Introduction
The advent of direct-acting antivirals (DAAs)
revolutionized the treatment of hepatitis C virus (HCV) and high
sustained virological response (SVR) rates may be achieved. The SVR
rates were reported to be 89.9% with daclatasvir/asunaprevir
(1), 95.8% with
sofosbuvir/ribavirin (2), and 98.5%
with sofosbuvir/ledipasvir (3).
Accumulating evidence suggests that SVR with DAA treatment reduces
the incidence of hepatocellular carcinoma (HCC) development
(4-6).
However, even after achieving SVR, some patients may develop
HCC.
Advanced liver fibrosis was reported to be the most
important risk factor for HCC development after SVR (7); therefore, evaluation of the degree of
liver fibrosis is important. Regarding the evaluation of fibrosis,
non-invasive methods (serum markers or transient elastography) were
recently adopted instead of invasive liver biopsy. Representative
fibrosis markers include type IV collagen (8), Wisteria floribunda
agglutinin-positive Mac-2-binding protein (M2BPGi) (9), and fibrosis-4 (FIB-4) index (10,11).
On the other hand, an enhanced liver fibrosis (ELF) score composed
of three liver fibrosis markers was developed to evaluate liver
fibrosis (12). The ELF score was
confirmed to be useful in patients with non-alcoholic fatty liver
(13), primary biliary
cholangitis/cirrhosis (14) and
chronic hepatitis C (15). It was
recently reported that the ELF score was comparable with transient
elastography in detecting advanced fibrosis (F≥3) in
treatment-naïve patients with chronic HCV infection (16). In addition, the usefulness of ELF
score as a predictor of HCC in the general population, particularly
in predicting non-viral-related HCC, was previously reported
(17).
Among these fibrosis markers, M2BPGi and FIB-4 index
were reported to be useful markers for the risk of HCC development
after HCV eradication (18,19). On the other hand, other than
fibrosis markers, α-fetoprotein (AFP) was also reported to be
useful for predicting HCC development after HCV eradication
(20). However, to the best of our
knowledge, there is no report confirming the usefulness of the ELF
score for predicting HCC development after HCV eradication. The aim
of the present study was to assess fibrosis markers, including ELF
score, type IV collagen, M2BPGi and FIB-4 index, tumor markers, and
biochemical tests associated with HCC development after viral
eradication. The time course of the changes in fibrosis markers
during and after DAA treatment was also examined.
Materials and methods
Subjects
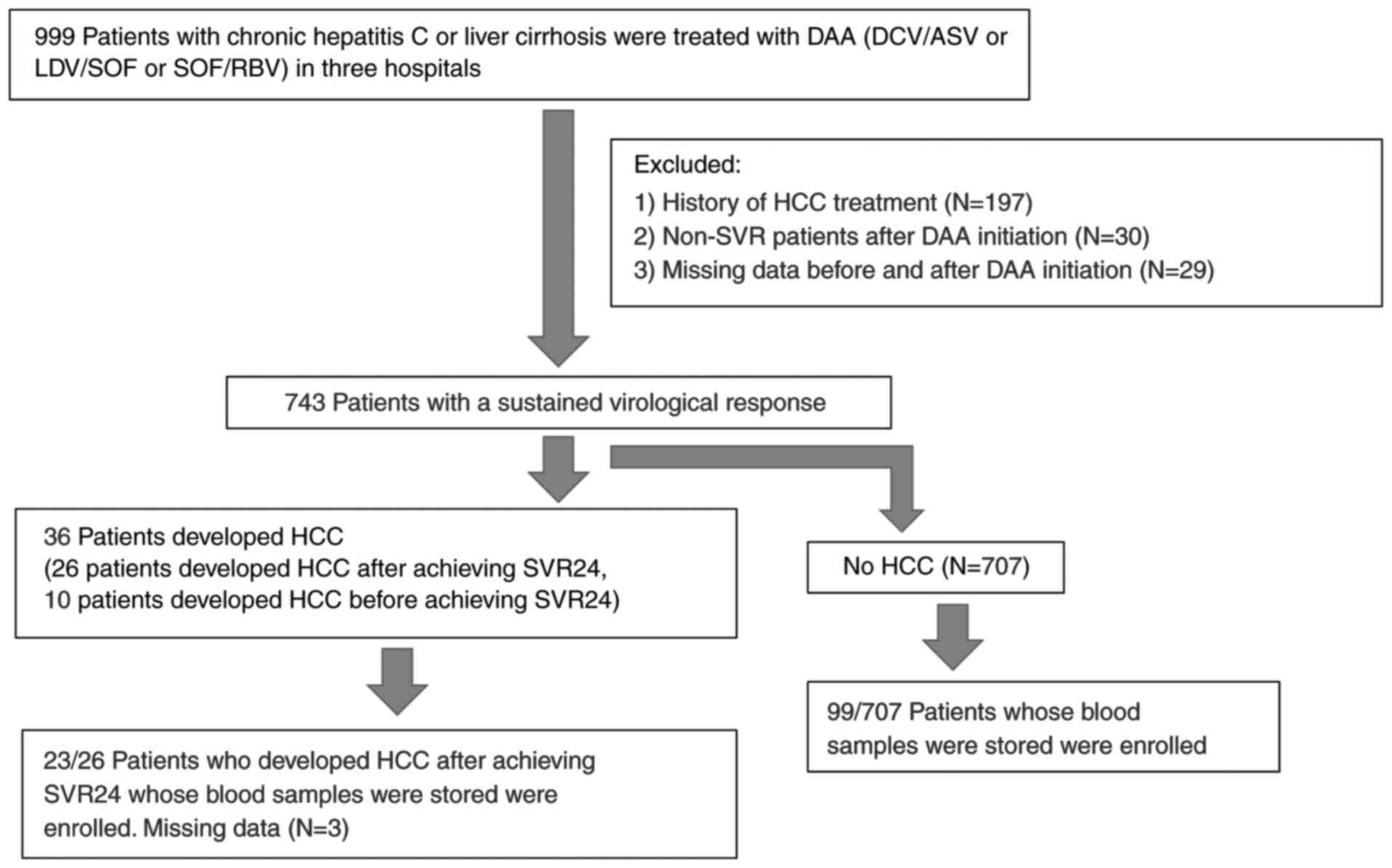
Patients with chronic hepatitis C or liver cirrhosis
from three hospitals in Japan (Kurume University Hospital, Yame
General Hospital and Chikugo City Hospital) who were initiated on
DAA therapy between October 2014 and September 2016 were selected.
A total of 999 patients with chronic hepatitis C or liver cirrhosis
were treated with DAA (daclatasvir plus asunaprevir, or ledipasvir
plus sofosbuvir, or sofosbuvir plus ribavirin). SVR was achieved in
743 patients. The diagnosis of liver cirrhosis was comprehensively
made based on biochemical test results, imaging findings and
physical findings on a case-by-case basis. Among the patients who
achieved SVR, 122 patients (50 male and 72 female patients, with a
mean age ± SD of 68.7±8.8 years; range, 32-83 years) whose blood
samples were stored were enrolled (Fig.
1). All the patients were positive for HCV antibody as
determined using chemiluminescence immunoassay
(Architect®; Abbott Japan Co., Ltd.). The HCV RNA levels
were measured using a COBAS Taq Man test (Roche Diagnostics).
Patients who had hepatitis B surface antigen, a history of HCC
prior to DAA therapy, or developed HCC within 24 weeks after DAA
therapy were excluded. Imaging surveillance (ultrasonography,
computed tomography or magnetic resonance imaging) were undertaken
every 3-6 months. Patients were followed up until HCC development
or the last visit before October 2018. The mean observation period
was 2.7 years after the initiation of DAA therapy. The present
study was conducted according to the guidelines of the Declaration
of Helsinki and was approved by the Ethics Committees of Kurume
University School of Medicine (approval no. 14178), Yame General
Hospital (approval no. 19-005), and Chikugo City Hospital (approval
no. 2019-09). Written informed consent was obtained from all
patients.
DAA therapy
The HCV treatment regimen of daclatasvir (60 mg once
daily) plus asunaprevir (100 mg twice daily) for 24 weeks, or
ledipasvir (90 mg) plus sofosbuvir (400 mg) for 12 weeks, was
administered to patients with HCV genotype 1. Sofosbuvir (400 mg)
plus ribavirin (weight-based dosing: 600 mg daily for patients
weighing ≤60 kg, 800 mg daily for patients weighing >60 and ≤80
kg, and 1,000 mg daily for patients weighing >80 kg) for 12
weeks was administered to patients with HCV genotype 2. SVR was
defined as undetectable serum HCV RNA at 24 weeks after completing
DAA therapy.
Liver fibrosis markers
Four fibrosis biomarkers (type IV collagen, M2BPGi,
FIB-4 index and ELF score) were assessed in 122 patients. For all
patients, the biomarkers were analyzed at baseline, at the end of
treatment (EOT) and at post-treatment week 24 (PTW24). Type IV
collagen was measured using a JCA-BM 8000 series automated immune
analyzer (Japan Electron Optics Laboratory Ltd.). M2BPGi was
measured using a HISCL-5000 (Sysmex Corporation). The FIB-4 index
was calculated as follows: Age (years) x aspartate aminotransferase
(AST; U/l)/platelet count (109/l) x alanine
aminotransferase (ALT; U/l)1/2 (10). The ELF
score consists of three fibrosis markers: Hyaluronic acid (HA),
amino-terminal propeptide of type III procollagen (PIIINP) and
tissue inhibitor of metalloproteinase type-1 (TIMP-1). The ELF
score (12) was measured using an
ADVIA Center XP automated immunoanalyzer and calculated
automatically using the following equation: ELF score=2.278 + 0.851
ln(CHA) + 0.751 ln(CPIIINP) + 0.394
ln(CTIMP1). Three biomarkers (type IV collagen, M2BPGi
and ELF score) were measured using stored blood samples. All
collected blood samples were stored at -30˚C until analysis.
Parameters associated with HCC
The following parameters were analyzed to identify
the factors associated with HCC: Sex, cirrhosis, age, type IV
collagen, M2BPGi, FIB-4 index, ELF score, AST, ALT, γ-glutamyl
transpeptidase, albumin, total bilirubin, prothrombin time,
platelets, AFP and des-γ-carboxy prothrombin. For all patients, all
parameters were assessed at baseline and PTW24.
Statistical analysis
Statistical analysis was performed using the JMP
software package (release 13; SAS Institute, Inc.). Mean values and
SDs were calculated for continuous data. For comparison of
variables, the Wilcoxon signed-rank test was performed as
appropriate. Factors associated with HCC risk were determined using
the Cox proportional hazard regression analysis. P<0.05 was
considered to indicate statistically significant. Diagnostic
accuracy was assessed using time-dependent receiver operating
characteristics (ROC) curves by examining the area under the ROC
curve (AUROC).
Results
Patient characteristics
The characteristics of the patients are summarized
in Table I. Of the 122 patients, 50
(41%) were male and 72 (59%) were female, with a mean age of
68.7±8.8 years. A total of 36 (30%) patients were diagnosed with
cirrhosis clinically.
 | Table IBaseline characteristics of patients
(n=122) with chronic hepatitis C. |
Table I
Baseline characteristics of patients
(n=122) with chronic hepatitis C.
| Characteristics | Values |
|---|
| Male, n (%) | 50 (41.0) |
| Cirrhosis/chronic
hepatitis, n | 36/86 |
| Genotype (1/2),
n | 119/3 |
| Treatment regimen,
n | |
|
Daclatasvir
+ asunaprevir | 113 |
|
Ledipasvir +
sofosbuvir | 6 |
|
Sofosbuvir +
ribavirin | 3 |
| Age (years) | 68.7±8.8 |
| Aspartate
aminotransferase (U/l) | 53±27 |
| Alanine
aminotransferase (U/l) | 51±36 |
| Albumin (g/dl) | 3.9±0.4 |
| Total bilirubin
(mg/dl) | 0.8±0.3 |
| Prothrombin time
(%) | 91±16 |
| Platelet count
(x104/ µl) | 12.4±5.0 |
| α-Fetoprotein
(ng/ml) | 16.9±30.5 |
| Des-γ-carboxy
prothrombin (mAU/ml) | 20.4±18.1 |
Changes in fibrosis biomarkers and
their association with HCC risk
The baseline/EOT/PTW24 fibrosis biomarkers (type IV
collagen, M2BPGi, FIB-4 index and ELF score including its
individual components, namely HA, PIIINP and TIMP-1) are shown in
Table II. There was a significant
decrease in all biomarkers at PTW24 compared with those at baseline
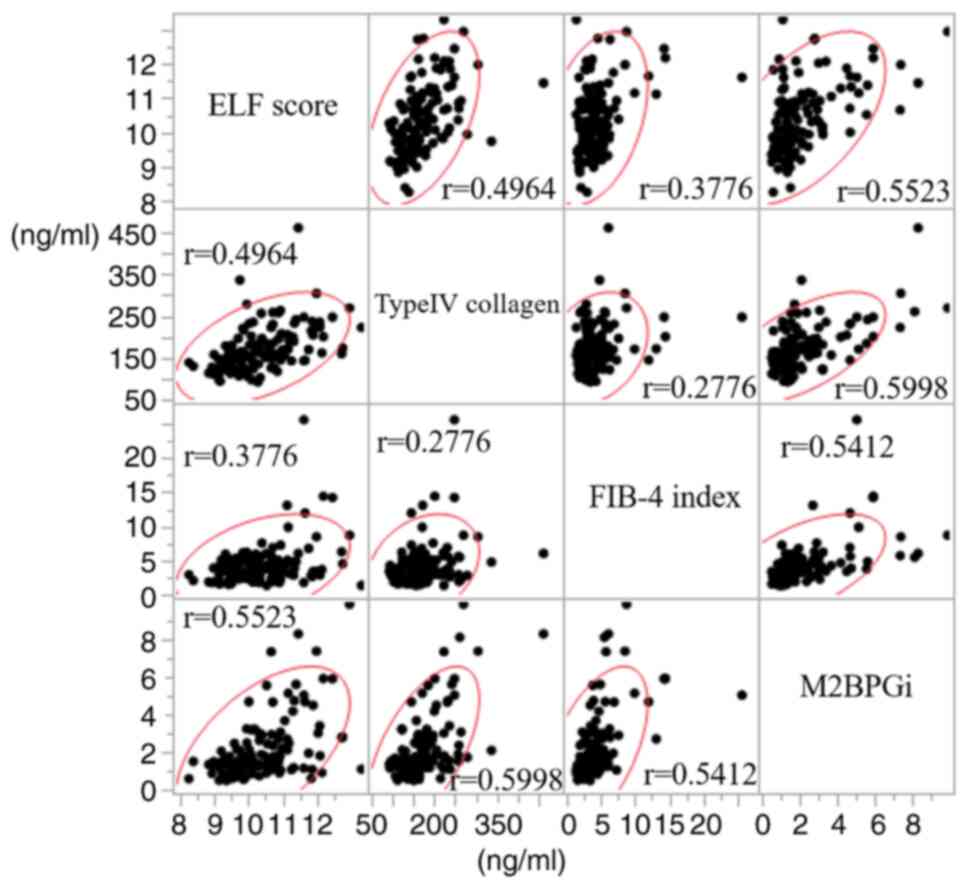
(P<0.0001). First, the correlations among the four fibrosis
markers were investigated. As a result, correlations were
identified among the four fibrosis markers, albeit weak. The
strongest correlation observed was between M2BPGi and type IV
collagen (r=0.5998). A scatterplot matrix is shown in Fig. 2. Second, factors associated with the
risk for HCC development were investigated (Table III). Of the 122 patients, 23 (19%)
developed HCC. A high baseline ELF score (P=0.0264), PTW24 ELF
score (P=0.0003), baseline FIB-4 index (P=0.0451), PTW24 AFP level
(P=0.0133), and baseline prothrombin time (P=0.0455) were
identified as risk factors for HCC development based on the
univariate analyses. A multivariate analysis was performed using
the four factors that were found to be significant in the
univariate analysis: PTW24 ELF score, baseline FIB-4 index, PTW24
AFP level and baseline prothrombin time. Based on the multivariate
analysis, a high PTW24 ELF score was the only significant risk
factor for HCC development (P=0.0035; hazard ratio=1.89; 95%
confidence interval: 1.24-2.85).
 | Table IIChanges in biomarkers measured at
baseline, end of treatment, and at 24 weeks after DAA therapy.
P-values are for the comparison between baseline and 24 weeks after
treatment. |
Table II
Changes in biomarkers measured at
baseline, end of treatment, and at 24 weeks after DAA therapy.
P-values are for the comparison between baseline and 24 weeks after
treatment.
| Biomarkers | Baseline | End of
treatment | 24 weeks after
treatment | P-value |
|---|
| Type IV collagen
(ng/ml) | 213±85 | 190±67 | 174±55 | <0.0001 |
| M2BPGi | 4.8±3.5 | 2.7±2.0 | 2.2±1.8 | <0.0001 |
| FIB-4 index | 5.31±3.82 | 4.36±2.79 | 4.24±3.09 | <0.0001 |
| ELF score | 11.5±1.2 | 10.8±1.1 | 10.4±1.0 | <0.0001 |
| HA (ng/ml) | 534±780 | 350±687 | 224±272 | <0.0001 |
| PIIINP (ng/ml) | 15.3±7.7 | 12.7±8.3 | 10.6±5.3 | <0.0001 |
| TIMP-1 (ng/ml) | 338±291 | 248±74 | 233±69 | <0.0001 |
 | Table IIIFactors associated with the risk for
HCC development. |
Table III
Factors associated with the risk for
HCC development.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Factors | HCC+
(n=23) | HCC-
(n=99) | HR | 95% CI | P-value | P-value, HR, 95%
CI |
|---|
| Sex (male/female),
n | 10/13 | 40/59 | 1.17 | 0.50-2.67 | 0.707 | |
| Liver
cirrhosis/chronic hepatitis, n | 9/14 | 27/72 | 1.57 | 0.65-3.59 | 0.299 | |
| Age, years | 69.6±7.3 | 68.5±9.0 | 1.00 | 0.95-1.06 | 0.915 | |
| Pre AST (U/l) | 64.1±38.5 | 50.9±22.7 | 1.01 | 1.00-1.02 | 0.070 | |
| PTW24 AST
(U/l) | 28.9±10.2 | 26.9±9.0 | 1.03 | 0.99-1.08 | 0.153 | |
| Pre ALT (U/l) | 61.3±49.8 | 48.9±31.4 | 1.01 | 1.00-1.01 | 0.210 | |
| PTW24 ALT
(U/l) | 19.6±8.6 | 19.6±11.5 | 1.01 | 0.97-1.05 | 0.581 | |
| Pre γ-GTP
(U/l) | 47±32 | 40±40 | 1.01 | 1.00-1.02 | 0.100 | |
| PTW24 γ-GTP
(U/l) | 32±25 | 26±27 | 1.01 | 1.00-1.02 | 0.155 | |
| Pre Alb (g/dl) | 3.8±0.4 | 4.0±0.4 | 0.39 | 0.14-1.07 | 0.067 | |
| PTW24 Alb
(g/dl) | 4.2±0.3 | 4.3±0.3 | 0.48 | 0.16-1.53 | 0.210 | |
| Pre T.Bil
(mg/dl) | 0.9±0.3 | 0.8±0.3 | 1.34 | 0.33-4.50 | 0.669 | |
| PTW24 T.Bil
(mg/dl) | 0.8±0.3 | 0.9±0.3 | 0.39 | 0.07-1.61 | 0.215 | |
| Pre PTa (%) | 84.6±14.8 | 92.5±15.6 | 0.97 | 0.95-1.00 | 0.0455 | |
| PTW24 PT (%) | 87.8±15.4 | 92.4±13.9 | 0.98 | 0.95-1.01 | 0.217 | |
| Pre Plt
(x104/µl) | 11.2±5.2 | 12.5±4.7 | 0.95 | 0.86-1.04 | 0.254 | |
| PTW24 Plt
(x104/µl) | 12.0±5.6 | 13.1±4.3 | 0.96 | 0.87-1.05 | 0.388 | |
| Pre AFP
(ng/ml) | 23.7±39.4 | 15.2±27.8 | 1.00 | 0.99-1.01 | 0.522 | |
| PTW24
AFPa (ng/ml) | 8.1±11.0 | 4.4±2.6 | 1.06 | 1.02-1.09 | 0.0133 | |
| Pre DCP
(mAU/ml) | 20.1±8.3 | 20.5±19.6 | 1.01 | 0.97-1.04 | 0.457 | |
| PTW24 DCP
(mAU/ml) | 23±10 | 21±16 | 1.03 | 1.00-1.05 | 0.0758 | |
| Pre type IV
(collagen (ng/ml) | 225±62 | 210±89 | 1.00 | 1.00-1.01 | 0.522 | |
| PTW24 type IV
collagen (ng/ml) | 192±42 | 169±56 | 1.00 | 1.00-1.01 | 0.120 | |
| Pre M2BPGi | 5.43±3.02 | 4.69±3.58 | 1.05 | 0.93-1.16 | 0.417 | |
| PTW24 M2BPGi | 2.98±1.61 | 2.05±1.76 | 1.18 | 0.98-1.39 | 0.0764 | |
| Pre FIB-4
indexa | 7.2±6.1 | 4.9±2.9 | 1.08 | 1.00-1.15 | 0.0451 | |
| PTW24 FIB-4
index | 5.6±5.0 | 3.9±2.3 | 1.09 | 0.99-1.17 | 0.0879 | |
| Pre ELF score | 12.0±1.1 | 11.3±1.2 | 1.47 | 1.05-2.10 | 0.0264 | |
| PTW24 ELF
scorea | 11.2±1.0 | 10.3±1.0 | 1.96 | 1.38-2.77 | 0.0003 | 0.0035, 1.89,
1.24-2.85 |
| PTW24 HA
(ng/ml) | 414±434 | 180±193 | 1.00 | 1.00-1.00 | 0.0007 | |
| PTW24 PIIINP
(ng/ml) | 13.4±5.9 | 10.0±5.0 | 1.08 | 1.02-1.13 | 0.009 | |
| PTW24 TIMP-1
(ng/ml) | 277±63 | 223±66 | 1.01 | 1.00-1.01 | 0.0033 | |
Diagnostic accuracy of fibrosis
biomarkers for predicting HCC development
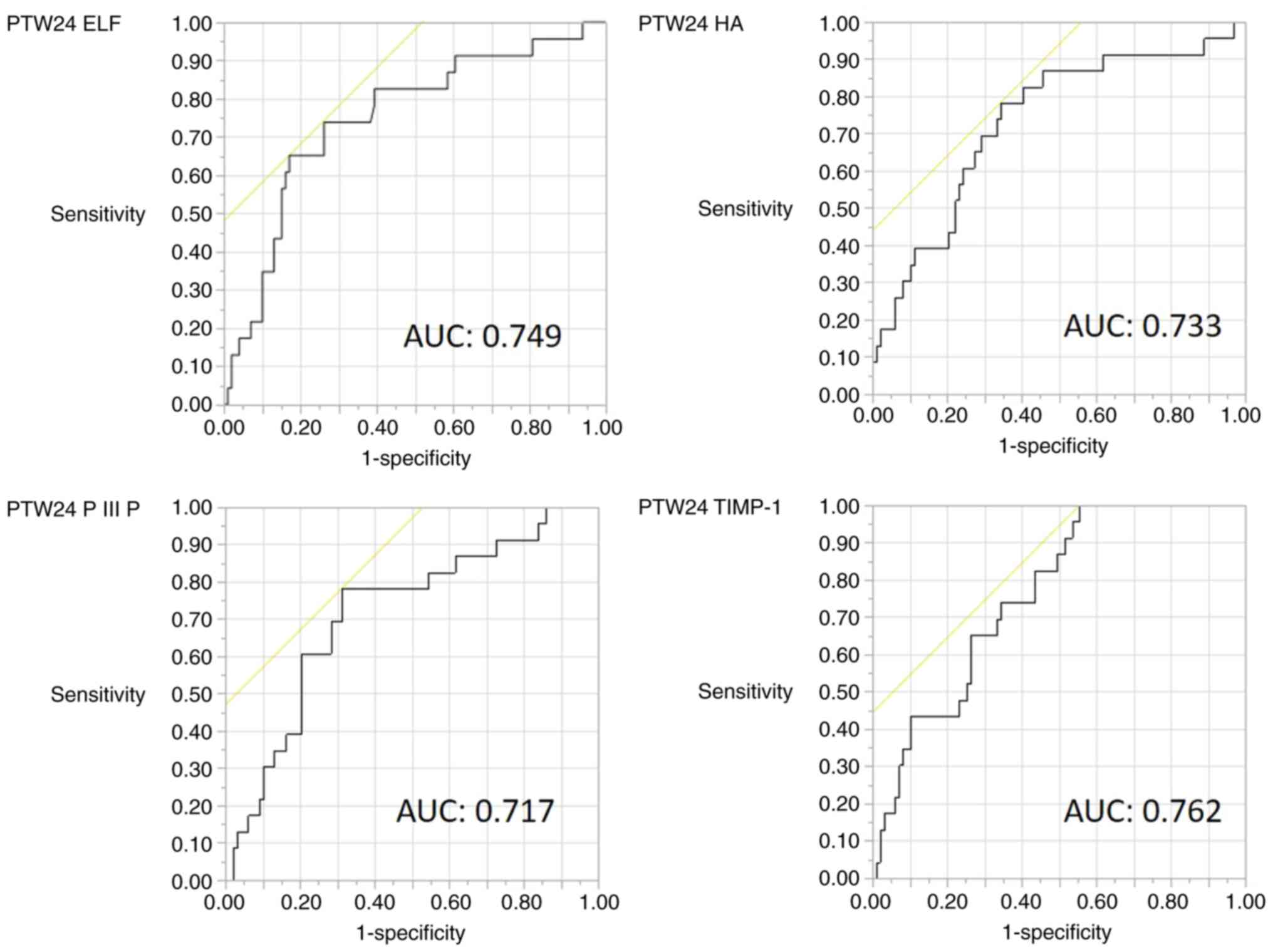
As the ELF score is composed of three fibrosis
markers, it was examined which component was mostly involved. The
diagnostic accuracy of the PTW24 ELF score, PTW24 HA, PTW24 PIIINP
and PTW24 TIMP-1 for predicting HCC development is shown in
Table IV. The cut-off value of the
PTW24 ELF score of 10.96 had a sensitivity of 65.2% and specificity
of 82.8% for predicting HCC development. PTW24 TIMP-1 had a high
sensitivity (100%) but low specificity (44.4%). The AUROC of PTW24
ELF and PTW24 TIMP-1 was 0.75 and 0.76, respectively. The ROC
curves of PTW24 ELF, PTW24 HA, PTW24 PIIINP and PTW24 TIMP-1 are
shown in Fig. 3.
 | Table IVDiagnostic accuracy of the PTW24 ELF
score, PTW24 HA, PTW24 PIIINP and PTW24 TIMP-1 for predicting
hepatocellular carcinoma development. |
Table IV
Diagnostic accuracy of the PTW24 ELF
score, PTW24 HA, PTW24 PIIINP and PTW24 TIMP-1 for predicting
hepatocellular carcinoma development.
| Diagnostic
measures | Cut-off | Sensitivity | Specificity | AUC | PPV | NPV | P-value |
|---|
| PTW24 ELF
score | 10.96 | 0.652 | 0.828 | 0.749 | 0.469 | 0.911 | 0.0002 |
| PTW24 HA
(ng/ml) | 153.6 | 0.783 | 0.657 | 0.733 | 0.346 | 0.928 | 0.0009 |
| PTW24 PIIINP
(ng/ml) | 10.5 | 0.783 | 0.687 | 0.717 | 0.367 | 0.932 | 0.0104 |
| PTW24 TIMP-1
(ng/ml) | 199.1 | 1 | 0.444 | 0.762 | 0.295 | 1 | 0.0013 |
Discussion
The objective of the present study was to measure
fibrosis markers, tumor markers and biochemistry parameters in
patients with chronic hepatitis C who achieved SVR with DAA therapy
in order to identify useful markers for predicting HCC development.
The ELF score at 24 weeks after the completion of DAA therapy was
demonstrated to be such a marker. In addition, the time course of
the changes in fibrosis markers during DAA therapy was
investigated, and the levels of all the markers decreased after the
completion of therapy.
As regards important markers for the prediction of
HCC development after SVR, among fibrosis markers, M2BPGi (9) after the completion of DAA therapy was
reported to be useful (18,21). In addition, a pre-FIB-4 index of
3.25 was previously shown to exhibit a significant association with
HCC development (19). In addition
to fibrosis markers, the usefulness of AFP after treatment was also
previously reported (20).
Therefore, M2BPGi, FIB-4 index and AFP, which were
previously reported to be useful for predicting HCC development
after SVR, were investigated in the present study. However, only
the ELF score at 24 weeks after treatment was extracted by
multivariate analysis and demonstrated to be the most useful marker
for predicting HCC development.
The ELF score (12)
is calculated from 3 hepatic fibrosis markers, HA, PIIINP, and
TIMP-1, and these markers have been reported to be useful for
evaluating fibrosis in varying etiologies of chronic liver disease
such as non-alcoholic fatty liver disease (NAFLD), primary biliary
cholangitis/cirrhosis and chronic hepatitis C (13-15).
HA is a glycosaminoglycan involved in fibrogenesis by hepatic
stellate cells, PIIINP is a marker of inflammation and early
fibrogenesis, and TIMP-1 inhibits fibrinolysis, thereby increasing
fiber deposition. The usefulness of HA, PIIINP and TIMP-1 as
fibrosis markers in NAFLD was previously reported (22-24).
Furthermore, HA, PIIINP and TIMP-1 have all been reported to be
useful for the evaluation of fibrosis in chronic hepatitis C
(25-27).
Therefore, an increase in HA, PIIINP and TIMP-1 levels is
considered to reflect fibrosis in NAFLD and chronic hepatitis C.
Individual parameters may coincidentally increase due to the
presence of other diseases; however, evaluation of liver fibrosis
based on the ELF score is stable, as this score is calculated by
summing up all three fibrosis markers (12). This may be the reason why only the
ELF score was identified as the predictor of HCC development by
multivariate analysis.
The longitudinal change in fibrosis markers during
DAA therapy were next investigated, and all biomarkers were found
to be significantly decreased 24 weeks after DAA therapy.
In patients who achieved SVR by DAA therapy, M2BPGi
(28), ELF score (29) [and its components HA (28,29)
and TIMP-1(29)], type IV collagen
(28) and FIB-4 index (30-32)
were found to be significantly decreased after therapy, consistent
with the findings of the present study. In our study, PIIINP also
significantly decreased after therapy. The decreases in the levels
of these fibrosis markers may be due to the improvement of
fibrosis. On the other hand, regarding the improvement of fibrosis
stage based on histological examination of liver biopsy, it
improves by 0.282 stages per year in patients who achieve SVR by
IFN therapy (33), indicating that
a long time is required for the improvement of liver fibrosis at
the tissue level. The fibrosis markers investigated in the present
study had decreased by 10-58% at 24 weeks after the end of therapy.
The reduction rate in the levels of fibrosis markers from baseline
to the end of therapy was higher compared with that from the end of
therapy to 24 weeks after the end of therapy, suggesting that the
decrease in fibrosis markers from baseline to the end of DAA
therapy may reflect both inflammation and fibrosis in liver tissue.
Indeed, in a previous study comparing the degree of liver fibrosis
and fibrosis markers in patients with chronic hepatitis C, the ELF
score, M2BPGi and FIB4-index were considered to reflect both
fibrosis and inflammation (34).
These findings indicate that the fibrosis markers at PTW24 may
reflect the fibrosis level with greater precision. Previous reports
have demonstrated that hepatocarcinogenesis was closely associated
with the degree of liver fibrosis rather than that of inflammation
(35-37).
Therefore, the ELF score at PTW24 was selected as a predictor of
HCC development after HCV eradication.
As regards the limitations of the present study, the
total number of patients in the study was relatively small, but the
HCC development rate was high (19%). Age and cirrhosis were not
found to be associated with HCC development, although these are
known risk factors associated with the development of this type of
cancer. In addition, a pre-FIB-4 index of 3.25 was previously found
to exhibit a significant association with HCC development (19). Finally, there may exist selection
bias, as only patients with stored serum samples were selected.
In conclusion, the most useful parameter for
predicting hepatocarcinogenesis after DAA therapy for chronic
hepatitis C was found to be the ELF score at 24 weeks after
therapy. In addition, the four investigated fibrosis markers
decreased after DAA therapy. The number of patients who achieve SVR
by DAA therapy is expected to increase in the future; therefore,
the incidence of HCC development after SVR is also expected to
increase. Although DAA administration improves inflammation and
fibrosis, careful follow-up is required for patients with a high
ELF score ≥10.96 at 24 weeks after DAA therapy owing to the high
risk for HCC development.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
TK and TI contributed to the study concept and
design; TK, TI, KA, TAH, RK, TS, SM, NO and TT contributed to data
acquisition and analysis; TT revised the manuscript. TK and TI have
seen and can confirm the authenticity of the raw data. All the
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethics Committee of Kurume University School of Medicine (approval
no. 14178), Yame General Hospital (approval no. 19-005), and
Chikugo City Hospital (approval no. 2019-09). Written informed
consent was obtained from all the patients enrolled in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ji F, Wei B, Yeo YH, Ogawa E, Zou B, Stave
CD, Li Z, Dang S, Furusyo N, Cheung RC and Nguyen MH: Systematic
review with meta-analysis: Effectiveness and tolerability of
interferon-free direct-acting antiviral regimens for chronic
hepatitis C genotype 1 in routine clinical practice in Asia.
Aliment Pharmacol Ther. 47:550–562. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wei B, Ji F, Yeo YH, Ogawa E, Zou B, Stave
CD, Dang S, Li Z, Furusyo N, Cheung RC and Nguyen MH: Real-world
effectiveness of sofosbuvir plus ribavirin for chronic hepatitis C
genotype 2 in Asia: A systematic review and meta-analysis. BMJ Open
Gastroenterol. 5(e000207)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mizokami M, Liu LJ, Fujiyama N, Littman M,
Yuan J, Sekiya T, Hedskog C and Ng LJ: Real-world safety and
effectiveness of ledipasvir/sofosbuvir for the treatment of chronic
hepatitis C virus genotype 1 in Japan. J Viral Hepat. 28:129–141.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Calvaruso V, Cabibbo G, Cacciola I, Petta
S, Madonia S, Bellia A, Tinè F, Distefano M, Licata A,
Giannitrapani L, et al: Incidence of hepatocellular carcinoma in
patients With HCV-associated cirrhosis treated with direct-acting
antiviral agents. Gastroenterology. 155:411–421.e4. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li DK, Ren Y, Fierer DS, Rutledge S,
Shaikh OS, Lo Re V III, Simon T, Abou-Samra AB, Chung RT and Butt
AA: The short-term incidence of hepatocellular carcinoma is not
increased after hepatitis C treatment with direct-acting
antivirals: An ERCHIVES study. Hepatology. 67:2244–2253.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ioannou GN, Green PK and Berry K: HCV
eradication induced by direct-acting antiviral agents reduces the
risk of hepatocellular carcinoma. J Hepatol: Sep 5, 2017 (Epub
ahead of print).
|
|
7
|
Kanwal F, Kramer J, Asch SM, Chayanupatkul
M, Cao Y and El-Serag HB: Risk of hepatocellular cancer in HCV
patients treated with direct-acting antiviral agents.
Gastroenterology. 153:996–1005.e1. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yabu K, Kiyosawa K, Mori H, Matsumoto A,
Yoshizawa K, Tanaka E and Furuta S: Serum collagen type IV for the
assessment of fibrosis and resistance to interferon therapy in
chronic hepatitis C. Scand J Gastroenterol. 29:474–479.
1994.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yamasaki K, Tateyama M, Abiru S, Komori A,
Nagaoka S, Saeki A, Hashimoto S, Sasaki R, Bekki S, Kugiyama Y, et
al: Elevated serum levels of Wisteria floribunda
agglutinin-positive human Mac-2 binding protein predict the
development of hepatocellular carcinoma in hepatitis C patients.
Hepatology. 60:1563–1570. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vallet-Pichard A, Mallet V, Nalpas B,
Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H and Pol S:
FIB-4: An inexpensive and accurate marker of fibrosis in HCV
infection. Comparison with liver biopsy and fibrotest. Hepatology.
46:32–36. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li X, Xu H and Gao P: Fibrosis index based
on 4 factors (FIB-4) predicts liver cirrhosis and hepatocellular
carcinoma in chronic hepatitis C virus (HCV) patients. Med Sci
Monit. 25:7243–7250. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rosenberg WM, Voelker M, Thiel R, Becka M,
Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M and Arthur MJ:
European Liver Fibrosis Group. Serum markers detect the presence of
liver fibrosis: A cohort study. Gastroenterology. 127:1704–1713.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guha IN, Parkes J, Roderick P,
Chattopadhyay D, Cross R, Harris S, Kaye P, Burt AD, Ryder SD,
Aithal GP, et al: Noninvasive markers of fibrosis in nonalcoholic
fatty liver disease: Validating the European liver fibrosis panel
and exploring simple markers. Hepatology. 47:455–460.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mayo MJ, Parkes J, Adams-Huet B, Combes B,
Mills AS, Markin RS, Rubin R, Wheeler D, Contos M, West AB, et al:
Prediction of clinical outcomes in primary biliary cirrhosis by
serum enhanced liver fibrosis assay. Hepatology. 48:1549–1557.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Parkes J, Guha IN, Roderick P, Harris S,
Cross R, Manos MM, Irving W, Zaitoun A, Wheatley M, Ryder S and
Rosenberg W: Enhanced liver fibrosis (ELF) test accurately
identifies liver fibrosis in patients with chronic hepatitis C. J
Viral Hepat. 18:23–31. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Omran D, Yosry A, Darweesh SK, Nabeel MM,
El-Beshlawey M, Saif S, Fared A, Hassany M and Zayed RA: Enhanced
liver fibrosis test using ELISA assay accurately discriminates
advanced stage of liver fibrosis as determined by transient
elastography fibroscan in treatment naïve chronic HCV patients.
Clin Exp Med. 18:45–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Loo WM, Goh GB, Wang Y, Yuan JM, Ong L,
Dan YY and Koh WP: Enhanced liver fibrosis score as a predictor of
hepatocellular carcinoma. Clin Chem. 64:1404–1405. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nagata H, Nakagawa M, Asahina Y, Sato A,
Asano Y, Tsunoda T, Miyoshi M, Kaneko S, Otani S, Kawai-Kitahata F,
et al: Effect of interferon-based and -free therapy on early
occurrence and recurrence of hepatocellular carcinoma in chronic
hepatitis C. J Hepatol. 67:933–939. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ioannou GN, Beste LA, Green PK, Singal AG,
Tapper EB, Waljee AK, Sterling RK, Feld JJ, Kaplan DE, Taddei TH
and Berry K: Increased risk for hepatocellular carcinoma persists
up to 10 years after HCV eradication in patients with baseline
cirrhosis or high FIB-4 scores. Gastroenterology. 157:1264–1278.e4.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Asahina Y, Tsuchiya K, Nishimura T,
Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K,
Nakanishi H, et al: α-fetoprotein levels after interferon therapy
and risk of hepatocarcinogenesis in chronic hepatitis C.
Hepatology. 58:1253–1262. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yasui Y, Kurosaki M, Komiyama Y, Takada H,
Tamaki N, Watakabe K, Okada M, Wang W, Shimizu T, Kubota Y, et al:
Wisteria floribunda agglutinin-positive Mac-2 binding
protein predicts early occurrence of hepatocellular carcinoma after
sustained virologic response by direct-acting antivirals for
hepatitis C virus. Hepatol Res. 48:1131–1139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dvorak K, Stritesky J, Petrtyl J, Vitek L,
Sroubkova R, Lenicek M, Smid V, Haluzik M and Bruha R: Use of
non-invasive parameters of non-alcoholic steatohepatitis and liver
fibrosis in daily practice-an exploratory case-control study. PLoS
One. 9(e111551)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tanwar S, Trembling PM, Guha IN, Parkes J,
Kaye P, Burt AD, Ryder SD, Aithal GP, Day CP and Rosenberg WM:
Validation of terminal peptide of procollagen III for the detection
and assessment of nonalcoholic steatohepatitis in patients with
nonalcoholic fatty liver disease. Hepatology. 57:103–111.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yilmaz Y and Eren F: Serum biomarkers of
fibrosis and extracellular matrix remodeling in patients with
nonalcoholic fatty liver disease: Association with liver histology.
Eur J Gastroenterol Hepatol. 31:43–46. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
McHutchison JG, Blatt LM, de Medina M,
Craig JR, Conrad A, Schiff ER and Tong MJ: Measurement of serum
hyaluronic acid in patients with chronic hepatitis C and its
relationship to liver histology. Consensus interferon study group.
J Gastroenterol Hepatol. 15:945–951. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Leroy V, Monier F, Bottari S, Trocme C,
Sturm N, Hilleret MN, Morel F and Zarski JP: Circulating matrix
metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2
as serum markers of liver fibrosis in patients with chronic
hepatitis C: Comparison with PIIINP and hyaluronic acid. Am J
Gastroenterol. 99:271–279. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Boeker KH, Haberkorn CI, Michels D,
Flemming P, Manns MP and Lichtinghagen R: Diagnostic potential of
circulating TIMP-1 and MMP-2 as markers of liver fibrosis in
patients with chronic hepatitis C. Clin Chim Acta. 316:71–81.
2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Miyaki E, Imamura M, Hiraga N, Murakami E,
Kawaoka T, Tsuge M, Hiramatsu A, Kawakami Y, Aikata H, Hayes CN and
Chayama K: Daclatasvir and asunaprevir treatment improves liver
function parameters and reduces liver fibrosis markers in chronic
hepatitis C patients. Hepatol Res. 46:758–764. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bernuth S, Yagmur E, Schuppan D, Sprinzl
MF, Zimmermann A, Schad A, Kittner JM, Weyer V, Knapstein J,
Schattenberg JM, et al: Early changes in dynamic biomarkers of
liver fibrosis in hepatitis C virus-infected patients treated with
sofosbuvir. Dig Liver Dis. 48:291–297. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yamazaki T, Joshita S, Umemura T, Usami Y,
Sugiura A, Fujimori N, Kimura T, Matsumoto A, Igarashi K, Ota M and
Tanaka E: Changes in serum levels of autotaxin with direct-acting
antiviral therapy in patients with chronic hepatitis C. PLoS One.
13(e0195632)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hsu WF, Lai HC, Su WP, Lin CH, Chuang PH,
Chen SH, Chen HY, Wang HW, Huang GT and Peng CY: Rapid decline of
noninvasive fibrosis index values in patients with hepatitis C
receiving treatment with direct-acting antiviral agents. BMC
Gastroenterol. 19(63)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bachofner JA, Valli PV, Kröger A, Bergamin
I, Künzler P, Baserga A, Braun D, Seifert B, Moncsek A, Fehr J, et
al: Direct antiviral agent treatment of chronic hepatitis C results
in rapid regression of transient elastography and fibrosis markers
fibrosis-4 score and aspartate aminotransferase-platelet ratio
index. Liver Int. 37:369–376. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shiratori Y, Imazeki F, Moriyama M, Yano
M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G,
et al: Histologic improvement of fibrosis in patients with
hepatitis C who have sustained response to interferon therapy. Ann
Intern Med. 132:517–524. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fujita K, Kuroda N, Morishita A, Oura K,
Tadokoro T, Nomura T, Yoneyama H, Arai T, Himoto T, Watanabe S and
Masaki T: Fibrosis staging using direct serum biomarkers is
influenced by hepatitis activity grading in hepatitis C virus
infection. J Clin Med. 7(267)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hiramatsu N, Oze T and Takehara T:
Suppression of hepatocellular carcinoma development in hepatitis C
patients given interferon-based antiviral therapy. Hepatol Res.
45:152–161. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Motoyama H, Tamori A, Kubo S,
Uchida-Kobayashi S, Takemura S, Tanaka S, Ohfuji S, Teranishi Y,
Kozuka R, Kawamura E, et al: Stagnation of histopathological
improvement is a predictor of hepatocellular carcinoma development
after hepatitis C virus eradication. PLoS One.
13(e0194163)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yamaguchi T, Matsuzaki K, Inokuchi R,
Kawamura R, Yoshida K, Murata M, Fujisawa J, Fukushima N, Sata M,
Kage M, et al: Phosphorylated Smad2 and Smad3 signaling: Shifting
between tumor suppression and fibro-carcinogenesis in chronic
hepatitis C. Hepatol Res. 43:1327–1342. 2013.PubMed/NCBI View Article : Google Scholar
|