Introduction
Although squamous cell carcinoma (SCC) of the head
and neck (HNSCC) is predominantly a locoregional disease, the
incidence of distant metastasis in high-risk patients (≥3 lymph
node metastases, bilateral lymph node metastases, lymph nodes
metastases >6 cm, low jugular lymph node metastases,
locoregional tumor recurrence, or second primary tumor) is
relatively high, ranging from 8.9 to 23.8% (1-4).
Previously, distant metastases from HNSCC were mainly considered to
be lung metastases, as the lung is the most frequent site,
accounting for 59% of all distant metastases (5,6). Bone
metastases (BM) from HNSCC have attracted little attention, as
their incidence was reported to be low (1.3%) in the literature
(7), and BM from HNSCC were
considered to occur as part of widespread metastatic disease
(6). However, the recent increase
in the availability of fluorodeoxyglucose-positron emission
tomography for HNSCC staging has increased the incidence of
detection of clinically relevant BM from HNSCC to 3.4-4.8%
(8-10).
Conversely, data regarding the prognosis after BM development are
limited. Only a few studies are available, which have reported
dismal prognosis, with a median overall survival (OS) of 6.0-6.6
months (8,11), whereas a full investigation of
variable prognostic factors has not been performed to date, to the
best of our knowledge. Therefore, the purpose of the present study
was to identify clinical factors predicting longer survival in the
largest cohort of patients with BM from HNSCC to date.
Materials and methods
Patients
The present study was approved by our Institutional
Review Board (Osaka University Hospital Interventional and
Observational Studies Review Committee; reference no. 19341). The
need for informed consent was waived due to the retrospective
nature of this study. The data of 518 otolaryngology patients with
suspected or confirmed diagnosis of BM were collected from the
hospital and radiology information system databases of two
university hospitals (Kindai University Hospital and Osaka
University Hospital), by using the search terms ‘bone metastasis’
as the disease name, ‘from January 2010 to December 2020’ as the
date of diagnosis, and ‘otolaryngology’ as the department. After
reviewing the individual electronic charts, a total of 108 Japanese
patients with BM from HNSCC were identified according to the
following criteria: i) Histological diagnosis of the primary tumor
as SCC, ii) histological diagnosis of the bone lesion as SCC, or
iii) diagnosis of BM on imaging. The exclusion criteria were as
follows: i) Presence of other synchronous malignancies that could
develop distant metastases and ii) age <20 years. After
excluding patients lost to follow-up, a total of 97 patients were
finally included.
Definition of variables affecting
OS
The following possible predictive factors for longer
OS were selected by reviewing the literature describing the
prognostic factors for recurrent and/or metastatic HNSCC as
follows: Good performance status (PS) (0-1) (12-15),
administration of systemic chemotherapy (6,11,12),
less prominent weight loss (<10%) (13,14),
bone-exclusive metastasis (absence of metastases to other organs)
(10,16), distant metastasis only (locoregional
control in patients with metachronous BM) (17,18),
positive human papillomavirus (HPV) status in oropharyngeal SCC
(19-21),
nasopharyngeal primary site (22),
poor tumor cell differentiation (13), and a longer time interval from the
primary diagnosis (>19 months) (18).
Additional possible predictive factors for longer OS
were selected by reviewing the literature reporting prognostic
factors for BM from other common type of cancer, including lung,
breast, prostate, esophageal, gastrointestinal, colorectal and
pancreatic cancer. These included single BM (23-28),
good PS (23,25,26,29),
systemic chemotherapy (29-31),
absence of skeletal-related events (SREs) (23,32),
younger age (<61 years) (33),
distribution of metastatic sites confined to the vicinity of the
primary tumor (24), lower serum
alkaline phosphatase (ALP) level (26), a longer time interval from the
primary diagnosis (31), and
osteoblastic morphology on CT images (34).
Data acquisition
Data on the baseline characteristics of the patients
and clinical characteristics of BM were collected. Baseline
characteristics included the patient's sex, age, primary site,
histological differentiation of the primary tumor and the HPV
status of oropharyngeal SCC. Clinical characteristics of BM
included the time interval from primary diagnosis to BM, the
disease extent in patients with metachronous BM (distant metastasis
only or distant metastasis and locoregional disease), the presence
of distant metastasis to other organs, number of BM, the BM sites,
morphological patterns on CT, the PS at the time of BM development,
SREs at the time of developing BMs, and administration of systemic
chemotherapy for BM. A positive HPV status was determined by
positive expression of p16 on immunohistochemistry, defined as
strong and diffuse nuclear and cytoplasmic staining in ≥70% of
tumor cells (35). Metachronous
metastasis was defined as BM found at >60 days from the time of
primary diagnosis. The BM sites were divided into five areas: Above
the clavicle (craniofacial bones and cervical spine), shoulder and
thorax (clavicle, scapula, sternum and ribs), thoracolumbar spine,
pelvis (ilium, ischium, pubis, hip and sacrum-coccyx), and the
extremities. Morphological patterns on CT images were evaluated
independently by two radiologists and classified into osteolytic,
intertrabecular, mixed and osteoblastic types. Discrepancies
between the assessments of the two radiologists were resolved
through consensus. PS was classified according to the Eastern
Cooperative Oncology Group criteria, and a good PS was defined as 0
and 1. SREs were defined as pathological fractures, spinal cord
compression, hypercalcemia, or the requirement for radiation
therapy or surgery for symptomatic BM (36). OS was defined from the date of BM
diagnosis to the date of death from any cause or the end of data
collection (December 31, 2020). As regards body weight loss and
serum ALP level, sufficient data for statistical analyses were not
available.
Statistical analysis
The Kaplan-Meier method was used to estimate
cumulative survival, depict survival curves, and calculate the
median 1- and 2-year OS rates. Univariate and multivariate analyses
of the associations between the variables and OS were conducted
using a log-rank test and a Cox proportional hazards model,
respectively. Factors with P<0.1 on univariate testing were
evaluated using multivariate analysis. The reason for adopting the
high threshold of P<0.1 in the univariate screening was to
eliminate the influence of potential confounders. P<0.05 on the
multivariate analysis was considered to indicate a statistically
significant difference. All statistical analyses were performed
using the IBM SPSS Statistics software, version 24 (IBM Corp.).
Results
Baseline characteristics of patients
and clinical characteristics of BM
The baseline characteristics of the patients are
presented in Table I. The patients
included 80 men (80.5%) and 17 women (19.5%), with a mean age of
63.8 years (range: 21-87 years). The primary sites were the
nasopharynx in 19 patients (19.6%), oropharynx in 16 (16.5%), oral
cavity in 20 (20.6%), hypopharynx in 26 (26.8%), larynx in 9 (9.3%)
and other sites in 7 patients (7.2%). The histological
differentiation was high in 22 patients (22.7%), moderate in 23
(23.7%), poor in 30 (30.9%) and undifferentiated in 1 patient
(1.0%), whereas data on differentiation were not available (N/A) in
21 patients (21.7%). Of the 16 patients with oropharyngeal SCC, the
HPV status was positive in 7 and negative in 9 patients. The
clinical characteristics of BM are summarized in Table II. Regarding the time interval from
the primary diagnosis to BM development, synchronous BM were
observed in 36 patients (37.1%) and metachronous BM in 61 patients
(62.9%). Of the 61 patients with metachronous BM, 31 (32.0%)
developed BM 1 year after the initial diagnosis. As regards the
disease extent of patients with metachronous BM, 28 patients
(45.9%) had only distant metastases (locoregional disease was
controlled) and 33 patients (54.1%) had both distant metastases and
locoregional disease. A total of 43 patients (44.3%) presented with
bone-exclusive metastasis, whereas 54 patients (55.7%) had distant
metastasis to other organs, including the lung in 38, liver in 20,
and other sites in 41 patients. BM were single in 40 patients
(41.2%) and multiple in 57 patients (58.8%). BM were located above
the clavicle (cervical spine and craniomaxillofacial bones) in 28
patients (28.9%), the shoulder and thorax in 39 (40.2%), the
thoracolumbar spine in 62 (63.9%), the pelvis in 39 (40.2%), and
the extremities in 17 patients (17.5%). In 13 of the 28 patients
with BM above the clavicle, the BM were confined to the area above
the clavicle. The morphological types on CT imaging were osteolytic
in 45 patients (46.4%), intertrabecular in 31 (32.0%), mixed in 7
(7.2%) and osteoblastic in 14 patients (14.4%). The PS was 0 in 25
patients (25.8%), 1 in 37 (38.1%), 2 in 8 (8.2%), 3 in 13 (13.4%),
4 in 3 (3.1%), and N/A in 11 patients (11.4%). SREs were not
observed in 67 patients (69.1%) and occurred in 30 patients
(30.9%). SREs included pathological fracture in 2, neurological
symptoms in 5, hypercalcemia in 7, required radiotherapy in 16 and
required surgery in 5 patients. Systemic chemotherapy was
administered to 62 patients (63.9%).
 | Table IBaseline characteristics of patients
with BM from HNSCC (n=97). |
Table I
Baseline characteristics of patients
with BM from HNSCC (n=97).
|
Characteristics | No. (%) |
|---|
| Sex | |
|
Male | 80 (80.5) |
|
Female | 17 (19.5) |
| Age, years [mean
(range)] | 63.8 (21-87) |
|
<60 | 29 (29.9) |
|
≥60 | 68 (70.1) |
| Primary site | |
|
Nasopharynx | 19 (19.6) |
|
Oropharynx | 16 (16.5) |
|
Oral
cavity | 20 (20.6) |
|
Hypopharynx | 26 (26.8) |
|
Larynx | 9 (9.3) |
|
Sinonasal
cavity | 6 (6.2) |
|
Unknown | 1 (1.0) |
| Histological
differentiation | |
|
High | 22 (22.7) |
|
Moderate | 23 (23.7) |
|
Poor | 30 (30.9) |
|
Undifferentiated | 1 (1.0) |
|
Not
available | 21 (21.7) |
| Human papilloma
virus status (oropharyngeal SCC, n=16) | |
|
Positive | 7 |
|
Negative | 9 |
| BM, bone
metastasis; HNSCC, head and neck squamous cell carcinoma. | |
 | Table IIClinical characteristics of BM
(n=97). |
Table II
Clinical characteristics of BM
(n=97).
|
Characteristics | No. (%) |
|---|
| Time interval from
primary diagnosis | |
|
Synchronous
metastases | 36 (37.1) |
|
2 months to
1 year | 30 (30.9) |
|
≥1 year | 31 (32.0) |
| Disease extent
(metachronous metastases, n=61) | |
|
Only distant
metastases | 28 (45.9) |
|
Distant
metastases and locoregional disease | 33 (54.1) |
| Metastases to other
organs | |
|
None
(bone-exclusive metastasis) | 43 (44.3) |
|
Present | 54 (55.7) |
| Number of BM | |
|
Single | 40 (41.2) |
|
Multiple | 57 (58.8) |
| BM sites | |
|
Above the
clavicle/only above the clavicle | 28 (28.9)/13
(13.4) |
|
Shoulder and
thorax | 39 (40.2) |
|
Thoracolumbar
spine | 62 (63.9) |
|
Pelvis | 39 (40.2) |
|
Extremities | 17 (17.5) |
| CT morphology | |
|
Osteolytic
type | 45 (46.4) |
|
Intertrabecular
type | 31 (32.0) |
|
Mixed
type | 7 (7.2) |
|
Osteoblastic
type | 14 (14.4) |
| Performance
status | |
|
0 | 25 (25.8) |
|
1 | 37 (38.1) |
|
2 | 8 (8.2) |
|
3 | 13 (13.4) |
|
4 | 3 (3.1) |
|
Not
available | 11 (11.4) |
| Skeletal-related
events | |
|
None | 67 (69.1) |
|
Pathological
fracture | 2 (2.1) |
|
Neurological
symptoms | 5 (5.2) |
|
Hypercalcemia | 7 (7.2) |
|
Requirement
for radiotherapy or surgery | 21 (21.6) |
| Systemic
chemotherapy for BM | |
|
Received | 62 (63.9) |
|
Not
received | 35 (36.1) |
Overall survival and prognostic
factors
The mean and median follow-up periods were 21.8 and
17 months, respectively (range, 1-119 months); 85 patients (85.6%)
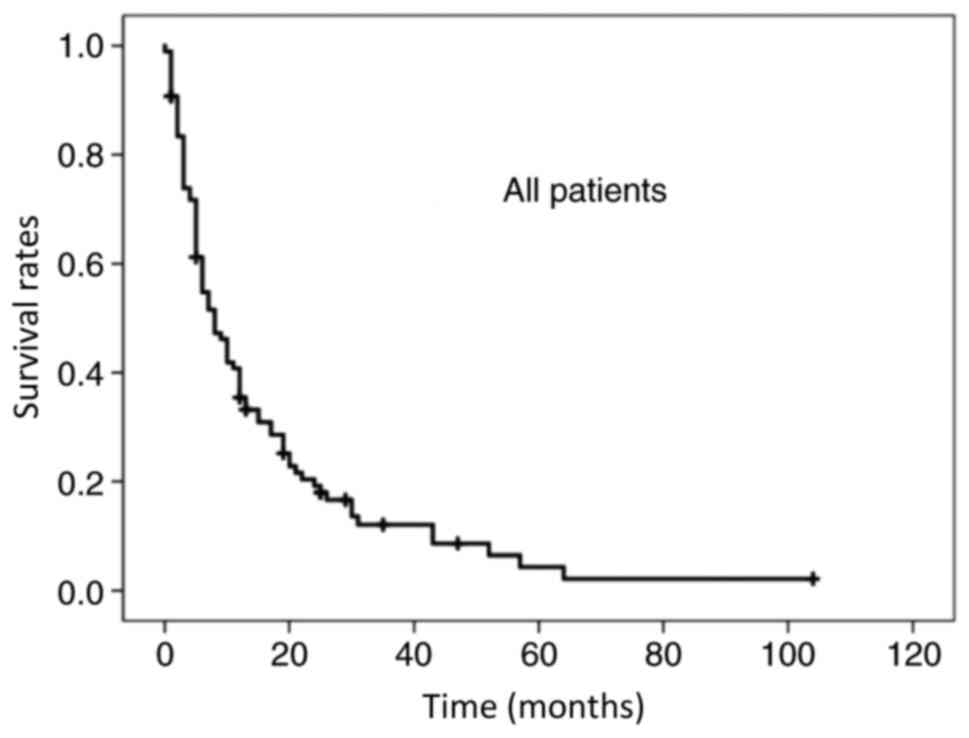
succumbed to the disease. The median OS time was 7 months, and the
1- and 2-year OS rates for all patients were 35.4 and 19.2%,
respectively (Fig. 1). Tables III and IV present the univariate and multivariate
analysis results for the possible predictive factors of longer OS.
In the univariate analyses, the following factors had P<0.05 or
P<0.1: Nasopharynx as the primary site, bone-exclusive
metastasis, single BM, osteoblastic morphology, a good PS (0-1) and
systemic chemotherapy. After multivariate analyses, single BM
[hazard ratio (HR)=0.543; 95% confidence interval (CI):
0.330-0.894; P=0.016], a good PS (HR=0.458; 95% CI: 0.267-0.787;
P=0.005), and administration of systemic chemotherapy (HR=0.547;
95% CI: 0.321-0.93; P=0.026) were independent predictors of longer
OS. The overall median OS time for patients with single BM was 10
months, and the 1- and 2-year OS rates were 41.1 and 24.1%,
respectively. For patients with a good PS, the overall median OS
was 10 months, and the 1- and 2-year OS rates were 47.8 and 27.3%,
respectively. For patients receiving systemic chemotherapy, the
overall median OS was 10.5 months, and the 1- and 2-year OS rates
were 42.8 and 27.1%, respectively. Due to the limited influence of
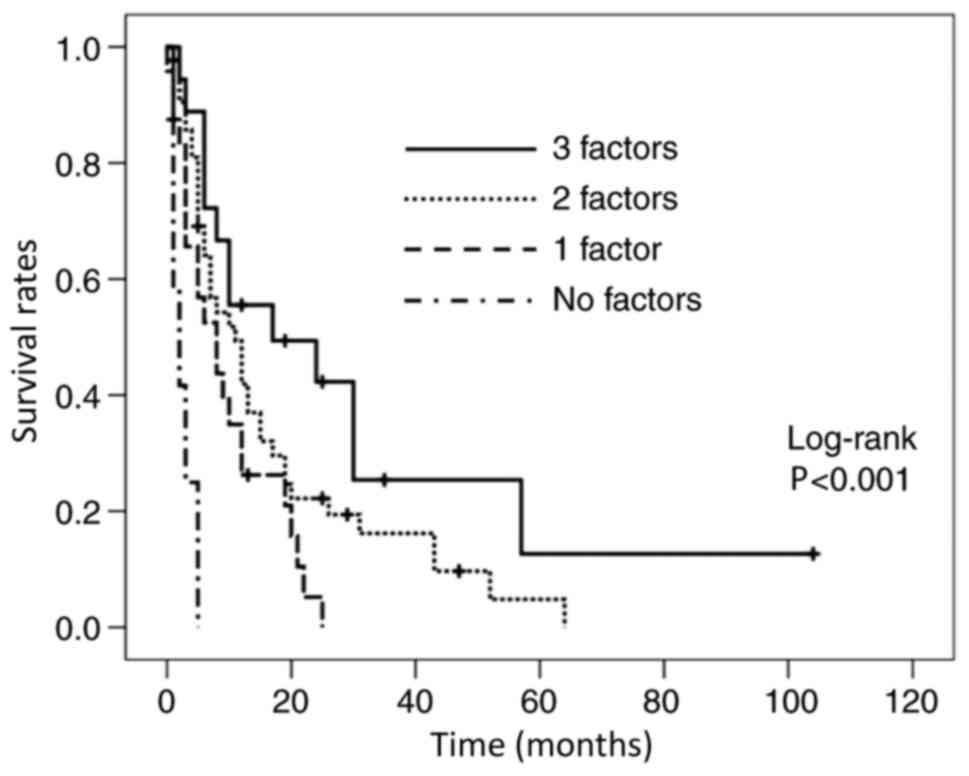
each independent predictor, the patients were stratified according
to the number of the predictors and Kaplan-Meier curves were drawn
(Fig. 2). The median OS in patients
with all three predictive factors (n=18) was 14.5 months, in those
with two factors (n=43) it was 10 months, in those with one factor
(n=24) it was 7 months, and in those with no factor (n=12) the OS
was 2 months (Table V).
 | Table IIIUnivariate analysis of possible
predictive factors for longer OS. |
Table III
Univariate analysis of possible
predictive factors for longer OS.
| Factors | Number (%) | Median OS
(months) | 1-year OS rate
(%) | 2-year OS rate
(%) | P-value |
|---|
| Age, years | | | | | 0.106 |
|
<60 | 29 (29.9) | 11 | 44.8 | 29.9 | |
|
≥60 | 68 (70.1) | 6 | 31.3 | 14.3 | |
| Primary site | | | | | 0.037 |
|
Nasopharynx | 19 (19.6) | 10 | 42.1 | 42.1 | |
|
Others | 78 (80.4) | 6.5 | 33.8 | 12.7 | |
| Histological
differentiation | | | | | 0.710 |
|
High to
moderate | 45 (59.2) | 8 | 39.2 | 13.8 | |
|
Poor to
undifferentiated | 31 (40.8) | 6 | 26.7 | 16.7 | |
| HPV status | | | | | 0.851 |
|
Positive HPV
in oropharyngeal cancer | 7 (7.2) | 10 | 17.1 | 0.0 | |
|
Others | 90 (92.8) | 7 | 36.5 | 20.4 | |
| Time interval from
primary diagnosis | | | | | 0.258 |
|
≥1 year | 31 (32.0) | 7 | 42.2 | 19.8 | |
|
<1
year | 66 (68.0) | 7.5 | 32.4 | 18.8 | |
| Disease extent | | | | | 0.176 |
|
Only distant
metastases | 28 (28.9) | 11 | 44.6 | 24.3 | |
|
Distant
metastases and locoregional disease | 69 (71.1) | 6 | 31.7 | 17.1 | |
| Metastases to other
organs | | | | | 0.001 |
|
None
(bone-exclusive metastasis) | 43 (44.3) | 10 | 45.3 | 35.2 | |
|
Present | 54 (55.7) | 6 | 27.4 | 5.1 | |
| Number of BM | | | | | 0.073 |
|
Single | 40 (41.2) | 10 | 41.1 | 24.1 | |
|
Multiple | 57 (58.8) | 6 | 31.4 | 15.8 | |
| BM sites | | | | | 0.532 |
|
Only above
the clavicle | 13 (13.4) | 10 | 46.2 | 27.7 | |
|
Others | 84 (86.6) | 7 | 33.7 | 17.9 | |
| CT morphology of
BM | | | | | 0.064 |
|
Osteoblastic
type | 14 (14.4) | 16.5 | 54.5 | 46.8 | |
|
Others | 83 (85.6) | 6 | 32.2 | 14.4 | |
| Performance
status | | | | | <0.001 |
|
0-1 | 62 (72.1) | 10 | 47.8 | 27.3 | |
|
2-4 | 24 (27.9) | 4.5 | 8.3 | 4.2 | |
| Skeletal-related
events | | | | | 0.273 |
|
Present | 30 (30.9) | 5.5 | 29.6 | 14.8 | |
|
None | 67 (69.1) | 10 | 37.9 | 21.1 | |
| Systemic
chemotherapy for BM | | | | | <0.001 |
|
Received | 62 (63.9) | 10.5 | 42.8 | 27.1 | |
|
Not
received | 35 (36.1) | 3 | 21.6 | 3.6 | |
 | Table IVMultivariate analysis using a Cox
proportional hazards model. |
Table IV
Multivariate analysis using a Cox
proportional hazards model.
| | 95% CI |
|---|
| Factors | P-value | Hazard ratio | Upper limit | Lower limit |
|---|
| Primary site | 0.118 | 0.617 | 1.131 | 0.267 |
| Metastases to other
organs | 0.365 | 0.781 | 1.333 | 0.458 |
| Number of BM | 0.016 | 0.543 | 0.894 | 0.330 |
| CT morphology of
BM | 0.120 | 0.579 | 1.154 | 0.290 |
| Performance
status | 0.005 | 0.458 | 0.787 | 0.267 |
| Systemic
chemotherapy for BM | 0.026 | 0.547 | 0.931 | 0.321 |
 | Table VStratification according to the
number of predictive factors of longer OS. |
Table V
Stratification according to the
number of predictive factors of longer OS.
| Number of
factors | Number of patients
(%) | Median OS
(months) | 1-year OS rate
(%) | 2-year OS rate
(%) |
|---|
| 3 | 18(19) | 14.5 | 55.6 | 42.3 |
| 2 | 43(44) | 10 | 41.9 | 22.2 |
| 1 | 24(25) | 7 | 26.3 | 5.3 |
| None | 12(12) | 2 | 0 | 0 |
Discussion
In addition to the dismal prognosis in patients with
BM from HNSCC, BM are clinically important because they represent a
major cause of morbidities, such as severe pain, pathological
fractures, spinal cord compression and hypercalcemia. Therefore,
elucidating the prognostic characteristics of BM from HNSCC is
helpful for appropriate management. Data of 10 years from two
reference centers were reviewed to comprise the largest patient
cohort of BM from HNSCC to date, which allowed for multivariate
analyses. Consequently, three independent factors for longer OS,
including single BM, a good PS and systemic chemotherapy, were
identified.
Comparable to the results of the present study,
single or few BM have been reported as predictors of longer OS in
patients with BM from several common types of cancer, including
lung, breast, prostate and gastrointestinal cancers (23-28).
A possible explanation is that the bone is not a vital organ, which
indicates that a relatively lower tumor volume (single or only a
few BM) may not affect the general condition of the patients.
Another possible explanation is that cases with a single BM without
metastases to other organs can be classed as an oligometastatic
state, which is an intermediate state between the purely localized
and widely metastatic state. The oligometastatic state, termed as a
limited number of metastases restricted to a single organ, is
increasingly considered to be associated with a better prognosis in
common types of cancer, such as colorectal or prostate cancer
(37,38). Although the concept of
oligometastases in HNSCC is not well established, our data may
support the concept that oligometastatic HNSCC has a better
prognosis and is potentially amenable to local therapy (8,39,40).
Therefore, although further prospective analysis is needed, local
resection followed by systemic chemotherapy may be a viable
treatment option for patients with oligo BM from HNSCC.
Consistent with several previous studies indicating
that PS was a favorable prognostic factor for recurrent and
metastatic HNSCC (12-15),
the current study confirmed that PS was a predictive factor for
longer OS, even in patients with BM. PS has also been reported as a
prognostic factor in patients with BM from other common types of
cancer, including breast, lung and gastric cancers (23,25,26,29).
This is explained by the fact that a good PS is associated with a
lower risk of pulmonary infection (41), a higher tolerance to chemotherapy,
and the selection of more aggressive chemotherapy (42). A good PS may also indicate that the
BM may not be that widely disseminated so as to affect daily
activities.
Palliative therapy is the usual management strategy
for patients with HNSCC who develop distant metastases (5,6), and
systemic chemotherapy has been reported to improve OS to a certain
degree (6,11,12,17).
Compared with lung metastases and locoregional recurrence, systemic
chemotherapy was expected to contribute more to BM from HNSCC, as
the red marrow, in which BM develop, has a richer blood supply
compared with the lung and locoregional area. Although the
prognostic influence of systemic chemotherapy reached statistical
significance in the present study, it had only a limited influence,
with a median OS of 10.5 months. This result is largely consistent
with previous analyses of chemotherapy for overall recurrent and/or
metastatic HNSCC (6,11,12).
Therefore, the indication of systemic chemotherapy for BM from
HNSCC should be carefully considered by taking into account its
adverse effects. Moreover, novel therapies for BM from HNSCC are
warranted.
In accordance with the short life expectancy of
patients with distant metastatic HNSCC (6,10,11),
the median OS in all our patients was 7 months. Even in patients
with predictors for longer OS, the prognosis was still dismal, with
a median overall survival of <11 months. Therefore, the data
that were further extracted on the patient group with all the three
predictors were added, and it was found that they had a relatively
longer median OS of 14.5 months. These data appear to suggest that
prognosis is determined by multiple factors, and they may serve as
a reference for patient counseling on survival expectations.
One major change in HNSCC over the last two decades
is the increase in the number of patients with HPV-positive
oropharyngeal SCC and the confirmation of their longer survival,
resulting in the distinction of staging and treatment guidelines
for oropharyngeal SCC depending on HPV status (43,44).
Additionally, recent studies reported that HPV-positive recurrent
and/or metastatic HNSCCs, including non-oropharyngeal SCC, were
also characterized by longer survival compared with their
HPV-negative counterparts (19-21).
However, a positive HPV status was not a predictor for longer OS in
the present study, which may be explained by the small number of
patients with HPV-positive oropharyngeal SCC. Therefore, further
evaluation in a larger patient cohort, including non-oropharyngeal
SCC cases with known HPV status is needed.
The major limitation of the present study was the
inherent bias of its retrospective design. For example, the
demographic data showed varied primary locations with inhomogeneous
proportions, and the patients received various managements for BM,
including palliative radiotherapy, systemic chemotherapy and
supportive care. Additionally, several factors that may affect
survival could not be evaluated owing to the lack of data.
Therefore, based on our results, further prospective studies in
selected cohorts are warranted. Another limitation is that
histological confirmation of BM was performed in only 6 cases. The
diagnosis of BM from HNSCC by imaging studies alone cannot fully
exclude other bone diseases, such as multiple myeloma or metastases
from other occult malignancies. However, histopathological
confirmation of all cases is impractical, and even unethical in
patients who are in a poor condition and eligible for palliative
treatment.
In conclusion, to the best of our knowledge, the
present study was the first to evaluate the variable potential
factors predicting longer OS in patients with BM from HNSCC. Single
BM, a good PS and administration of systemic chemotherapy were
independent factors for longer OS, but the median survival did not
exceed 11 months, whereas the selected group of patients with all
three factors had a relatively longer median OS of 14.5 months.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TS wrote the manuscript, and performed data
collection and analysis; NK designed the study and wrote the
manuscript; HD performed data analysis; HT, AA, CM, YM, KS, HO and
KI performed data collection; HI edited and critically reviewed the
manuscript for important intellectual content; NT edited and
critically reviewed the manuscript for important intellectual
content, and supervised the study. TS and NK confirm the
authenticity of the raw data. All the authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board (Osaka University Hospital Interventional and
Observational Studies Review Committee; reference no. 19341), which
waived the requirement for informed consent given the retrospective
nature of the study.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
References
|
1
|
Takes RP, Rinaldo A, Silver CE, Haigentz M
Jr, Woolgar JA, Triantafyllou A, Mondin V, Paccagnella D, Bree R,
Shaha AR, et al: Distant metastases from head and neck squamous
cell carcinoma. Part I. Basic aspects. Oral Oncol. 48:775–779.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Senft A, Yildirim G, Hoekstra OS,
Castelijns JA, Leemans CR and de Bree R: The adverse impact of
surveillance intervals on the sensitivity of FDG-PET/CT for the
detection of distant metastases in head and neck cancer patients.
Eur Arch Otorhinolaryngol. 274:1113–1120. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Peters TT, Senft A, Hoekstra OS,
Castelijns JA, Witte BI, Leemans CR and de Bree R: Pretreatment
screening on distant metastases and head and neck cancer patients:
Validation of risk factors and influence on survival. Oral Oncol.
51:267–271. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Deurvorst SE, Hoekstra OS, Castelijns JA,
Witte BI, Leemans CR and de Bree R: Clinical value of
18FDG PET/CT in screening for distant metastases in head
and neck squamous cell carcinoma. Clin Otolaryngol. 43:875–881.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haigentz M Jr, Hartl DM, Silver CE,
Langendijk JA, Strojan P, Paleri V, de Bree R, Machiels JP, Hamoir
M, Rinaldo A, et al: Distant metastases from head and neck squamous
cell carcinoma. Part III. Treatment. Oral Oncol. 48:787–793.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wiegand S, Zimmermann A, Wilhelm T and
Werner JA: Survival after distant metastasis in head and neck
cancer. Anticancer Res. 35:5499–5502. 2015.PubMed/NCBI
|
|
7
|
Pietropaoli MP, Damron TA and Vermont AI:
Bone metastases from squamous cell carcinoma of the head and neck.
J Surg Oncol. 75:136–141. 2000.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Suzuki A, Kashiwagi N, Doi H, Ishii K, Doi
K, Kitano M, Kozuka T, Hyodo T, Tsurusaki M, Yagyu Y and Nakanishi
K: Patterns of bone metastases from head and neck squamous cell
carcinoma. Auris Nasus Larynx. 47:262–267. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Al-Bulushi NK and Abouzied ME: Comparison
of 18F-FDG PET/CT scan and 99mTc-MDP bone scintigraphy in detecting
bone metastasis in head and neck tumors. Nucl Med Commun.
37:583–588. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee DH, Kim MJ, Roh JL, Kim SB, Choi SH,
Nam SY and Kim SY: Distant metastases and survival prediction in
head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg.
147:870–875. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bollig CA, Newberry CI, Galloway TL,
Zitsch RP, Hanly EK, Zhu VL, Pagedar N, Nallani R, Bur AM, Spanos
WC and Jorgensen JB: Prognostic impact of metastatic site and
pattern in patients with metastatic head and neck cancer.
Laryngoscope. 131:E1838–E1846. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Argiris A, Li Y and Forastiere A:
Prognostic factors and long-term survivorship in patients with
recurrent or metastatic carcinoma of the head and neck. Cancer.
101:2222–2229. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Recondo G, Armand JP, Tellez-Bernal E,
Domenge C, Belehradek M, Vathaire FD, Wibault P, Richard JM and
Cvitkovic E: Recurrent and/or metastatic head and neck squamous
cell carcinoma: A clinical, univariate and multivariate analysis of
response and survival with cisplatin-based chemotherapy.
Laryngoscope. 101:494–501. 1991.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Grünwald V, Chirovsky D, Cheung WY,
Bertolini F, Ahn MJ, Yang MH, Castro G, Berrocal A, Sjoquist K,
Kuyas H, et al: Global treatment patterns and outcomes among
patients with recurrent and/or metastatic head and neck squamous
cell carcinoma: Results of the GLANCE H&N study. Oral Oncol.
102(104526)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Duprez F, Berwouts D, De Neve W, Bonte K,
Boterberg T, Deron P, Huvenne W, Rottey S and Mareel M: Distant
metastases in head and neck cancer. Head Neck. 39:1733–1743.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cho H, Nishiike S, Yamamoto Y, Takenaka Y,
Nakahara S, Yasui T, Hanamoto A and Inohara H: Docetaxel,
cisplatin, and fluorouracil for patients with inoperable recurrent
or metastatic head and neck squamous cell carcinoma. Auris Nasus
Larynx. 42:396–400. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mirabile A, Miceli R, Calderone RG, Locati
L, Bossi P, Bergamini C, Granata R, Perrone F, Mariani L and
Licitra L: Prognostic factors in recurrent or metastatic squamous
cell carcinoma of the head and neck. Head Neck. 41:1895–1902.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Argiris A, Li S, Ghebremichael M, Egloff
AM, Wang L, Forastiere AA, Burtness B and Mehra R: Prognostic
significance of human papillomavirus in recurrent or metastatic
head and neck cancer: An analysis of Eastern Cooperative Oncology
Group trials. Ann Oncol. 25:1410–1416. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vermorken JB, Psyrri A, Mesía R, Peyrade
F, Beier F, de Blas B, Celik I and Licitra L: Impact of tumor HPV
status on outcome in patients with recurrent and/or metastatic
squamous cell carcinoma of the head and neck receiving chemotherapy
with or without cetuximab: Retrospective analysis of the phase III
EXTREME trial. Ann Oncol. 25:801–807. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Deeken JF, Newkirk K, Harter KW, Marshall
MB, Banovac F, Johnson L, Wang H, Wang Y, Zhuang T, Ja AK, et al:
Effect of multimodality treatment on overall survival for patients
with metastatic or recurrent HPV-positive head and neck squamous
cell carcinoma. Head Neck. 37:630–635. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ahn MJ, Chirovsky D, Kuyas H, Auclair V,
Abounit S, Joo S, Shah R and Yang MH: Global longitudinal
assessment of treatment outcomes in recurrent/metastatic
nasopharyngeal carcinoma: GLANCE-NPC study. Future Oncol.
17:2015–2025. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang H, Zhu W, Biskup E, Yang W, Yang Z,
Wang Z, Qiu X, Zhang C and Hu G and Hu G: Incidence, risk factors
and prognostic characteristics of bone metastases and
skeletal-related events (SREs) in breast cancer patients: A
systematic review of the real world data. J Bone Oncol. 11:38–50.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Koizumi M, Yoshimoto M, Kasumi F and Ogata
E: Comparison between solitary and multiple skeletal metastatic
lesions of breast cancer patients. Ann Oncol. 14:1234–1240.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bae HM, Lee SH, Kim TM, Kim DW, Yang SC,
Wu HG, Kim YW and Heo DS: Prognostic factors for non-small cell
lung cancer with bone metastasis at the time of diagnosis. Lung
Cancer. 77:572–577. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang L and Gong Z: Clinical
characteristics and prognostic factors in bone metastases from lung
cancer. Med Sci Monit. 23:4087–4094. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Portales F, Thézenas S, Samalin E, Assenat
E, Mazard T and Ychou M: Bone metastases in gastrointestinal
cancer. Clin Exp Metastasis. 32:7–14. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yamamoto Y, Okuda Y, Kanaki T, Tanaka R,
Nagahara A, Nakai Y, Nakayama M, Kakimoto K and Nishimura K:
Clinical indicators for predicting prognosis after radium-223
administration in castration-resistant prostate cancer with bone
metastases. J Clin Oncol. 26:192–198. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Imura Y, Tateiwa D, Sugimoto N, Inoue A,
Wakamatsu T, Outani H, Tanaka T, Tamiya H, Yagi T, Naka N, et al:
Prognostic factors and skeletal-related events in patients with
bone metastasis from gastric cancer. Mol Clin Oncol.
13(31)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Imura Y, Yamamoto S, Wakamatsu T, Tanaka
T, Tamiya H, Sugimura K, Miyata H, Ishihara R, Yano M and Naka N:
Clinical features and prognostic factors in patients with
esophageal cancer with bone metastasis. Oncol Lett. 19:717–724.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Outani H, Akita H, Nakai T, Takada R,
Imura Y, Tanaka T, Tamiya H, Oshima K, Takahashi H, Ohkawa K, et
al: Clinical features and prognosis of patients with the bone
metastasis of pancreatic cancer: A single-institutional cohort
study. Pancreas. 47:e43–e46. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nørgaard M, Jensen AØ, Jacobsen JB, Cetin
K, Fryzek JP and Sørensen HT: Skeletal related events, bone
metastasis and survival of prostate cancer: A population based
cohort study in Denmark (1999 to 2007). J Urol. 184:162–167.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li X, Hu W, Sun H and Gou H: Survival
outcome and prognostic factors for colorectal cancer with
synchronous bone metastasis: A population-based study. Clin Exp
Metastasis. 38:89–95. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Santini D, Tampellini M, Vincenzi B,
Ibrahim T, Ortega C, Virzi V, Silvestris N, Berardi R, Masini C,
Calipari N, et al: Natural history of bone metastasis in colorectal
cancer: Final results of a large Italian bone metastases study. Ann
Oncol. 23:2072–2077. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C,
et al: Human papillomavirus and survival of patients with
oropharyngeal cancer. N Engl J Med. 363:24–35. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Coleman RE: Skeletal complications of
malignancy. Cancer. 80 (Suppl 8):S1588–S1594. 1997.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Huang F, Wu G and Yang K: Oligometastasis
and oligo-recurrence: More than a mirage. Radiat Oncol.
9(230)2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Weichselbaum RR and Hellman S:
Oligometastases revisited. Nat Rev Clin Oncol. 8:378–382.
2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Thomas TV, Packianathan S, Bhanat E,
Albert A, Abraham A, Gordy X, Kanakamedala M, Mehta D and
Vijayakumar S: Oligometastatic head and neck cancer: Comprehensive
review. Head Neck. 42:2194–2201. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Albergotti WG, Abberbock S, Mathews F,
Ferris RL, Johnson JT, Duvvuri U and Kim S: Oligometastatic status
as predictor of survival in metastatic human
papillomavirus-positive oropharyngeal carcinoma. Head Neck.
40:1685–1690. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Webb BJ, Dascomb K, Stenehjem E and Dean
N: Predicting risk of drug-resistant organisms in pneumonia: Moving
beyond the HCAP model. Respir Med. 109:1–10. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
La EM, Smyth EN, Talbird SE, Li L, Kaye
JA, Lin AB and Bowman L: Treatment patterns and health care
resource use in patients receiving multiple lines of therapy for
metastatic squamous cell carcinoma of the head and neck in the
United Kingdom. Eur J Cancer Care (Engl). 27(e12862)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cramer JD, Hicks KE, Rademaker AW, Patel
UA and Samant S: Validation of the eighth edition American Joint
Committee on cancer staging system for human
papillomavirus-associated oropharyngeal cancer. Head Neck.
40:457–466. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Head and Neck Cancer Study Group (HNCSG).
Monden N, Asakage T, Kiyota N, Homma A, Matsuura K, Hanai N,
Kodaira T, Zenda S, Fujii H, et al: A review of head and neck
cancer staging system in the TNM classification of malignant tumors
(eighth edition). Jpn J Clin Oncol. 49:589–595. 2019.PubMed/NCBI View Article : Google Scholar
|