Introduction
Epithelial ovarian carcinoma is a common cause of
cancer deaths in women worldwide. The predominant subtype is serous
ovarian carcinoma, which accounts for more than 50% of all ovarian
carcinomas (1). The diagnosis is
generally made at an advanced stage because of the insidious
disease onset and lack of effective screening strategies. The
standard treatment for these patients consists of primary debulking
surgery followed by platinum-based chemotherapy. The initial
response to first-line platinum-based chemotherapy is favorable;
however, most patients develop recurrences and resistance to
platinum-based chemotherapy, resulting in poor 5- and 10-year
survivals of ~32 and 15% respectively (1,2). There
are currently no biomarkers that reliably predict the response to
platinum-based chemotherapy. Identification of dependable
biomarkers is needed to facilitate planning optimal personalized
treatment strategies and predicting the prognosis of patients with
advanced serous ovarian carcinoma.
Systemic inflammation plays a crucial role in the
development and progression of several types of cancer. Systemic
inflammation can up-regulate cytokines and inflammatory mediators,
inhibit apoptosis, initiate angiogenesis, remodel the extracellular
matrix, and trigger DNA damage (3,4).
Biological indicators of the severity of systemic inflammation
include C-reactive protein, neutrophil-to-lymphocyte ratio (NLR),
platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and
platelet count (5-9).
Among these indicators, NLR has been attracting great interest
because it is reproducible and easy and inexpensive to measure in
routine clinical practice. Several researchers have reported the
association between a high NLR and poor prognosis in patients with
various types of carcinoma (9-12).
Furthermore, a high NLR is reportedly a useful predictor of poor
response to treatment and disease recurrence (13-15).
Several studies into the relationship between NLR
and prognosis of ovarian carcinoma have been published; however,
their conclusions are controversial. Most of these studies have
focused on pre-treatment NLRs. We evaluated the relationship
between NLR before initiating chemotherapy, after primary debulking
surgery, and sensitivity to platinum-based chemotherapy and
prognosis. We considered that the pre-chemotherapy NLR would more
accurately reflect the severity of inflammation and predict the
sensitivity to chemotherapy than the pre-surgery NLR. To the best
of our knowledge, this is the first reported investigation of this
hypothesis.
Materials and methods
Patients and data
This study was a retrospective study of data drawn
from patients' medical records. We reviewed the records of 50
patients with stage III or IV serous ovarian cancer treated at
Osaka City University Hospital between January 2005 and December
2012. High-grade serous ovarian carcinoma had been diagnosed
histologically in all patients. We excluded patients for whom
pre-treatment neutrophil or lymphocyte counts were unavailable. All
patients had undergone primary debulking surgery followed by six
3-weekly cycles of carboplatin plus paclitaxel. We collected
information on the following clinical variables: Age, Federation of
Gynaecology and Obstetrics (FIGO) stage, serum cancer antigen
(CA125) concentration, size of postoperative residual tumor,
leukocyte count, and response to platinum-based chemotherapy. The
NLR was defined as the neutrophil count divided by the lymphocyte
count and was calculated 2 days before initiation of chemotherapy,
which was commenced ~2 weeks after primary debulking surgery. A ROC
curve was generated to determine the cutoff value of NLR for
predicting sensitivity to platinum-based chemotherapy. Patients
were allocated to low-NLR group and high-NLR group on the basis of
this cutoff value.
We obtained informed consent for treatment from all
patients and received approval from the Institutional Review Board
of Osaka City University Hospital before initiating this study (IRB
no. 2020-288).
Chemotherapy and evaluation of the
effect of treatment
All patients underwent six 3-weekly cycles of
chemotherapy with paclitaxel plus carboplatin (175 mg/m2
of paclitaxel infused >3 h; dosage of carboplatin calculated
with an area under the curve of 5 and infused >1 h). Platinum
resistance was defined as <6 months between last dose of
platinum and recurrence and platinum sensitivity as longer than 6
months between last platinum dose and recurrence. DFS was defined
as the time between dates of diagnosis and disease recurrence.
Overall survival (OS) was defined as the time between dates of
diagnosis and death or most recent follow-up.
Statistical analysis
We compared characteristics, sensitivity to
platinum-based chemotherapy, OS, and DFS between patients in the
low-NLR and high-NLR groups. EZR software version 1.3 (Saitama
Medical Center, Jichi Medical University, Saitama, Japan) was used
for all statistical analyses. The data are represented as mean ±
standard deviation. Student's t-test was used to compare
differences between the data. Fisher's exact test was used to
identify differences in the distribution of categorical variables
between groups. Kaplan-Meier plots and log-rank tests were used to
analyze OS and DFS. P-value <0.05 was considered to denote
statistical significance.
Results
Patient characteristics
The characteristics of all patients are shown in
Table I. Their age ranged from 36
to 79 years (mean age, 60.64 years). The most prevalent disease
stage was IIIC, accounting for 74% of all patients.
Pre-chemotherapy NLRs ranged from 0.89 to 18.8 (mean, 4.04). Half
of the patients were sensitive to platinum-based chemotherapy and
the other resistant to it.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Value |
|---|
| Number of
patients | 50 |
| Age, years | |
|
Mean ±
SD | 60.64±10.89 |
|
Range | 36-79 |
| FIGO stage, n
(%) | |
|
IIIA | 1(2) |
|
IIIB | 4(8) |
|
IIIC | 37(74) |
|
IVA | 5(10) |
|
IVB | 3(6) |
| CA125 (U/ml) | |
|
Mean ±
SD | 2365.3±3063.9 |
|
Range | 62-12,300 |
| NLR before
chemotherapy | |
|
Mean ±
SD | 4.04±3.83 |
|
Range | 0.89-18.8 |
| Postoperative
residual tumor, n (%) | |
|
None | 5(10) |
|
≤1 cm | 12(24) |
|
>1
cm | 33(66) |
| Sensitivity to
platinum-based chemotherapy, n (%) | |
|
Sensitive | 25(50) |
|
Resistant | 25(50) |
ROC curve for determining NLR cutoff
value for predicting sensitivity to platinum-based
chemotherapy
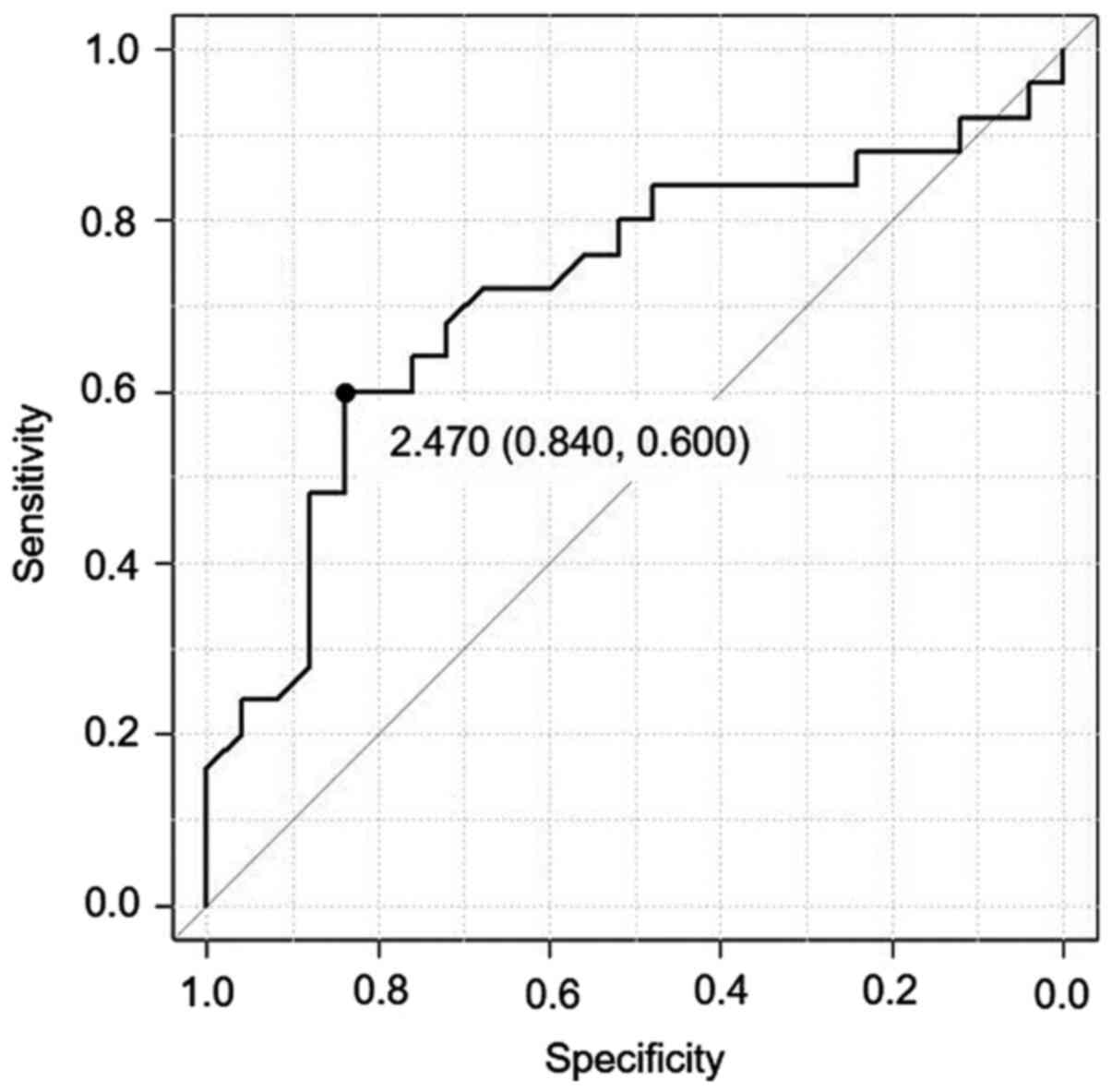
We generated an ROC curve to determine the cutoff
value for predicting sensitivity to platinum-based chemotherapy
(Fig. 1). This resulted in a cutoff
value of 2.47. The specificity for prediction of sensitivity to
platinum-based chemotherapy was 84% and the sensitivity of this
cutoff value was 60%. We therefore adopted an NLR cutoff of 2.47
for allocating patients to high-NLR (≥2.47) and low-NLR (<2.47)
groups.
Characteristics of high- and low-NLR
groups and comparison of platinum sensitivity between group
Table II shows
patient characteristics according to groups. We compared age, FIGO
stage, serum CA125 concentration, and size of postoperative
residual tumor. None of the studied factors differed significantly
between the groups, suggesting that the NLR value was the only
difference between them. Next, we compared sensitivity to
platinum-based chemotherapy between the two groups. Table III shows the number of patients
with platinum-sensitive and platinum-resistant disease in each
group. In the low-NLR group, 77.8% of patients were sensitive to
platinum-based chemotherapy, whereas in the high-NLR group 34.4%
were sensitive to it. Thus, the low-NLR group was significantly
more sensitive to platinum-based chemotherapy than was the high-NLR
group (P=0.007).
 | Table IICharacteristics of patients in the
low- and high-NLR groups. |
Table II
Characteristics of patients in the
low- and high-NLR groups.
| Characteristic | Low-NLR group
(<2.47) | High-NLR group
(≥2.47) | P-value |
|---|
| No. of
patients | 18 | 32 | - |
| Age, years | | | 0.821a |
|
Mean ±
SD | 61.11±9.76 | 60.38±11.62 | |
|
Range | 47-75 | 36-79 | |
| FIGO stage, n | | | 0.588b |
|
IIIA | 0 | 1 | |
|
IIIB | 1 | 3 | |
|
IIIC | 16 | 21 | |
|
IVA | 1 | 4 | |
|
IVB | 0 | 3 | |
| CA125 (U/ml) | | | 0.641a |
|
Mean ±
SD |
2,638.11±3,416.06 |
2,211.88±2,876.52 | |
| Postoperative
residual tumor, n | | | 0.115b |
|
None | 4 | 1 | |
|
≤1 cm | 4 | 8 | |
|
>1
cm | 10 | 23 | |
 | Table IIINumber of platinum-sensitive and
platinum-resistant patients in the low- and high-NLR groups. |
Table III
Number of platinum-sensitive and
platinum-resistant patients in the low- and high-NLR groups.
| Variable | Low-NLR group
(≤2.47) n=18 | High-NLR (≥2.47)
n=32 | P-value |
|---|
| Platinum-sensitive,
n (%) | 14 (77.8) | 11 (34.4) | 0.007a |
| Platinum-resistant,
n (%) | 4 (22.2) | 21 (65.6) | |
Comparison of prognosis between high-
and low-NLR groups
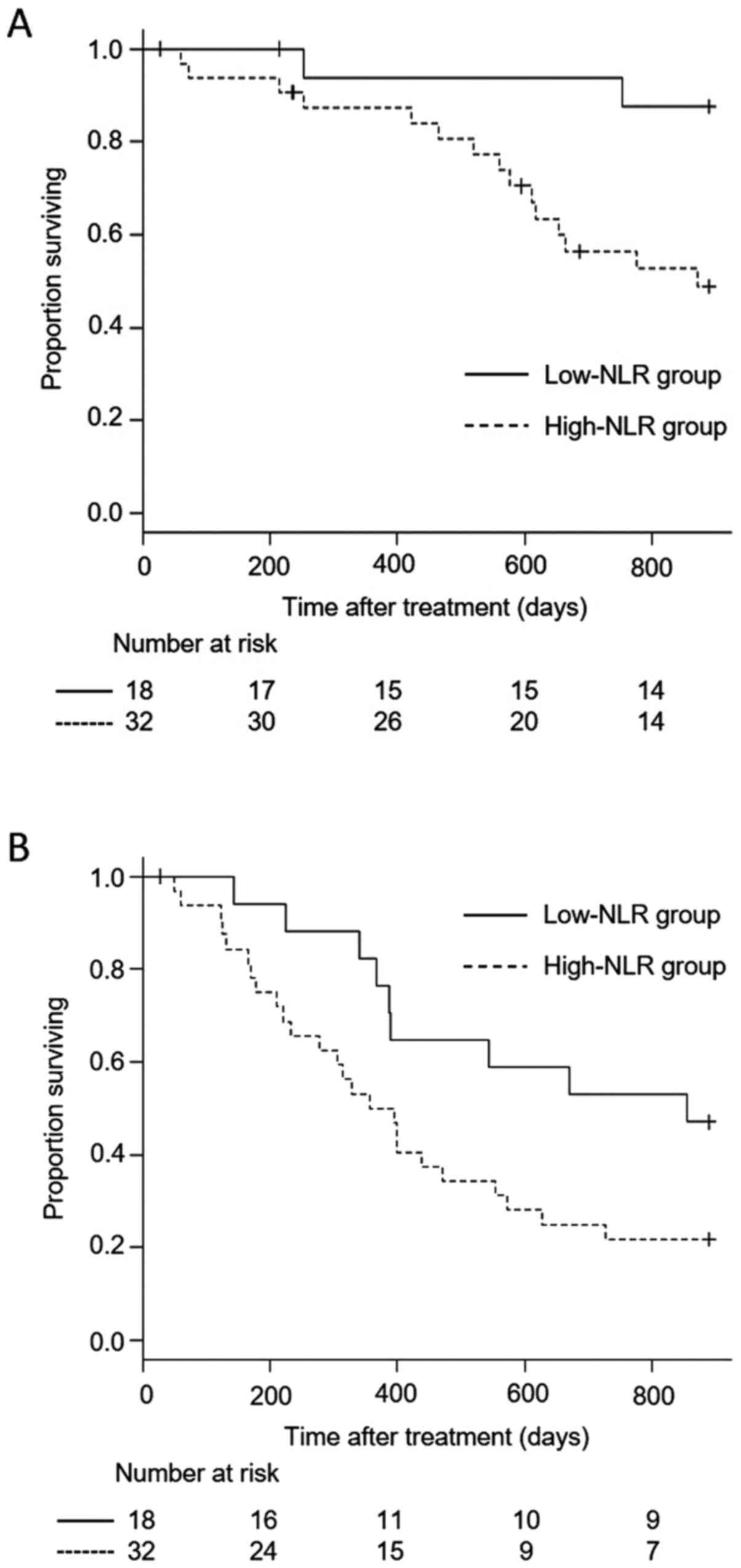
Fig. 2 shows the OS
and DFS of the two groups. The low-NLR group had significantly
better OS and DFS than did the high-NLR group (P=0.013 and P=0.043,
respectively). These results suggest that the NLR can serve as a
biomarker for predicting the prognosis of patients with serous
ovarian carcinoma who undergo debulking surgery followed by
platinum-based chemotherapy.
Discussion
In this study of patients with ovarian serous
carcinoma who had undergone debulking surgery followed by
platinum-based chemotherapy, we investigated the ability of
pre-chemotherapy NLR to predict sensitivity to platinum-based
chemotherapy and prognosis. We found that the pre-chemotherapy NLR
predicted sensitivity to chemotherapy and prognosis when we used a
cutoff value of 2.47.
The crucial impact of inflammation and the
associated leukocyte recruitment on cancer development was first
reported in 1863 by Virchow. It is now clear that
inflammation-related neutrophils and immunocytes are components of
the tumor microenvironment and play an essential role in the
neoplastic process by fostering proliferation of, and communication
between cancer cells and that microenvironment (3,16,17).
Tumor development and progression are initiated via DNA damage and
overproduction of cytokines such as vascular endothelial growth
factor (VEGF), tumor necrosis factor alpha (TNF-α), and interleukin
(IL)-2 and IL-6 (16,18). The inflammatory response involves
clustering of immune cells, including tumor-associated macrophages
and neutrophils, tumor-induced T cells, dendritic cells, and innate
lymphoid cells. This accumulation of cells prompts initiation and
progression of cancer and may simultaneously suppress cancer
progression (19-22).
Neutrophils account for 50-60% of leukocytes and
become more numerous in the presence of inflammation. An increase
in neutrophil count indicates systemic inflammation and has been
reported to be involved in tumor proliferation, invasion, and
angiogenesis (3,23,24).
Cancer-associated angiogenesis and cellular DNA damage are promoted
by cytotoxic mediators such as reactive oxygen species and
neutrophil elastase, which are released by neutrophils (25). Neutrophils recruit inflammatory
mediators, including IL-1, IL-6, TNF, and VEGF, and then inhibit
the cytotoxic activity of lymphocytes, impairing activation of
adaptive immunity (26,27). Neutrophils can facilitate tumor
development by remodeling the extracellular matrix, providing
pro-angiogenic factors, and inhibiting lymphocyte activity
(28).
Hematological markers that reflect systemic
inflammation, such as the NLR, have recently attracted considerable
interest as predictors of prognosis and sensitivity to chemotherapy
in patients with cancer. An increasing number of researchers have
reported a relationship between pre-operative NLR and prognosis in
patients with ovarian cancer. However, several studies have failed
to confirm this relationship (29).
Additionally, the mechanism(s) by which a high NLR contributes to
poor prognosis and poor sensitivity to chemotherapy is still poorly
understood. The NLR precisely expresses the balance between
neutrophils and immunocytes, a high NLR reflecting an up-regulated
innate immune response with increased concentrations of cytokines
and tumor macrophage infiltration. The NLR has therefore been
considered a novel prognostic indicator (30,31).
It is also reportedly associated with the responses of several
cancers to chemotherapy (32-35),
indicating it potentially has value as a biomarker for predicting
sensitivity to chemotherapy. Blood cell counts are routinely
checked at the beginning of diagnosis or treatment, making the NLR
a convenient, cost-effective, and reproducible marker in clinical
practice. However, varying means of determining optimal thresholds
have been used to predict prognosis and sensitivity to
chemotherapy. Some studies have used ROC analysis to calculate the
threshold, others have used the median NLR, and still other have
used the interquartile limits. The resultant lack of consensus on
threshold values, and therefore on definitions of normal and high
NLRs, has hindered the use of the NLR both in daily clinical
practice and in investigating the relationship between NLR and
prognosis in patients with ovarian cancer.
To the best of our knowledge, this is the first
study to investigate the relationships between pre-chemotherapeutic
NLR and sensitivity to platinum-based chemotherapy and prognosis in
patients with serous ovarian carcinoma. In this study, we used the
post-debulking surgery, pre-chemotherapy NLR rather than the NLR
before initiation of any treatment because we believe that the
former more accurately reflects the status of cancer-related
inflammation and therefore more reliably predicts tumor sensitivity
to chemotherapy. We found that pre-chemotherapy NLR is associated
with sensitivity to platinum-based chemotherapy and prognosis in
patients with advanced high-grade serous ovarian carcinoma. Several
studies have used the pre-treatment NLR to predict the sensitivity
to chemotherapy or prognosis. However, to the best of our
knowledge, no studies have investigated the post-debulking surgery,
pre-chemotherapy NLR.
The limitations of this study include that it was a
relatively small, single institution, retrospective study using
univariate analysis. Further investigation, including validation in
a second cohort, are needed before NLR can be confidently
recommended in clinical practice. However, we believe that our
findings contribute to progress in the use of the NLR to predict
sensitivity to platinum-based chemotherapy and prognosis in
patients with serous ovarian carcinoma.
Acknowledgements
Not applicable.
Funding
This study was funded by The Osaka Medical Research Foundation
for Intractable Diseases (grant no. 26-2-47).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TF and TS designed the present study. TF, MKaw, YA,
SN, MS, YI, HM, MY, MKas, YH, TI and TY collected and analyzed the
data. TF, MKaw and TS wrote the manuscript. TF and MKaw confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Osaka City University Hospital before its
initiation (approval no. 2020-288). Written informed consent for
all treatment was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peres LC, Cushing-Haugen KL, Köbel M,
Harris HR, Berchuck A, Rossing MA, Schildkraut JM and Doherty JA:
Invasive epithelial ovarian cancer survival by histotype and
disease stage. J Natl Cancer Inst. 111:60–68. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Buergy D, Wenz F, Groden C and Brockmann
MA: Tumor-platelet interaction in solid tumors. Int J Cancer.
130:2747–2760. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nishijima TF, Muss HB, Shachar SS, Tamura
K and Takamatsu Y: Prognostic value of lymphocyte-to-monocyte ratio
in patients with solid tumors: A systematic review and
meta-analysis. Cancer Treat Rev. 41:971–978. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shrotriya S, Walsh D, Bennani-Baiti N,
Thomas S and Lorton C: C-reactive protein is an important biomarker
for prognosis tumor recurrence and treatment response in adult
solid tumors: A systematic review. PLoS One.
10(e0143080)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Templeton AJ, Ace O, McNamara MG,
Al-Mubarak M, Vera-Badillo FE, Hermanns T, Seruga B, Ocaña A,
Tannock IF and Amir E: Prognostic role of platelet to lymphocyte
ratio in solid tumors: A systematic review and meta-analysis.
Cancer Epidemiol Biomarkers Prev. 23:1204–1212. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106(dju124)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Orditura M, Galizia G, Diana A, Saccone C,
Cobellis L, Ventriglia J, Iovino F, Romano C, Morgillo F, Mosca L,
et al: Neutrophil to lymphocyte ratio (NLR) for prediction of
distant metastasis-free survival (DMFS) in early breast cancer: A
propensity score-matched analysis. ESMO Open.
1(e000038)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wei B, Yao M, Xing C, Wang W, Yao J, Hong
Y, Liu Y and Fu P: The neutrophil lymphocyte ratio is associated
with breast cancer prognosis: An updated systematic review and
meta-analysis. Onco Targets Ther. 9:5567–5575. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tang L, Li X, Wang B, Luo G, Gu L, Chen L,
Liu K, Gao Y and Zhang X: Prognostic Value of
neutrophil-to-lymphocyte ratio in localized and advanced prostate
cancer: A systematic review and meta-analysis. PLoS One.
11(e0153981)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xue P, Kanai M, Mori Y, Nishimura T, Uza
N, Kodama Y, Kawaguchi Y, Takaori K, Matsumoto S, Uemoto S and
Chiba T: Neutrophil-to-lymphocyte ratio for predicting palliative
chemotherapy outcomes in advanced pancreatic cancer patients.
Cancer Med. 3:406–415. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Cho IR, Park JC, Park CH, Jo JH, Lee HJ,
Kim S, Shim CN, Lee H, Shin SK, Lee SK and Lee YC: Pre-treatment
neutrophil to lymphocyte ratio as a prognostic marker to predict
chemotherapeutic response and survival outcomes in metastatic
advanced gastric cancer. Gastric Cancer. 17:703–710.
2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nakamura K, Nagasaka T, Nishida T, Haruma
T, Ogawa C, Kusumoto T, Seki N and Hiramatsu Y: Neutrophil to
lymphocyte ratio in the pre-treatment phase of final-line
chemotherapy predicts the outcome of patients with recurrent
ovarian cancer. Oncol Lett. 11:3975–3981. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Menon S, Shin S and Dy G: Advances in
cancer immunotherapy in solid tumors. Cancers (Basel).
8(106)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kusumanto YH, Dam WA, Hospers GA, Meijer C
and Mulder NH: Platelets and granulocytes, in particular the
neutrophils, form important compartments for circulating vascular
endothelial growth factor. Angiogenesis. 6:283–287. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hagerling C, Casbon AJ and Werb Z:
Balancing the innate immune system in tumor development. Trends
Cell Biol. 25:214–220. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pillay J, Tak T, Kamp VM and Koenderman L:
Immune suppression by neutrophils and granulocytic myeloid-derived
suppressor cells: Similarities and differences. Cell Mol Life Sci.
70:3813–3827. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Walker JA, Barlow JL and McKenzie AN:
Innate lymphoid cells-how did we miss them? Nat Rev Immunol.
13:75–87. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lin EY and Pollard JW: Role of infiltrated
leucocytes in tumour growth and spread. Br J Cancer. 90:2053–2058.
2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Güngör N, Knaapen AM, Munnia A, Peluso M,
Haenen GR, Chiu RK, Godschalk RW and van Schooten FJ: Genotoxic
effects of neutrophils and hypochlorous acid. Mutagenesis.
25:149–154. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nicolás-Ávila J, Adrover JM and Hidalgo A:
Neutrophils in homeostasis, immunity, and cancer. Immunity.
46:15–28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Uribe-Querol E and Rosales C: Neutrophils
in cancer: Two sides of the same coin. J Immunol Res.
2015(983698)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Raungkaewmanee S, Tangjitgamol S,
Manusirivithaya S, Srijaipracharoen S and Thavaramara T: Platelet
to lymphocyte ratio as a prognostic factor for epithelial ovarian
cancer. J Gynecol Oncol. 23:265–273. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu R, Zheng S, Yuan Q, Zhu P, Li B, Lin
Q, Shi W, Min Y, Ge Q and Shao Y: The prognostic significance of
combined pretreatment fibrinogen and neutrophil-lymphocyte ratio in
various cancers: A systematic review and meta-analysis. Dis
Markers. 2020(4565379)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Peng SM, Yu N, Ren JJ, Xu JY, Chen GC,
Yang JR, Li ZN, Du HZ, Li DP, Zhang YS and Qin LQ: The Geriatric
Nutritional Risk Index as a prognostic factor in patients with
advanced non-small-cell lung cancer. Nutr Cancer: Dec 24, 2020
(Epub ahead of print). doi: 10.1080/01635581.2020.1865423.
|
|
32
|
Luo X, Yu B, Jiang N, Du Q, Ye X, Li H,
Wang WQ and Zhai Q: Chemotherapy-induced reduction of
neutrophil-to-lymphocyte ratio is associated with better survival
in pancreatic adenocarcinoma: A meta-analysis. Cancer Control.
27(1073274820977135)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nogueira-Costa G, Fernandes I, Gameiro R,
Gramaça J, Xavier AT and Pina I: Prognostic utility of
neutrophil-to-lymphocyte ratio in patients with metastatic
colorectal cancer treated using different modalities. Curr Oncol.
27:237–243. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Iwai N, Okuda T, Sakagami J, Harada T,
Ohara T, Taniguchi M, Sakai H, Oka K, Hara T, Tsuji T, et al:
Neutrophil to lymphocyte ratio predicts prognosis in unresectable
pancreatic cancer. Sci Rep. 10(18758)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vernieri C, Mennitto A, Prisciandaro M,
Huber V, Milano M, Rinaldi L, Cona MS, Maggi C, Ferrari B,
Manoukian S, et al: The neutrophil-to-lymphocyte and
platelet-to-lymphocyte ratios predict efficacy of platinum-based
chemotherapy in patients with metastatic triple negative breast
cancer. Sci Rep. 8(8703)2018.PubMed/NCBI View Article : Google Scholar
|