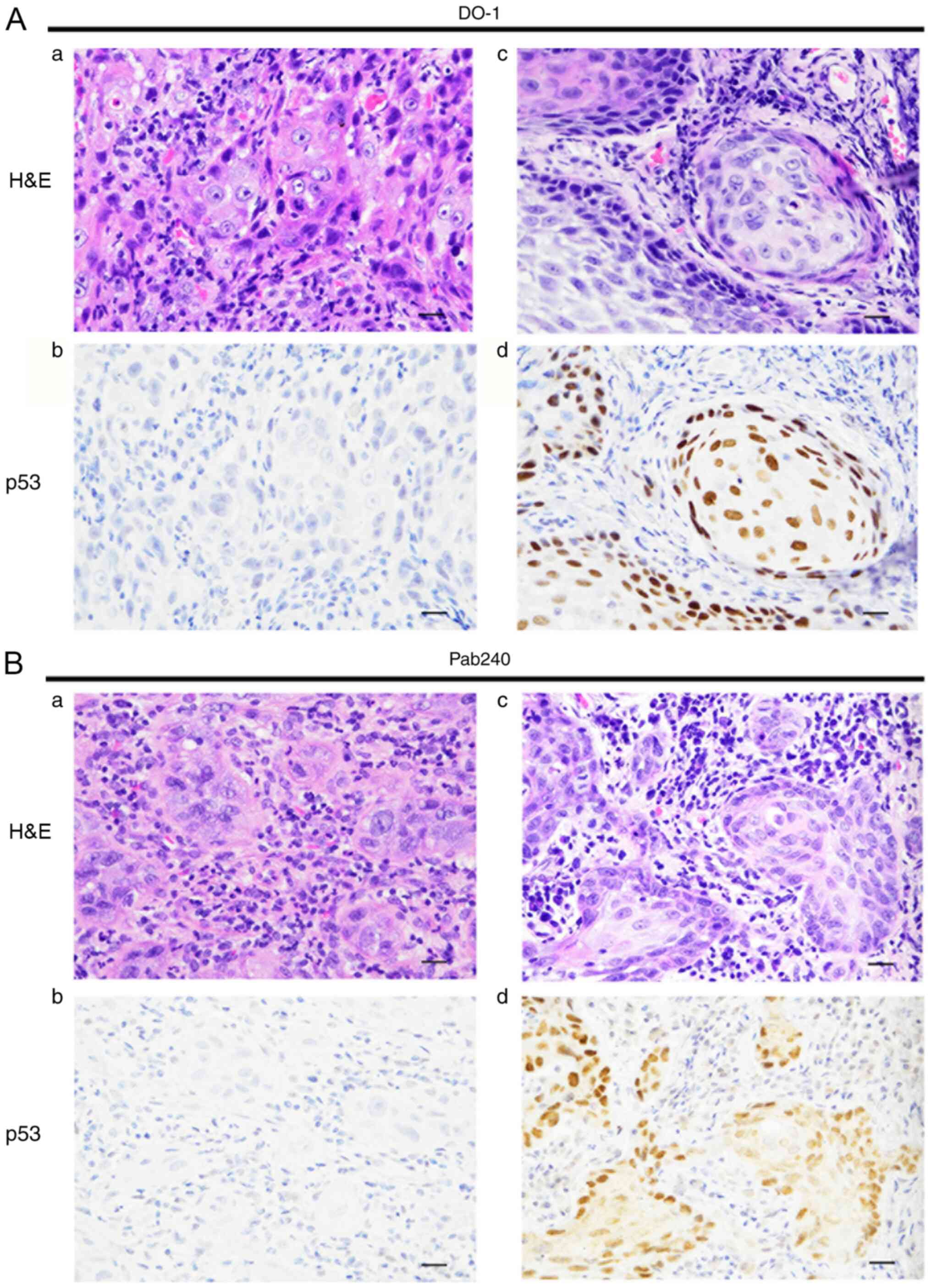
the expression patterns of mutated p53 in the primary tumors, IHC
staining of p53 was performed by using the clones DO-1 and PAb240,
which are representative clones for detecting TP53
mutations. The representative immunostaining patterns of p53 for
each clone are shown in Fig. 2A and
B. A total of 47 (50.0%) of the 94
patients with OSCC were positive for DO-1, while 33 (35.1%) were
positive for PAb240. In addition, 48 patients (51.1%) were positive
for either DO-1 or PAb240 (Table
II). There was a significant correlation between Ap53Ab status
and p53 staining pattern, which could be detected by the DO-1 clone
expression status in primary tumors (P=0.027; Table II).
 | Table IIAssociation between Ap53Ab status and
results of p53 immunohistochemical staining in 96 patients with
oral squamous cell carcinoma. |
Table II
Association between Ap53Ab status and
results of p53 immunohistochemical staining in 96 patients with
oral squamous cell carcinoma.
| | Ap53Ab status | |
|---|
| Antibody | Negative, n
(%) | Positive, n
(%) | P-value |
|---|
| Total (n=94) | 72 (76.6) | 22 (23.4) | |
| DO-1 | | | 0.027 |
|
Negative | 41 (56.9) | 6 (27.3) | |
|
Positive | 31 (43.1) | 16 (72.7) | |
| Pab240 | | | 0.126 |
|
Negative | 50 (69.4) | 11 (50.0) | |
|
Positive | 22 (30.6) | 11 (50.0) | |
| Either DO-1- or
Pab240-positive | | | 0.226 |
|
No | 38 (52.8) | 8 (36.4) | |
|
Yes | 34 (47.2) | 14 (63.6) | |
Associations between Ap53Ab status and
clinicopathological characteristics
To elucidate the clinical significance of the Ap53Ab
status in the 94 patients with OSCC, the correlations between
Ap53Ab status and the clinicopathological variables were examined.
The clinical background characteristics of the study patients were
divided into two groups, namely Ap53Ab-positive and Ap53Ab-negative
(Table III). The frequency of
Ap53Ab positivity was significantly higher in patients with
advanced cT-category, pN-category and pStage (P=0.04, P=0.010 and
P=0.013, respectively; Table
III).
 | Table IIIAssociation between Ap53Ab status and
clinicopathological factors in 94 patients with oral squamous cell
carcinoma. |
Table III
Association between Ap53Ab status and
clinicopathological factors in 94 patients with oral squamous cell
carcinoma.
| | Ap53Ab status | |
|---|
|
Characteristics | Total (n=94) | Negative, n
(%) | Positive, n
(%) | P-value |
|---|
| Total | | 72 (76.6) | 22 (23.4) | |
| Age (years) | | | | 1.000 |
|
Median | 66.8 | 66.3 | 68.5 | |
|
Range | | 24-86 | 45-88 | |
|
≤65 | 33 | 25 (34.7) | 8 (36.4) | |
|
>65 | 61 | 47 (65.3) | 14 (63.6) | |
| Sex | | | | 0.220 |
|
Male | 57 | 41 (56.9) | 16 (72.7) | |
|
Female | 37 | 31 (43.1) | 6 (27.3) | |
| Oral subsite | | | | 0.901 |
|
Tongue | 51 | 40 (55.6) | 11 (50.0) | |
|
Oral
floor | 9 | 6 (8.3) | 3 (13.6) | |
|
Mandible | 21 | 15 (20.8) | 6 (27.3) | |
|
Maxilla | 5 | 4 (5.6) | 1 (4.5) | |
|
Buccal
mucosa | 7 | 6 (8.3) | 1 (4.5) | |
|
Hard
palate | 1 | 1 (1.4) | 0 (0.0) | |
| Clinical
T-category | | | | 0.04a |
|
T1 | 21 | 19 (26.4) | 2 (9.1) | |
|
T2 | 50 | 40 (55.6) | 10 (45.5) | |
|
T3 | 13 | 8 (11.1) | 5 (22.7) | |
|
T4 | 10 | 5 (6.9) | 5 (22.7) | |
| Clinical
N-category | | | | 0.249 |
|
0 | 73 | 58 (80.6) | 15 (68.2) | |
|
≥1 | 21 | 14 (19.4) | 7 (31.8) | |
| Clinical stage | | | | 0.077 |
|
I | 21 | 19 (26.4) | 2 (9.1) | |
|
II | 42 | 34 (47.2) | 8 (36.4) | |
|
III | 14 | 9 (12.5) | 5 (22.7) | |
|
IV | 17 | 10 (13.9) | 7 (31.8) | |
| Pathological
T-category | | | | 0.054 |
|
T1 | 19 | 17 (23.6) | 2 (9.1) | |
|
T2 | 55 | 44 (61.1) | 11 (50.0) | |
|
T3 | 14 | 8 (11.1) | 6 (27.3) | |
|
T4 | 6 | 3 (4.2) | 3 (13.6) | |
| Pathological
N-category | | | | 0.010a |
|
0 | 84 | 68 (94.4) | 16 (72.7) | |
|
≥1 | 10 | 4 (5.6) | 6 (27.3) | |
| Pathological
stage | | | | 0.013a |
|
I | 19 | 17 (23.6) | 2 (9.1) | |
|
II | 52 | 43 (59.7) | 9 (40.9) | |
|
III | 12 | 7 (9.7) | 5 (22.7) | |
|
IV | 11 | 5 (6.9) | 6 (27.3) | |
|
Differentiation | | | | 0.361 |
|
High | 48 | 34 (47.2) | 14 (63.6) | |
|
Moderate | 42 | 34 (47.2) | 8 (36.4) | |
|
Poor | 4 | 4 (5.6) | 0 (0.0) | |
| Worst pattern of
invasion | | | | 0.335 |
|
1b
and 2c | 29 | 22 (30.6) | 7 (31.8) | |
|
3d | 41 | 34 (47.2) | 7 (31.8) | |
|
4e
and 5f | 24 | 16 (22.2) | 8 (36.4) | |
| Local
recurrence | | | | 0.694 |
|
No | 84 | 65 (90.3) | 19 (86.4) | |
|
Yes | 10 | 7 (9.7) | 3 (13.6) | |
| Delayed neck
metastasis | | | | 0.175 |
|
No | 82 | 64 (88.9) | 17 (77.3) | |
|
Yes | 12 | 8 (11.1) | 5 (22.7) | |
| Distant
metastasis | | | | 0.664 |
|
No | 87 | 67 (93.1) | 20 (90.9) | |
|
Yes | 13 | 5 (6.9) | 2 (9.1) | |
Associations between Ap53Ab status and
prognosis
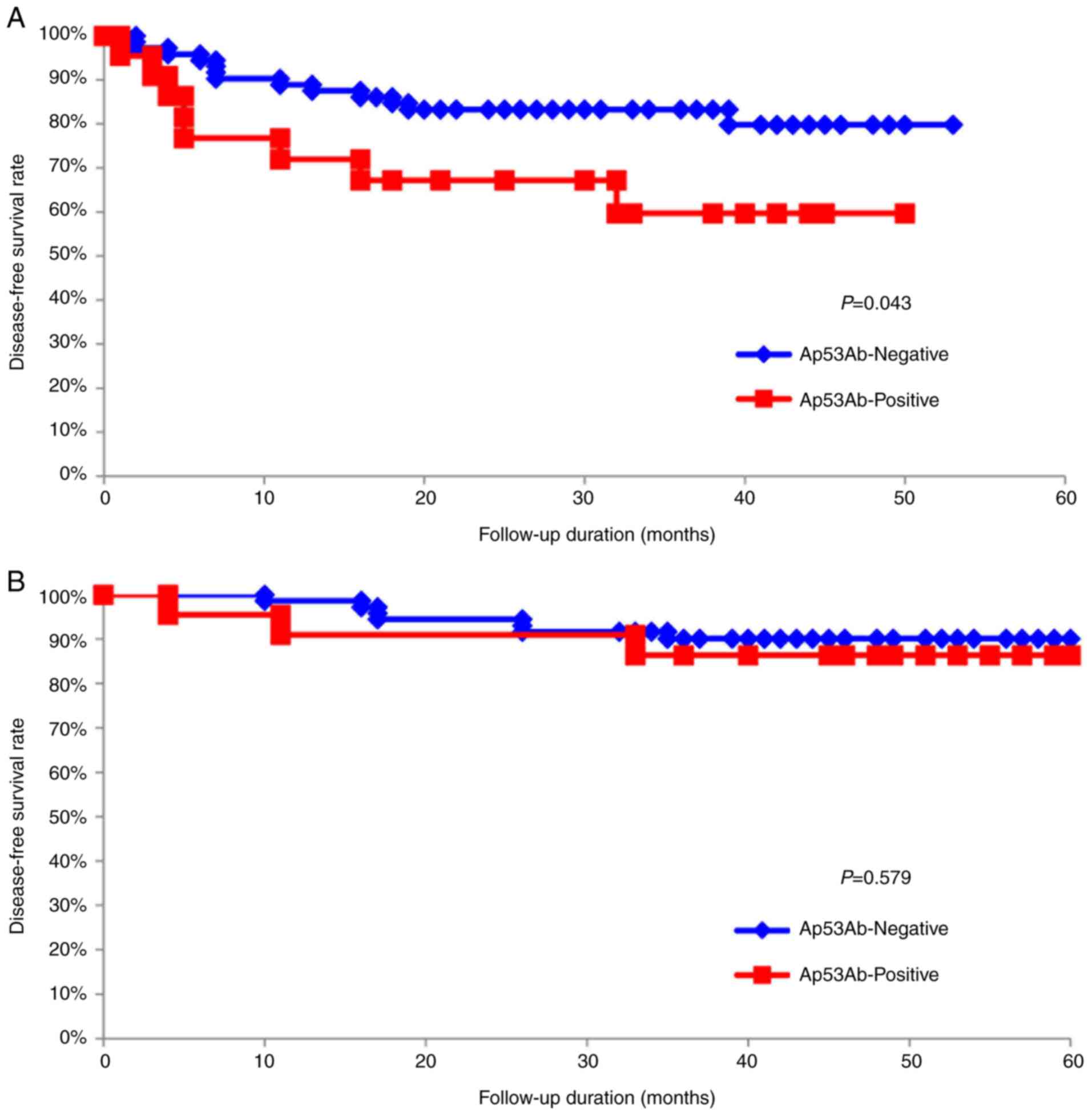
To assess the impact of Ap53Ab status on survival
time, the DFS and OS of the 94 patients with OSCC were analyzed by
using the Kaplan-Meier method. The 5-year DFS rates of patients
with an Ap53Ab-positive status were significantly lower compared
with those of patients with an Ap53Ab-negative status (P=0.043;
Fig. 3A). Conversely, there was no
statistically significant difference in the 5-year OS rates
(P=0.579; Fig. 3B). Moreover, the
multivariate analysis using the Cox proportional hazards regression
model on the 94 patients with OSCC revealed that the Ap53Ab status
[hazard ratio (HR)=2.807; 95% confidence interval (CI):
1.029-7.160; P=0.044], cN-category (HR=0.176; 95% CI: 0.035-0.678;
P=0.012), cStage (HR=4.412; 95% CI: 1.065-20.529; P=0.041) and
worst pattern of invasion (HR=3.579; 95% CI: 1.816-7.665;
P<0.001) were significant prognostic factors for DFS (Table IV).
 | Table IVMultivariate regression analysis
results for predicting disease-free survival in 94 patients with
oral squamous cell carcinoma. |
Table IV
Multivariate regression analysis
results for predicting disease-free survival in 94 patients with
oral squamous cell carcinoma.
| Variables | Assigned score | Hazard ratio (95%
CI) | P-value |
|---|
| Ap53Ab status | | | |
|
Negative | 0 | 2.807
(1.029-7.160) | 0.044a |
|
Positive | 1 | | |
| Primary site | | | |
|
Tongue | 0 | 0.859
(0.354-2.202) | 0.761 |
|
Others | 1 | | |
| Clinical
T-category | | | |
|
T1 | 1 | 0.454
(0.131-1.315) | 0.148 |
|
T2 | 2 | | |
|
T3 | 3 | | |
|
T4 | 4 | | |
| Clinical
N-category | | | |
|
N0 | 1 | 0.176
(0.035-0.678) | 0.012a |
|
≥N1 | 2 | | |
| Clinical stage | | | |
|
1 | 1 | 4.412
(1.065-20.529) | 0.041a |
|
2 | 2 | | |
|
3 and 4 | 3 | | |
| Worst pattern of
invasion | | | |
|
1c
and 2d | 1 | 3.579
(1.816-7.665) |
<0.001b |
|
3e | 2 | | |
|
4f
and 5g | 3 | | |
Discussion
From the late 1990s to the early 2000s, the
seropositivity rate of p53 was reported to be 15-30% for head and
neck SCC, including OSCC (9,19-27).
Although these results were based on the values obtained using
ELISA, these reports were not the result of a unified kit. In other
words, the differences in the Ap53Ab positivity rate may be
attributed to the measuring equipment. In 2003, Shimada et
al (7) reported a positive rate
of 35% for Ap53Ab in head and neck SCC when using the MESACUP
anti-p53Ab Test ELISA kit, which was approved by Japanese
authorities and has since been covered by the Japan Health
Insurance System. To make the study results universally applicable,
the procedures must use test equipment that is employed in daily
clinical practice. We therefore employed the MESACUP antip53Ab Test
ELISA kit in the present study, resulting in an Ap53Ab positivity
rate of 23%, which was the same as that previously reported
(7); however, to the best of our
knowledge, this study is the first to collect data based on daily
clinical practice in Japan specifically focusing on OSCC. Recently,
Takahashi et al (28)
reported decreased values in the majority of patients with
colorectal cancer who were positive for Ap53Ab (>1.3 U/ml) after
radical resection. The present results also demonstrated a decrease
in Ap53Ab titers among the majority of patients following surgery,
suggesting that Ap53Ab may be used as a monitoring marker in the
future.
The wild-type p53 protein has a short half-life
(20-30 min) and is rapidly degraded in cells, whereas the mutant
p53 protein has a significantly longer degradation time (several
hours) and accumulates in the nucleus. The mutant p53 protein can
therefore be identified as protein overexpression using IHC.
Conversely, the presence of the TP53 genetic abnormality may
be inferred from protein overexpression. In line with previous
reports (8,25,29), a
positive correlation between Ap53Ab status and p53 expression in
the primary tumors was observed in the present study. Our results
support the hypothesis that p53 accumulation in primary lesions may
be involved in p53 autoantibody production, as proposed by various
authors (20,30,31).
Other authors have reported that 10-30% of cases harboring
TP53 mutations (such as deletion, insertion, nonsense and
splicing site mutations) show negative immunoreaction for p53 IHC
staining (17,32-34).
The data from the present study demonstrated that p53
overexpression in primary tumors was present in ~70% of the
patients with Ap53Ab positivity (Table
II). Collectively, the findings of the present study indicate
that Ap53Ab may also predict the expression status of p53 in
primary OSCC, as well as in other malignancies. Moreover, the
MESACUP antip53Ab Test ELISA kit could be employed as a less
invasive diagnostic tool for determining the expression status of
p53 (or the presence or absence of mutations) in OSCC. However,
additional analyses are needed to elucidate the exact relationship
between Ap53Ab status and TP53 mutational status in tumor
samples.
There have been various reports on the correlation
between Ap53Ab and the clinical characteristics of head and neck
SCC, including OSCC. Wollenberg et al (22) reported that Ap53Ab seropositivity
was not dependent on histological grade, T-category or Union for
International Cancer Control stage. Gottschlich et al
(23) also reported that there was
no correlation between Ap53Ab status and clinical characteristics.
By contrast, Porrini et al (35) reported that Ap53Ab may be associated
with histopathological tumor grade, localization and recurrence. In
addition, Chow et al (26)
reported a positive correlation between Ap53Ab positivity and the
frequency of nodal metastasis. Yamazaki et al (27) demonstrated that Ap53Ab status was
significantly associated with secondary neck metastasis. In the
present study, the Ap53Ab status exhibited a significant
association with tumor size, lymph node metastasis and pathological
stage. In particular, the frequency of cases in the pathological
N-category was higher in the Ap53Ab-positive group compared with
that in the Ap53Ab-negative group (Table III). Recently, Sano et al
(36) reported that the TP53
mutation was associated with aggressive malignant behavior in head
and neck SCC, including OSCC. Taken together, the findings of
previous studies as well as the present study suggest that the
presence of Ap53Ab in the serum may indicate the presence of
TP53 mutations in the primary tumor, which is accompanied by
aggressive malignant phenotypes, such as enhanced proliferation,
invasion and metastatic potential, acquired by tumor cells.
Studies have reported that a positive Ap53Ab status
was associated with a poor prognosis in patients with various
malignancies, such as colorectal, esophageal, breast and oral
cancer (11,27,37,38).
In line with previous reports, we herein observed a significant
association between Ap53Ab status and prognosis, particularly in
terms of DFS (Fig. 3A). Indeed, a
positive association between positive Ap53Ab status and poor DFS
was observed. Moreover, positive Ap53Ab status was identified as
one of the independent prognostic factors in this study (Fig. 3A; Table
IV). The reason for these results may be associated with the
tendency for aggressive malignant phenotypes to be more frequent in
Ap53Ab-positive patients (Table
III). Furthermore, as the positive rate of Ap53Ab tended to be
high, not only in cases with pathological lymph node metastasis,
but also in cases with delayed neck metastasis, Ap53Ab may be a
potential predictive marker for occult neck metastasis in patients
with OSCC. Cumulatively, our data indicate that Ap53Ab status may
serve as a biomarker for predicting the prognosis of patients with
OSCC in terms of DFS. In support of this hypothesis, Ushigome et
al (5) recently reported the
usefulness of perioperative monitoring of tumor markers (including
Ap53Ab) in colorectal cancer. Therefore, we are currently in the
process of starting a prospective observational study to measure
the levels of Ap53Ab and SCC Ag, a typical OSCC tumor marker, over
time, in order to further elucidate their clinical significance in
OSCC.
In conclusion, despite the limitations due to the
small number of cases and the lack of tumor markers to compare, to
the best of our knowledge, the present study is the first to
re-evaluate the clinical significance of Ap53Ab in OSCC according
to the current daily clinical practice in Japan, in order to
further determine its potential usefulness.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
Financial support for the research, authorship and/or
publication of this article was provided by a Grant-in-Aid for
Scientific Research (C) (grant no. 18K09771) from the Japanese
Ministry of Education, Culture, Sport, Science and Technology.
Availability of data and materials
The datasets generated and/or analyzed in this study
are not publicly available due to privacy, but they are available
from the corresponding author on reasonable request.
Authors' contributions
Conceptualization, SG and RY; methodology, KY;
software, HA; validation, KY, SK and YN; formal analysis, MN;
investigation, AHiro HNakas; resources, AHira, KK; data curation,
RY; writing-original draft preparation, SG; writing-review and
editing, RY; visualization, JS; supervision, HidN; project
administration, HNakay; funding acquisition, RY. SG and RY have
seen and can confirm the authenticity of all the raw data. All the
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was conducted with the approval of
the Ethics Committee of Kumamoto University (approval no.
RINRI:1427), in accordance with the guidelines for Good Clinical
Practice and the Declaration of Helsinki. The present study is a
retrospective analysis, which does not require individual consent;
however, it guarantees the participation in the study and offers
the opportunity to refuse participation in an opt-out format
(RINRI1427).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Krimmel M, Hoffmann J, Krimmel C,
Cornelius CP and Schwenzer N: Relevance of SCC-Ag, CEA, CA 19.9 and
CA 125 for diagnosis and follow-up in oral cancer. J
Craniomaxillofac Surg. 26:243–248. 1998.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Soussi T: p53 antibodies in the sera of
patients with various types of cancer: A review. Cancer Res.
60:1777–1788. 2000.PubMed/NCBI
|
|
4
|
Lubin R, Schlichtholz B, Teillaud JL,
Garay E, Bussel A and Wild CP: p53 antibodies in patients with
various types of cancer: Assay, identification, and
characterization. Clin Cancer Res. 1:1463–1469. 1995.PubMed/NCBI
|
|
5
|
Ushigome M, Shimada H, Miura Y, Yoshida K,
Kaneko T, Koda T, Nagashima Y, Suzuki T, Kagami S and Funahashi K:
Changing pattern of tumor markers in recurrent colorectal cancer
patients before surgery to recurrence: Serum p53 antibodies, CA19-9
and CEA. Int J Clin Oncol. 25:622–632. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Couch ME, Ferris RL, Brennan JA, Koch WM,
Jaffee EM, Leibowitz MS, Nepom GT, Erlich HA and Sidransky D:
Alteration of cellular and humoral immunity by mutant p53 protein
and processed mutant peptide in head and neck cancer. Clin Cancer
Res. 13:7199–7206. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shimada H, Ochiai T and Nomura F: Japan
p53 Antibody Research Group. Titration of serum p53 antibodies in
1,085 patients with various types of malignant tumors: A
multiinstitutional analysis by the Japan p53 antibody research
group. Cancer. 97:682–689. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ralhan R, Agarwal S, Nath N, Mathur M,
Wasylyk B and Srivastava A: Correlation between p53 gene mutations
and circulating antibodies in betel- and tobacco-consuming North
Indian population. Oral Oncol. 37:243–250. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sainger RN, Shah MH, Desai AA, Shukla SN,
Shah PM, Telang SD and Patel PS: Clinical significance of serum p53
antibodies in oral cancer. Tumori. 92:134–139. 2006.PubMed/NCBI
|
|
10
|
Okada R, Otsuka Y, Wakabayashi T, Shinoda
M, Aoki T, Murakami M, Arizumi S, Yamamoto M, Aramaki O, Takayama
T, et al: Six autoantibodies as potential serum biomarkers of
hepatocellular carcinoma: A prospective multicenter study. Int J
Cancer. 147:2578–2586. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takashi S, Satoshi Y, Akihiko O, Naoya Y,
Yusuke T, Kentaro M, Yu O, Yasuaki N, Koichi Y, Takashi F, et al:
Clinical impact of preoperative serum p53 antibody titers in 1487
patients with surgically treated esophageal squamous cell
carcinoma: A multi-institutional study. Esophagus. 18:65–71.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oshima Y, Suzuki T, Yajima S, Nanami T,
Shiratori F, Funahashi K and Shimada H: Serum p53 antibody: Useful
for detecting gastric cancer but not for predicting prognosis after
surgery. Surg Today. 50:1402–1408. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou G, Liu Z and Myers JN: TP53 mutations
in head and neck squamous cell carcinoma and their impact on
disease progression and treatment response. J Cell Biochem.
117:2682–2692. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC (eds), et al: AJCC Cancer Staging Manual. 8th edition.
Springer International Publishing, New York, NY, 2017.
|
|
15
|
El-Naggar AK, Chan JC, Grandis JR, Takata
T, Grandis J and Slootweg P (eds): WHO Classification of Head and
Neck Tumours. 4th edition. IARC, Lyon, 2017.
|
|
16
|
Nasierowska-Guttmejer A, Trzeciak L,
Nowacki MP and Ostrowski J: p53 protein accumulation and p53 gene
mutation in colorectal cancer. Pathol Oncol Res. 6:275–279.
2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bertorelle R, Esposito G, Belluco C,
Bonaldi L, Del Mistro A, Nitti D, Lise M and Chieco-Bianchi L: p53
gene alterations and protein accumulation in colorectal cancer.
Clin Mol Pathol. 49:M85–M90. 1996.PubMed/NCBI
|
|
18
|
Poeta ML, Manola J, Goldwasser MA,
Forastiere A, Benoit N, Califano JA, Ridge JA, Goodwin J, Kenady D,
Saunders J, et al: TP53 mutations and survival in squamous-cell
carcinoma of the head and neck. N Engl J Med. 357:2552–2561.
2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bourhis J, Lubin R, Roche B, Koscielny S,
Bosq J, Dubois I, Talbot M, Marandas P, Schwaab G, Wibault P, et
al: Analysis of p53 serum antibodies in patients with head and neck
squamous cell carcinoma. J Natl Cancer Inst. 88:1228–1233.
1996.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Maass JD, Gottschlich S, Goeroegh T,
Lippert BM and Werner JA: Head and neck cancer and
p53-immunogenicity. Anticancer Res. 17:2873–2874. 1997.PubMed/NCBI
|
|
21
|
Werner JA, Gottschlich S, Folz BJ,
Goeroegh T, Lippert BM, Maass JD and Rudert H: p53 serum antibodies
as prognostic indicator in head and neck cancer. Cancer Immunol
Immunother. 44:112–116. 1997.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wollenberg B, Jan NV, Pitzke P, Reiter W
and Stieber P: Anti-p53 antibodies in serum of smokers and head and
neck cancer patients. Anticancer Res. 17:413–418. 1997.PubMed/NCBI
|
|
23
|
Gottschlich S, Folz BJ, Goeroegh T,
Lippert BM, Maass JD and Werner JA: A new prognostic indicator for
head and neck cancer-p53 serum antibodies? Anticancer Res.
19:2703–2705. 1999.PubMed/NCBI
|
|
24
|
Gottschlich S, Maune S, Maass JD, Görögh
T, Hoffmann M, Hoffmann-Fazel A, Meyer J, Werner JA and Rudert H:
Serum p53 autoantibodies in the follow-up of head and neck cancer
patients. Oncology. 59:31–35. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Warnakulasuriya S, Soussi T, Maher R,
Johnson N and Tavassoli M: Expression of p53 in oral squamous cell
carcinoma is associated with the presence of IgG and IgA p53
autoantibodies in sera and saliva of the patients. J Pathol.
192:52–57. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chow V, Yuen AP, Lam KY, Ho WK and Wei WI:
Prognostic significance of serum p53 protein and p53 antibody in
patients with surgical treatment for head and neck squamous cell
carcinoma. Head Neck. 23:286–291. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Yamazaki Y, Chiba I, Ishikawa M, Satoh C,
Notani K, Ohiro Y, Totsuka Y, Mizuno S and Kitagawa Y: Serum p53
antibodies as a prognostic indicator in oral squamous cell
carcinoma. Odontology. 96:32–37. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Takahashi R, Sakamoto K, Sugimoto K,
Motegi S, Tsukamoto R, Ichikawa R, Okazawa Y, Aoki J, Ishiyama S,
Takahashi M, et al: Significance of serum p53 antibody as a tumor
marker in colorectal cancer. Dis Markers.
2019(2721876)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
von Brevern MC, Hollstein MC, Cawley HM,
De Benedetti VM, Bennett WP, Liang L, He AG, Zhu SM, Tursz T, Janin
N and Trivers GE: Circulating anti-p53 antibodies in esophageal
cancer patients are found predominantly in individuals with p53
core domain mutations in their tumors. Cancer Res. 56:4917–4921.
1996.PubMed/NCBI
|
|
30
|
Davidoff AM, Iglehart JD and Marks JR:
Immune response to p53 is dependent upon p53/HSP70 complexes in
breast cancers. Proc Natl Acad Sci USA. 89:3439–3442.
1992.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schlichtholz B, Legros Y, Gillet D,
Gaillard C, Marty M, Lane D, Calvo F and Soussi T: The immune
response to p53 in breast cancer patients is directed against
immunodominant epitopes unrelated to the mutational hot spot.
Cancer Res. 52:6380–6384. 1992.PubMed/NCBI
|
|
32
|
George B, Datar RH, Wu L, Cai J, Patten N,
Beil SJ, Groshen S, Stein J, Skinner D, Jones PA, et al: p53 gene
and protein status: The role of p53 alterations in predicting
outcome in patients with bladder cancer. J Clin Oncol.
25:5352–5358. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dix B, Robbins P, Carrello S, House A and
Iacopetta B: Comparison of p53 gene mutation and protein
overexpression in colorectal carcinomas. Br J Cancer. 70:585–590.
1994.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yemelyanova A, Vang R, Kshirsagar M, Lu D,
Marks MA, Shih IeM and Kurman RJ: Immunohistochemical staining
patterns of p53 can serve as a surrogate marker for TP53 mutations
in ovarian carcinoma: An immunohistochemical and nucleotide
sequencing analysis. Mod Pathol. 24:1248–1253. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Porrini R, Vercellino V, Rocchetti V, Renò
F, Giorda E, Pomato E, Cannas M and Sabbatini M: Serum anti-p53
antibodies as a diagnostic tumor marker: Observations in patients
with malignant and premalignant oral cavity lesions. Minerva
Stomatol. 59:233–243. 2010.PubMed/NCBI
|
|
36
|
Sano D, Xie TX, Ow TJ, Zhao M, Pickering
CR, Zhou G, Sandulache VC, Wheeler DA, Gibbs RA, Caulin C and Myers
JN: Disruptive TP53 mutation is associated with aggressive disease
characteristics in an orthotopic murine model of oral tongue
cancer. Clin Cancer Res. 17:6658–6670. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yamaguchi T, Takii Y and Maruyama S:
Usefulness of serum p53 antibody measurement in colorectal cancer:
An examination of 1384 primary colorectal cancer patients. Surg
Today. 44:1529–1535. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sirotković-Skerlev M, Plavetić ND, Sedlić
F, Kuna SK, Vrbanec D, Belev B, Pleština S, Kovač Z and Kulić A:
Prognostic value of circulating Bcl-2 and anti-p53 antibodies in
patients with breast cancer: A long term follow-up (17.5 years).
Cancer Biomark. 30:95–104. 2021.PubMed/NCBI View Article : Google Scholar
|