Introduction
For the past 3 decades, we have been witnessing
dynamic changes in the treatment of locally advanced rectal cancer
(LARC) from single modality treatment using surgery alone to
multimodality treatment including radiation, chemotherapy and total
mesorectal excision (TME). These changes led to an improvement in
outcome for stage II and III rectal cancer (1).
TME has made a revolution in the management of
rectal cancer as it has shown a decrease in local recurrence from
30-50% to ~5% and allowed the patients to have sphincter preserving
surgery (2). The use of adjuvant
chemotherapy is advisable, but it is still controversial as studies
have not shown an improvement in distant recurrence, overall
survival (OS) and disease-free survival (DFS) (3). Compliance to adjuvant treatment has
been a major concern in our daily practice and this may have
affected the survival outcome. In fact, some studies have shown
that a 4-week delay in post-operative treatment correlated with a
decrease in OS of ~14% (4-6).
In terms of sequencing, German trial, along with
similar trials, showed that preoperative chemo-radiation therapy
combined with TME have shown better compliance, less toxicity and
less local pelvic recurrence rate as compared to post-operative
radiation treatment. Moreover, they detected an increase in
pathologic complete response (pCR) rate with chemo-radiation
therapy that correlates with better oncologic outcomes and may play
an important role in organ preservation strategy (7-11).
Currently, the accepted standard of care for
treatment of LARC is neoadjuvant chemo-radiation followed by TME
and adjuvant chemotherapy. Despite the improvement in local control
resulting from the association of different treatment regimens, the
death rate from rectal cancer from distant metastasis is still
elevated compared to local failure (12). While this multimodality approach
improved local control, no impact was noted on the OS, with distant
metastasis remaining the most concerning issue with a cumulative
incidence of 30% in 10 years and with an overall DFS of 68% at 10
years (13). Therefore, better
systemic control is needed. This could be potentially achieved by
focusing more on micrometastasis early in the course of treatment,
hence delivering chemotherapy in pre-operative setting. This
regimen, known as total neoadjuvant therapy (TNT), consists of
delivering chemotherapy and radiation therapy in neoadjuvant
setting. TNT was proposed to treat micrometastasis, increase pCR
rate and increase compliance to treatment.
The idea of TNT was developed in the RAPIDO trial
that was first launched in 2011(14). It showed impressive results that
were presented at ASCO 2020 and that may lead to a change in
standard of care with TNT regimen. In this trial, TNT was
associated with lower disease-related treatment failure, distant
metastasis rate and doubling of pathologic complete response (pCR)
rate compared to standard of care (15).
In this retrospective study, we assess the
difference in pCR rates, compliance to chemotherapy, tumor
downstaging and DFS between TNT and chemoradiation therapy (CRT)
for LARC at the American University of Beirut Medical Center
(AUBMC). We present this article in accordance with the STROBE
reporting checklist.
Materials and methods
Study design and patient
selection
This study is a retrospective chart review of
patients diagnosed with LARC at the American University of Beirut
Medical Center between January 1st, 2011, and June 1st, 2019.
Patients ≥18 years old and with cT3/4 or cT2-node-positive were
included. In total, 81 patients were included in the study and
patients' demographics, treatment course and clinical outcomes were
recorded. Charlson Comorbidity Index (CCI) was also calculated for
every patient. The study was conducted in accordance with the
Declaration of Helsinki (as revised in 2013). The study was
approved via expedited review by the local Institutional Review
Board (IRB) committee at AUBMC IRB ID: BIO-2019-0024.
Study objectives
The primary endpoint is the pathologic complete
response (pCR) rate between the two different treatment modalities.
pCR was defined as the absence of any viable tumor cell within the
tumor bed in the setting of chemotherapy effect (16). The secondary endpoints are the
compliance to chemotherapy, tumor downstaging and DFS.
Treatment modalities
The change in treatment modalities between patients
is due to advancement in the field. Patients included were divided
into either TNT or CRT groups. The TNT is defined as short course
radiotherapy (5x5 Gy) before or after 6 cycles of mFOLFOX
(oxaliplatin, fluorouracil and folinic acid) followed by TME. TME
was performed 4 weeks after neoadjuvant therapy in the TNT group.
The total duration of treatment between was 16-18 weeks. The CRT is
defined as concurrent long course radiotherapy (45 Gy divided in 25
fractions over 5 weeks) and capecitabine followed by TME and
adjuvant chemotherapy. TME involves the removal of the rectum
together with surrounding mesorectum (lymphovascular fatty tissue)
through a precise dissection along the pelvic visceral fascia
(17). Treatment modalities were
standardized between patients within the same treatment group.
Statistical analysis
A biomedical statistician performed the statistical
review of the study. Continuous variables were summarized by their
median, mean and range. Categorical variables were described by
counts and relative frequencies. Crosstabulations in the form of
2x2 tables were plotted to compared and detect differences between
the two groups in outcome, downstaging and pathology. DFS curve was
plotted using the Kaplan-Meier curve, the log rank was used to
check for significant difference between the studied groups. DFS
time was defined as the time from initial diagnosis to disease
progression or the end of follow-up (censored observations who did
not reach the progression event). A value of P<0.05 was
considered significant in all analyses. All statistical analysis
was performed using the SPSS v.25.0 statistical package.
Results
Patient characteristics and
distribution
Of the 81 patients diagnosed with LARC, 48 (59.3%)
were males and 33 (40.7%) were females with the average age 59
(34-81) and 55 (25-85) years, respectively. Patients were
distributed into two treatment groups; 26 patients received TNT and
55 received CRT with the average age 51 and 60 years, respectively
(Table I). The median follow-up
periods for the TNT and CRT groups were 22.7 and 47.8 months,
respectively. The mean CCI was 3.96 in the CRT group and 3.23 in
the TNT group (P<0.05).
 | Table IPatient demographics. |
Table I
Patient demographics.
| | Treatment
modality |
|---|
| Variables | TNT | CRT |
|---|
| Median age, years
(range) | 51 (25-75) | 60 (34-85) |
| Sex, n (%) | | |
|
Male | 16 (61.5) | 32 (58.2) |
|
Female | 10 (38.5) | 23 (41.8) |
| Tumor
differentiation, n (%) | | |
|
Well | 4 (15.3) | 6 (10.9) |
|
Moderate | 15 (57.7) | 39 (70.9) |
|
Poor | 6 (23.1) | 1 (1.8) |
|
Not
documented | 1 (3.9) | 9 (16.4) |
| Clinical stage, n
(%) | | |
|
T2N1 | 0 (0.0) | 1 (1.8) |
|
T3N0 | 2 (7.7) | 9 (16.4) |
|
T3N1 | 9 (34.6) | 34 (61.8) |
|
T3N2 | 9 (34.6) | 6 (10.9) |
|
T3Nx | 1 (3.9) | 3 (5.5) |
|
T4N0 | 0 (0.0) | 1 (1.8) |
|
T4N1 | 2 (7.7) | 1 (1.8) |
|
T4N2 | 3 (11.5) | 0 (0.0) |
|
T4Nx | 0 (0.0) | 0 (0.0) |
Pathologic and survival outcomes
Of the 26 patients that received TNT, 10 (38.5%)
patients had a pCR, while of the 55 patients that received CRT, 15
(27.3%) patients had a pCR (P=0.22). On the other hand, 7 (26.9%)
patients from the TNT group and 17 (30.9%) patients from the CRT
group had a pathologic node-positive (P=0.46). Moreover,
downstaging to pT0N0 and pT1N0 was achieved in 11 (42.3%) and 19
(35.8%) patients in the TNT and CRT groups, respectively (P=0.33)
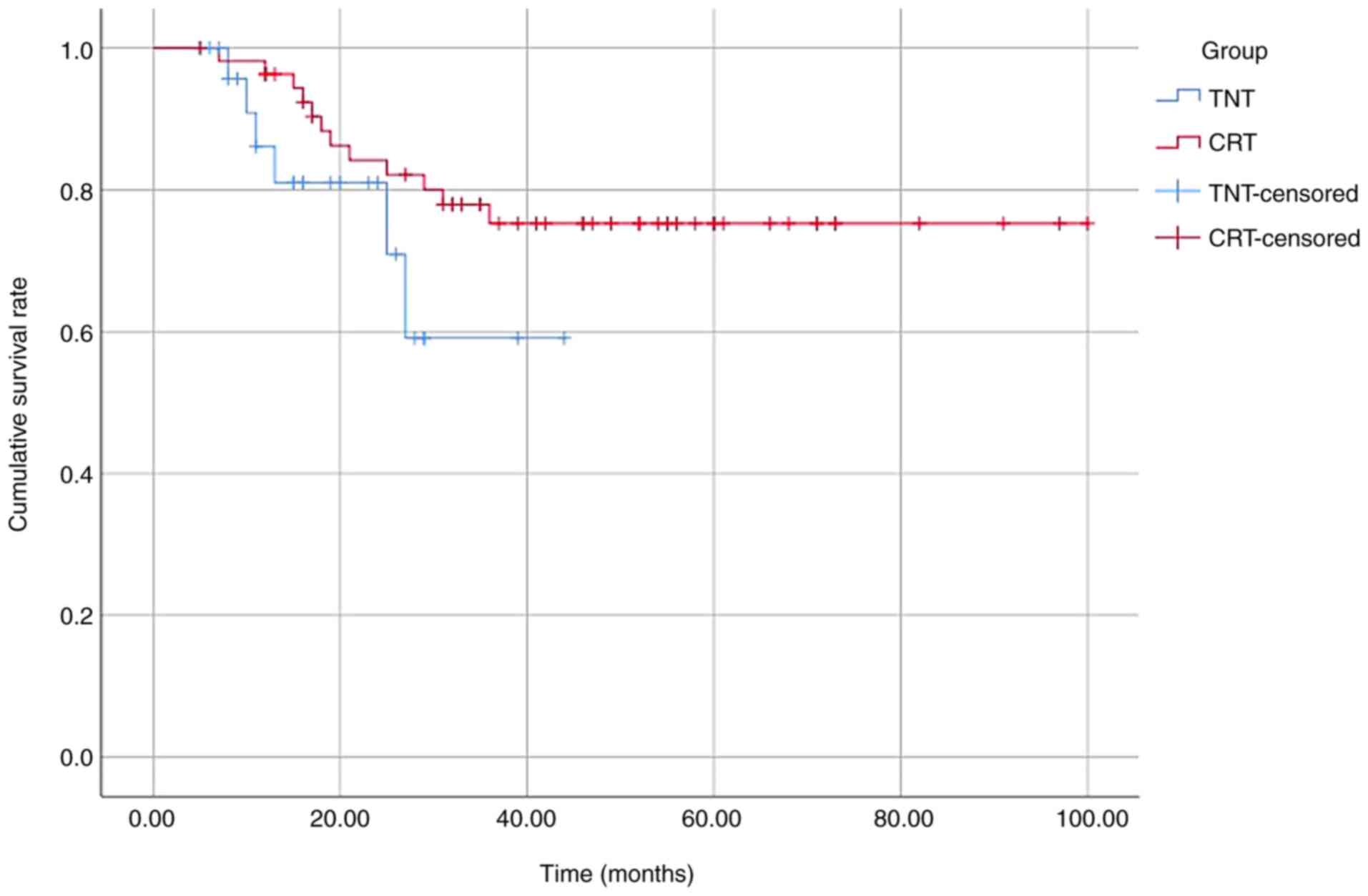
(Table II). The 2-year DFS rate
was 81 and 84% in the TNT and CRT groups, respectively (P=0.15)
(Fig. 1).
 | Table IITreatment outcome. |
Table II
Treatment outcome.
| | Treatment
modality |
|---|
| Variables | TNT | CRT |
|---|
| Pathologic
response | | |
|
Complete
response (TRG 0) | 10 (38.5) | 15 (27.3) |
|
Near
complete response (TRG 1) | 3 (11.5) | 5 (9.1) |
|
Other
response (TRG 2 and 3) | 13 (50.0) | 35 (63.6) |
| Pathologic
staging | | |
|
ypT0N0 | 10 (38.5) | 15 (27.3) |
|
ypTisN0 | 0 (0.0) | 1 (1.8) |
|
ypT1N0 | 1 (3.9) | 3 (5.5) |
|
ypT2N0 | 2 (7.7) | 3 (5.5) |
|
ypT3N0 | 6 (23.1) | 16 (29.1) |
|
ypT1N1 | 2 (7.7) | 0 (0.0) |
|
ypT2N1 | 0 (0.0) | 5 (9.1) |
|
ypT3N1 | 1 (3.9) | 6 (10.9) |
|
ypT3N2 | 1 (3.9) | 3 (5.5) |
|
ypT4N1 | 1 (3.9) | 1 (1.8) |
|
ypT4N2 | 2 (7.7) | 1 (1.8) |
|
ypTxN1 | 0 (0.0) | 1 (1.8) |
Compliance to chemotherapy
Of the 55 patients that received CRT, 30 (54.5%)
patients received any number of cycles of adjuvant chemotherapy and
22 (40%) of which received a full course of chemotherapy. All of
the 26 patients in the TNT group received neoadjuvant chemotherapy
with 22 (84.6%) of which receiving a full course (P<0.01). None
of CRT patients and 1 patient from the TNT group had a dose
reduction.
Discussion
Clinical outcomes in patients with LARC have
improved markedly especially significant decrease in local failure
with the development in treatment regimens. Sauer et al
(13) proved in a randomized trial
that neoadjuvant chemoradiation was significantly superior in local
control in comparison to adjuvant chemoradiation, but with no
difference in OS or DFS. Then, in the phase III randomized trials,
EORTC22921 and I-CNR-RT, the addition of adjuvant fluorouracil and
folinic acid to preoperative chemoradiation did not improve OS and
DFS in comparison to regular surveillance post-surgery (18,19).
Moreover, the PETACC-6 phase III trial compared neoadjuvant
chemoradiation with capecitabine followed by 6 cycles of adjuvant
capecitabine with or without oxaliplatin, before and after surgery.
This trial also showed that the addition of oxaliplatin to
capecitabine did not improve OS and DFS (20). Finally, in the German CAO/ARO/AIO-04
phase III trial, the addition of oxaliplatin to preoperative
chemoradiation and adjuvant fluorouracil and folinic acid, led to a
significant improvement in DFS and OS, even though the addition of
oxaliplatin to capecitabine-based chemoradiation is not the
standard of care (21).
Furthermore, the compliance rates to adjuvant chemotherapy were
low. In a multicenter retrospective review, only 44.1% received
adjuvant chemotherapy after neoadjuvant chemoradiation and rectal
surgery and, of those, only 56% were compliant to treatment
(22). In comparison, in our
cohort, 54.5% of the patients in the CRT group received adjuvant
chemotherapy and 40% of which were compliant to treatment. While on
the other hand, 100% of the patients in the TNT group received
neoadjuvant chemotherapy and 84% were compliant to treatment
(P<0.01).
Furthermore, in a phase II trial, Marco et al
(23) showed that giving mFOLFOX6
after chemoradiation and before surgery was associated with better
compliance and DFS rates. Indeed, in another phase II trial,
delaying the surgery by giving up to 6 cycles of mFOLFOX after
chemoradiation was associated with superior pCR rates in comparison
to patients not receiving neoadjuvant chemotherapy (24). Similar to the findings in previous
studies, the main causes of noncompliance in our patients were
post-operative complications, drug-related toxicities and patients'
preferences.
In a randomized trial, Ngan et al (25) compared the local recurrence rates
between short-course radiotherapy (25 Gy in 5 fractions) and
long-course chemoradiation (50.4 Gy in 28 fractions). No difference
was noted between the 3-year local recurrence rates, OS, distant
recurrence rate or late toxicity, all in favor of considering
short-course radiotherapy to decrease the treatment time without
any change in clinical outcomes (25).
Moreover, the Stockholm III trial compared by
randomization short-course radiation immediately before surgery,
short-course radiation with delayed surgery and long-course
radiotherapy (50 Gy in 25 fractions) with delayed surgery. It
showed that postoperative complications were significantly less in
the group receiving short-course radiotherapy with delayed surgery,
making this regimen practical (26). On the other hand, the Polish II
randomized trial showed no difference, after 8 years, between
short-course radiotherapy followed by neoadjuvant chemotherapy and
upfront chemoradiotherapy in OS or DFS (27).
In an effort to truly assess the potential of
neoadjuvant chemotherapy, Fokas et al (28) compared, in a randomized phase II
trial, neoadjuvant chemotherapy given before or after
chemoradiation followed by surgery. Chemoradiation followed by
neoadjuvant chemotherapy and surgery was associated with superior
pCR rates and better compliance with chemoradiation but worse
compliance with chemotherapy (28).
Most recently, in a randomized trial, short-course radiotherapy
followed by neoadjuvant chemotherapy then by TME was compared to
long-course radiotherapy followed by TME and optional adjuvant
chemotherapy. The RAPIDO trial showed that the TNT treatment
modality was significantly superior in pCR rate (28 vs. 14%) and
had a 7% decrease in disease-related treatment failure (14). In our cohort, the pCR rate was
numerically higher for the TNT group without statistical
significance, which can be due to the small sample size. On the
other hand, in a systemic review comparing the two treatment
modalities, the pooled pCR rates were found to be 32.4 and 22.3%,
while in our cohort the rates were 38.5 and 27.3% in the TNT and
CRT groups, respectively (29).
Moreover, the difference in the 2-years DFS rates between the two
modalities was not significant, while only one study showed an
improved DFS in the TNT group (29). In fact, the RAPIDO trial showed that
the 3-year OS rate was the same in both groups (14). Furthermore, our data showed that the
TNT group had a numerically higher rate of tumor downstaging than
the CRT group but without statistical significance. Chapman et
al (30) compared the
neoadjuvant rectal score between the two treatment modalities and
showed that TNT is superior to the standard CRT in tumor
downstaging between clinical and pathologic stage.
Furthermore, organ preservation has been advocated
with the elevated rates of complete response reached with TNT.
Patients in the OPRA trial were randomized into neoadjuvant
chemotherapy before or after chemoradiation, and then patients with
complete or near-complete response were offered watchful waiting.
It showed that patients receiving upfront chemoradiation followed
by neoadjuvant chemotherapy resulted in a significantly superior
rates of organ preservation (31).
Currently, a clinical trial is the assessing the
efficacy and toxicity of short-course radiation concurrently with
5-fluorouracil infusion for the treatment of LARC (NCT04370418)
(32). Moreover, Zhang et al
(33) are evaluating the efficacy
and safety of dose escalation of short-course radiotherapy, from 25
to 40 Gy in 5 fractions, followed by chemotherapy and surgery.
Additionally, immune checkpoint inhibitors have been gaining a
great deal of attention lately. The VOLTAGE trial is assessing
nivolumab (anti-programmed death-1) monotherapy after
chemoradiation followed by surgery in LARC patients. The
preliminary results showed a promising 30 and 60% pCR rates in
microsatellite stable and unstable patients, respectively (34). Also, the DUREC trial is assessing
the addition of durvalumab (anti-programmed death-ligand 1) to
induction chemotherapy and short-course radiotherapy or long-course
chemoradiation followed by surgery (35). Moreover, in a phase II trial,
avelumab (anti-programmed death-ligand 1) was combined with
neoadjuvant mFOLFOX after short-course radiotherapy followed by
surgery. The preliminary results showed a promising 25% pCR rate
and 50% major response rate (36).
There are several limitations facing this study.
First, this is a retrospective chart review with a small sample
size, therefore more patients are needed with extended follow up
periods to compare 5-year DFS and OS. In addition, there is some
inhomogeneity within the TNT group as some patients received
chemotherapy before radiotherapy unlike others who received
radiotherapy before chemotherapy. In addition, the 2 groups were
different in terms of comorbidity index which makes the comparison
challenging. Moreover, the short median follow-up period for the
TNT group is due to the novelty of this modality which has only
been used recently.
In conclusion, our data show that TNT is superior to
CRT in chemotherapy compliance. In addition, the pCR and tumor
downstaging rates in the TNT group were numerically higher than the
CRT group. No difference was noted in the 2-year DFS rates between
the two treatment modalities. Finally, more studies are needed to
truly compare the disease-free and OS rates between the two
treatment modalities.
Acknowledgements
This abstract was presented at the European Society
of Medical Oncology ESMO 22nd World Congress on Gastrointestinal
Cancer, which was held virtually between July 1st and 4th, 2020,
and was published as Abstract no. P-134 in Annals of Oncology.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to the IRB
restrictions but are available from the corresponding author on
reasonable request.
Authors' contributions
AS conceived and designed the study. MAD and MC
provided administrative support. AS provided study materials or
patients. ZEH, YH, YB and MK collected and assembled data. ZEH, YH,
YB, MK, DM, ST, MAD, MC and AS analyzed and interpreted data. All
authors wrote the manuscript. AS and MC confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki (as revised in 2013). The study was
approved via expedited review by the Biomedical Institutional
Review Board Committee of the American University of Beirut Medical
Center (IRB ID, BIO-2019-0024; Beirut, Lebanon). In this
retrospective study, the IRB previously granted a waiver of the
requirement to obtain informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sauer R, Becker H, Hohenberger W, Rödel C,
Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF,
et al: Preoperative vs. postoperative chemoradiotherapy for rectal
cancer. N Engl J Med. 351:1731–1740. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ruo L and Guillem JG: Major 20th-century
advancements in the management of rectal cancer. Dis Colon Rectum.
42:563–578. 1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Maas M, Nelemans PJ, Valentini V, Crane
CH, Capirci C, Rödel C, Nash GM, Kuo LJ, Glynne-Jones R,
García-Aguilar J, et al: Adjuvant chemotherapy in rectal cancer:
Defining subgroups who may benefit after neoadjuvant chemoradiation
and resection: A pooled analysis of 3,313 patients. Int J Cancer.
137:212–220. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Biagi JJ, Raphael MJ, Mackillop WJ, Kong
W, King WD and Booth CM: Association between time to initiation of
adjuvant chemotherapy and survival in colorectal cancer: A
systematic review and meta-analysis. JAMA. 305:2335–2342.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Breugom AJ, Swets M, Bosset JF, Collette
L, Sainato A, Cionini L, Glynne-Jones R, Counsell N, Bastiaannet E,
van den Broek CB, et al: Adjuvant chemotherapy after preoperative
(chemo)radiotherapy and surgery for patients with rectal cancer: A
systematic review and meta-analysis of individual patient data.
Lancet Oncol. 16:200–207. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bujko K, Glimelius B, Valentini V,
Michalski W and Spalek M: Postoperative chemotherapy in patients
with rectal cancer receiving preoperative radio(chemo)therapy: A
meta-analysis of randomized trials comparing surgery ± a
fluoropyrimidine and surgery + a fluoropyrimidine ± oxaliplatin.
Eur J Surg Oncol. 41:713–723. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
van Gijn W, Marijnen CA, Nagtegaal ID,
Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius
B and van de Velde CJ: Dutch Colorectal Cancer Group. Preoperative
radiotherapy combined with total mesorectal excision for resectable
rectal cancer: 12-year follow-up of the multicentre, randomised
controlled TME trial. Lancet Oncol. 12:575–582. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kapiteijn E, Marijnen CA, Nagtegaal ID,
Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B,
van Krieken JH, et al: Preoperative radiotherapy combined with
total mesorectal excision for resectable rectal cancer. N Engl J
Med. 345:638–646. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hartley A, Ho KF, McConkey C and Geh JI:
Pathological complete response following pre-operative
chemoradiotherapy in rectal cancer: Analysis of phase II/III
trials. Br J Radiol. 78:934–938. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cotte E, Passot G, Decullier E, Maurice C,
Glehen O, François Y, Lorchel F, Chapet O and Gerard JP: Pathologic
response, when increased by longer interval, is a marker but not
the cause of good prognosis in rectal cancer: 17-year follow-up of
the Lyon R90-01 randomized trial. Int J Radiat Oncol Biol Phys.
94:544–553. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Park IJ, You YN, Agarwal A, Skibber JM,
Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH, et
al: Neoadjuvant treatment response as an early response indicator
for patients with rectal cancer. J Clin Oncol. 30:1770–1776.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pretzsch E, Bösch F, Neumann J, Ganschow
P, Bazhin A, Guba M, Werner J and Angele M: Mechanisms of
metastasis in colorectal cancer and metastatic organotropism:
Hematogenous vs. peritoneal spread. J Oncol.
2019(7407190)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sauer R, Liersch T, Merkel S, Fietkau R,
Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann
H, et al: Preoperative vs. postoperative chemoradiotherapy for
locally advanced rectal cancer: Results of the German
CAO/ARO/AIO-94 randomized phase III trial after a median follow-up
of 11 years. J Clin Oncol. 30:1926–1933. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hospers G, Bahadoer RR, Dijkstra EA, Etten
BV, Marijnen C, Putter H, Meershoek-Klein Kranenbarg E, Roodvoets
AG, Nagtegaal ID, Regina GH Beets-Tan, et al: Short-course
radiotherapy followed by chemotherapy before TME in locally
advanced rectal cancer: The randomized RAPIDO trial. J Clin Oncol.
38 (Suppl 15)(S4006)2020.
|
|
15
|
Bahadoer RR, Dijkstra EA, van Etten B,
Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID,
Beets-Tan RGH, Blomqvist LK, et al: Short-course radiotherapy
followed by chemotherapy before total mesorectal excision (TME) vs.
preoperative chemoradiotherapy, TME, and optional adjuvant
chemotherapy in locally advanced rectal cancer (RAPIDO): A
randomised, open-label, phase 3 trial. Lancet Oncol. 22:29–42.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Becker K, Mueller JD, Schulmacher C, Ott
K, Fink U, Busch R, Böttcher K, Siewert JR and Höfler H:
Histomorphology and grading of regression in gastric carcinoma
treated with neoadjuvant chemotherapy. Cancer. 98:1521–1530.
2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Delibegovic S: Introduction to total
mesorectal excision. Med Arch. 71:434–438. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bosset JF, Calais G, Mineur L, Maingon P,
Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC,
Bolla M, et al: Fluorouracil-based adjuvant chemotherapy after
preoperative chemoradiotherapy in rectal cancer: Long-term results
of the EORTC 22921 randomised study. Lancet Oncol. 15:184–190.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sainato A, Cernusco Luna Nunzia V,
Valentini V, De Paoli A, Maurizi ER, Lupattelli M, Aristei C,
Vidali C, Conti M, Galardi A, et al: No benefit of adjuvant
Fluorouracil Leucovorin chemotherapy after neoadjuvant
chemoradiotherapy in locally advanced cancer of the rectum (LARC):
Long term results of a randomized trial (I-CNR-RT). Radiother
Oncol. 113:223–229. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schmoll HJ, Haustermans K, Price TJ,
Nordlinger B, Hofheinz R, Daisne JF, Janssens J, Brenner B, Schmidt
P, Reinel H, et al: Preoperative chemoradiotherapy and
postoperative chemotherapy with capecitabine +/-oxaliplatin in
locally advanced rectal cancer: Final results of PETACC-6. J Clin
Oncol. 36 (Suppl 15)(S3500)2018.
|
|
21
|
Rödel C, Graeven U, Fietkau R, Hohenberger
W, Hothorn T, Arnold D, Hofheinz RD, Ghadimi M, Wolff HA,
Lang-Welzenbach M, et al: Oxaliplatin added to fluorouracil-based
preoperative chemoradiotherapy and postoperative chemotherapy of
locally advanced rectal cancer (the German CAO/ARO/AIO-04 study):
Final results of the multicentre, open-label, randomised, phase 3
trial. Lancet Oncol. 16:979–989. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mari GM, Maggioni D, Crippa J, Costanzi
ATM, Scotti MA, Giardini V, Garancini M, Cocozza E, Borroni G,
Benzoni I, et al: Compliance to adjuvant chemotherapy of patients
who underwent surgery for rectal cancer: Report from a
Multi-institutional Research Network. World J Surg. 43:2544–2551.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Marco MR, Zhou L, Patil S, Marcet JE,
Varma MG, Oommen S, Cataldo PA, Hunt SR, Kumar A, Herzig DO, et al:
Consolidation mFOLFOX6 chemotherapy after chemoradiotherapy
improves survival in patients with locally advanced rectal cancer:
Final results of a multicenter phase II trial. Dis Colon Rectum.
61:1146–1155. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Garcia-Aguilar J, Chow OS, Smith DD,
Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides
T, Hunt SR, et al: Effect of adding mFOLFOX6 after neoadjuvant
chemoradiation in locally advanced rectal cancer: A multicentre,
phase 2 trial. Lancet Oncol. 16:957–966. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ngan SY, Burmeister B, Fisher RJ, Solomon
M, Goldstein D, Joseph D, Ackland SP, Schache D, McClure B,
McLachlan SA, et al: Randomized trial of short-course radiotherapy
vs. long-course chemoradiation comparing rates of local recurrence
in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology
Group trial 01.04. J Clin Oncol. 30:3827–3833. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Erlandsson J, Holm T, Pettersson D,
Berglund Å, Cedermark B, Radu C, Johansson H, Machado M, Hjern F,
Hallböök O, et al: Optimal fractionation of preoperative
radiotherapy and timing to surgery for rectal cancer (Stockholm
III): A multicentre, randomised, non-blinded, phase 3,
non-inferiority trial. Lancet Oncol. 18:336–346. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ciseł B, Pietrzak L, Michalski W, Wyrwicz
L, Rutkowski A, Kosakowska E, Cencelewicz A, Spałek M, Polkowski W,
Jankiewicz M, et al: Long-course preoperative chemoradiation vs.
5x5 Gy and consolidation chemotherapy for clinical T4 and fixed
clinical T3 rectal cancer: Long-term results of the randomized
Polish II study. Ann Oncol. 30:1298–1303. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fokas E, Allgäuer M, Polat B, Klautke G,
Grabenbauer GG, Fietkau R, Kuhnt T, Staib L, Brunner T, Grosu AL,
et al: Randomized phase II trial of chemoradiotherapy plus
induction or consolidation chemotherapy as total neoadjuvant
therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin
Oncol. 37:3212–3222. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Manthravadi S, Sun W, Saeed A, Baranda JC
and Kasi A: Total neoadjuvant therapy compared with standard
therapy in locally advanced rectal cancer: A systematic review and
meta-analysis. J Clin Oncol. 37 (Suppl 4)(S709)2019.
|
|
30
|
Chapman W Jr, Roxburgh C, Makhdoom B, Roy
A, Youssef FF, Brady P, Olsen J, Kim H, Pedersen K, Mutch H, et al:
Rectal cancer downstaging is significantly improved with different
regimens of total neoadjuvant therapy. Int J Radiation Oncol Biol
Phys. 102 (Suppl):S65–S66. 2018.
|
|
31
|
Garcia-Aguilar J, Patil S, Kim JK, Yuval
JB, Thompson H, Verheij F, Lee M and Saltz LB: on behalf of the
OPRA Consortium. Preliminary results of the organ preservation of
rectal adenocarcinoma (OPRA) trial. J Clin Oncol. 38 (Suppl
15)(S4008)2020.
|
|
32
|
Shui L, Yang X, Li J, Yi C, Sun Q and Zhu
H: Gut microbiome as a potential factor for modulating resistance
to cancer immunotherapy. Front Immunol. 10(2989)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang MX, Li XB, Guan BJ, Guan GX, Lin XY,
Wu XD, Chi P and Xu BH: Dose escalation of preoperative
short-course radiotherapy followed by neoadjuvant chemotherapy in
locally advanced rectal cancer: Protocol for an open-label,
single-centre, phase I clinical trial. BMJ Open.
9(e025944)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yuki S, Bando H, Tsukada Y, Inamori K,
Komatsu Y, Homma S, Uemura M, Kato T, Kotani D, Fukuoka S, et al:
Short-term results of VOLTAGE-A: Nivolumab monotherapy and
subsequent radical surgery following preoperative chemoradiotherapy
in patients with microsatellite stable and microsatellite
instability-high locally advanced rectal cancer. J Clin Oncol. 38
(Suppl 15)(4100)2020.
|
|
35
|
Capdevila J, Declara IM, Martinez MCR,
Maurel J, Hernando J, Alonso V, Graña Suárez B, Plazas JG, Losa F,
Vera R, et al: Phase II study of durvalumab plus total neoadjuvant
therapy (TNT) in locally advanced rectal cancer: The GEMCAD-1703
DUREC trial. J Clin Oncol. 38 (Suppl 15)(TPS4122)2020.
|
|
36
|
Shamseddine A, Zeidan Y, Khalifeh IM,
Kattan JG, Turfa R, Mukherji D, Naji Temraz S, Jamali F, Hani Shaib
Y, Soweid A, et al: Short-course radiation followed by mFOLFOX-6
plus avelumab for locally advanced rectal adenocarcinoma. J Clin
Oncol. 38 (Suppl 4)(S139)2020.PubMed/NCBI View Article : Google Scholar
|