Introduction
Colorectal carcinoma (CRC), a malignant tumour of
the colon and rectum, is one of the commonest types of cancer in
economically developed countries (1). The number of newly diagnosed cases of
cancer is growing worldwide each year (19.3 million in 2020) and it
is expected that it will continue to increase as the human
population grows and ages (2). CRC
is the third commonest type of cancer worldwide and poses a
significant health risk to the population (1). In ~50% of the cases of CRC, the
initial phase of therapy is successful, but the long-term survival
of the patients is influenced by metastases formation (3), especially in higher T stages.
Resection of the primary tumour is essential for radical treatment
for a chance of long-term survival. Rectal resection for cancer is
still associated with considerable morbidity. Acute leak (AL) is
probably the most serious complication, which is associated with
increased postoperative mortality, long-term consequences and
negative impact on function (4)
and oncological outcomes (5-7).
A large proportion of patients with AL (20%) end up with an
unplanned definitive stoma (8) and
increased economic costs associated with treatment for
complications and a prolonged stay at the Intensive Care Unit
should be taken into account (9,10).
Several papers analysing preoperative, intraoperative and
postoperative factors associated with the development of AL have
been published (11-13).
It can be said that being male, neoadjuvant radiotherapy and low
localisation of anastomosis are generally accepted risk factors for
AL development (14).
The stability of the extracellular matrix (ECM)
serves a pivotal role in colon cancer progression. One important
factor that regulates cancer-related events at different
tumorigenesis stages is the presence of collagens, which are the
main components of this three-dimensional network (15). As the most abundant proteins,
collagens mainly serve structural roles and contribute to
mechanical properties, organisation and the shape of tissues
(15). Today, the 28 known
collagen (COL) types are classified into four subfamilies on the
basis of their supramolecular assemblies (16). COLI, COLIII and COLV are mainly
produced by fibroblasts, while COLIV is predominantly expressed by
epithelial and endothelial cells (17,18).
In healthy tissue, the ECM is constantly undergoing remodelling to
maintain tissue integrity and function and new collagens are
synthesised to replace old collagens destined for degradation
(19). During the process of
tumorigenesis, the ECM undergoes structural changes (19). The content and distribution of
collagen is modified to further coordinate the biological
properties of cancer cells, including various gene mutations,
transcription factors, signal transduction pathways and receptors
(20). The balance between
collagen formation and degradation is closely monitored by
proteolytic enzymes called matrix metalloproteinases (MMPs). The
eight structural classes of MMPs involve a family of zinc-dependent
endopeptidases that consist of >21 human MMPs and numerous
homologues from other species. These enzymes participate in tissue
remodelling under physiological and pathological conditions by
degradation of major protein components of the ECM and basement
membranes. MMPs are upregulated in almost every type of human
cancer and can promote cancer progression by increasing cancer cell
growth, migration, invasion, metastasis and angiogenesis (21,22).
The functions of MMPs are controlled by endogenous
tissue-specific inhibitors and secreted proteins, called tissue
inhibitors of metalloproteinases (23). By binding to enzymatically active
MMPs, tissue inhibitors of metalloproteinases (TIMPs) inhibit their
proteolytic activity, which is essential for the homeostasis of the
ECM (24). It could be
hypothesized that MMPs and TIMPs may not only serve an important
role in tumour invasion and metastasis but also in carcinogenesis.
Previous studies have demonstrated that high preoperative serum or
plasma MMP-2, MMP-9 and TIMP-1 antigen levels are strong predictive
factors for poor prognosis in patients with carcinoma (25) and their determination and
identification might be useful for patients with a higher risk for
cancer recurrence (26).
The objective of the present study was to analyse
the gene expression of MMPs (MMP1-2-8-10-13), TIMPs
(TIMP1-2-4) and collagen (COL1A1 and COL3A1)
and their correlation with biochemistry variables in rectal tissue
after surgical resection of rectal adenocarcinoma.
Materials and methods
Patients and tissue specimens
All analysed samples (Table I) were obtained from consecutive
patients who underwent a laparoscopic low anterior resection with
total mesorectal excision for rectal adenocarcinoma. The
anastomosis was constructed by the double stapling method (27). Preoperative staging was performed
by either computed tomography or magnetic resonance imaging of the
abdomen and the pelvis. Patients with locally advanced tumours, T3
tumours and T4 tumours with or without involvement of the lymph
nodes received preoperative full course chemoradiotherapy (CHRT) to
downstage the tumour.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variable | [ART-] | [ART+] |
|---|
| Number of
patients | 12 | 17 |
| Age | | |
|
Range | 45-77 | 35-70 |
|
Mean ±
SEM | 60±11 | 57±11 |
| Sex | | |
|
Female | 5 | 4 |
|
Male | 7 | 13 |
| BMI (mean ±
SEM) | 27±5 | 26±4 |
| Proteins in plasma
(mean ± SEM) | | |
|
Albumin | 39±6 (g/l) | 40±4 (g/l) |
|
CRP | 5±3 (mg/l) | 4±3 (mg/l) |
|
Total | 65±9 (g/l) | 71±6 (g/l) |
| Leukocytes
(mean) |
8x109/l |
6.4x109/l |
| Collagen (mean ±
SEM) | 119±67 (µg/mg) | 174±82 (µg/mg) |
| Plasma iron (mean ±
SEM) | 13±5 (µmol/l) | 16±9 (µmol/l) |
If patients had undergone CHRT, restaging was
performed within 6 weeks of CHRT completion and surgery was
performed 10 weeks after CHRT completion. The standard CHRT regimen
involved irradiation with 45 Gy in 25 fractions to the pelvic area,
the rectum and the pararectal, presacral and internal iliac lymph
nodes. A supplementary boost dose of radiation was applied to the
tumour itself (5.4 Gy in 3 fractions) for a total dose of 50.4 Gy.
Radiotherapy was potentiated by chemotherapy with continuous
5-fluorouracil infusion at a dose of 200 mg/m2/24 h for
the entire duration of radiotherapy.
Surgical procedures took place in the between March
2019 and December 2020 at two medical institutions: The University
Hospital Martin and Faculty Hospital with an outpatient clinic in
Zilina. After terminating the resection phase of the surgical
procedure, two small samples of the intestinal wall (normal
adjacent tissue) were cut from the specimen by an operating
surgeon. Adjacent rectal tissues (ARTs) were obtained 10.0-15.00 cm
away from the primary tumour and immediately frozen and stored at
-80˚C for quantitative polymerase chain reaction (qPCR) and
estimation of hydroxyproline. The total number recruited for the
present study (adjacent rectal tissues of patients with rectal
adenocarcinoma) was 29, of which 12 were without therapy and 17
with neo-adjuvant therapy. The present study was performed
according to the Guidelines of the World Medical Association
Declaration of Helsinki. The Ethics committee of Jessenius Faculty
of Medicine CU in Martin with certification code no. IRB00005636
approved the study and written informed consent was given by all
patients.
Collagen concentration in rectal
tissue of patients
Hydroxyproline, a major component of collagen, was
quantified as a measure of the collagen content using the Total
collagen Colorimetric Assay kit (BioVision, Inc.). The assay is
based on the acid hydrolysis of samples to form hydroxyproline.
Adjacent rectal samples were homogenized in dH2O, using
100 µl H2O for every 10 mg of tissue (a of total 80 mg
of tissue was used).
To a 100 µl sample of homogenate, 100 µl of
concentrated HCl (~12 M) was added in a pressure-tight,
Teflon-capped vial and hydrolysed at 120˚C for 3 h. A total of 10
µl of each hydrolysed sample was transferred to a 96-well plate and
evaporated to dryness under a vacuum. A total of 100 µl of the
chloramine T reagent was added to each sample and standard and
incubated at room temperature for 5 min. Then, 100 µl of the
p-dimethylaminobenzaldehyde (DMAB) reagent was added to each well
and incubated for 90 min at 60˚C. Finally, absorbance at 560 nm was
measured by Synergy HTX Multi-Mode Microplate Reader (BioTek
Instruments, Inc.). The collagen concentration was expressed in
total amount of collagen µg to mg dry tissue.
RNA extraction and reverse
transcription quantitative RT-qPCR
Colorectal tissue samples stored frozen at -80˚C in
stabilizing reagent (RNAlater; Qiagen, Inc.) were used for
total RNA isolation. The samples ~25 mg were disrupted in QIAZOL
Lysis reagent (Qiagen, Inc.) by Polytron PT 1600 E homogeniser
(Kinematica AG). The RNA was isolated by using RNeasy Plus
Universal kit (Qiagen, Inc.) and measured by spectrometry using a
Implen P300 NanoPhotometer (Implen GmbH). A total of 2 µg of
purified cellular RNA was converted to single stranded cDNA using
RT2 First Strand kit (Qiagen, Inc.) according to
protocol supplied by the manufacturer. The newly synthesized and
diluted cDNA was used for studying multigene expression MMPs,
tissue inhibitors of MMPs and collagens using the RT2
Profiler PCR Array (Qiagen, Inc.). Three endogenous control genes;
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-actin
(ACTB) and β2-microglobulin (B2M) present on the PCR
array were used for normalization. The set of primers for all human
genes were acquired from RT² qPCR Primer Assays database
(https://geneglobe.qiagen.com/sk/product-groups/rt2-qpcr-primer-assays;
Table II).
 | Table IIGenes and size of products used in
RT-qPCR assays acquired from Qiagen RT² qPCR Primer Assays
database. |
Table II
Genes and size of products used in
RT-qPCR assays acquired from Qiagen RT² qPCR Primer Assays
database.
| Abbreviation | Full name | Detected
transcripts | Band size | Reference
position |
|---|
| COL3A1 | Collagen, type III,
α 1 | 5,490 bp | 95 | 4,287
(NM_000090) |
| COL1A1 | Collagen, type I, α
1 | 5,927 bp | 75 | 3,760
(NM_000088) |
| ACTB | Actin, β | 1,852 bp | 174 | 730
(NM_001101) |
| MMP1 | Matrix
metallopeptidase 1 (interstitial collagenase) | 2,081 bp | 111 | 1,116
(NM_002421) |
| MMP2 | Matrix
metallopeptidase 2 (gelatinase A) | 3,558 bp | 69 | 1,594
(NM_004530) |
| MMP3 | Matrix
metallopeptidase 3 (stromelysin 1, progelatinase) | 1,828 bp | 70 | 1,173
(NM_002422) |
| MMP8 | Matrix
metallopeptidase 8 (neutrophil collagenase) | 3,056 bp | 92 | 1,192
(NM_002424) |
| MMP9 | Matrix
metallopeptidase 9 (gelatinase B) | 2,387 bp | 36 | 2,085
(NM_004994) |
| MMP10 | Matrix
metallopeptidase 10 (stromelysin 2) | 1,777 bp | 120 | 1,224
(NM_002425) |
| MMP13 | Matrix
metallopeptidase 13 (collagenase 3) | 2,735 bp | 61 | 1,380
(NM_002427) |
| TIMP1 | Tissue inhibitor of
metallopeptidase 1 | 931 bp | 91 | 243
(NM_003254) |
| TIMP2 | Tissue inhibitor of
metallopeptidase 2 | 3,670 bp | 92 | 2,480
(NM_003255) |
| TIMP4 | Tissue inhibitor of
metallopeptidase 4 | 1,667 bp | 128 | 1,316
(NM_003256) |
| GAPDH |
Glyceraldehyde-3-phosphate
dehydrogenase | 1,421 bp | 89 | 1,321
(NM_002046) |
| B2M |
β-2-microglobulin | 987 bp | 114 | 357
(NM_004048) |
A reaction mixture prepared according to
manufacturer's protocol containing cDNA template (800 ng) and
particular volume (1,240 µl) of SYBR Green ROX qPCR Mastermix
(Qiagen, Inc.) was immediately loaded into each well of 96-well
plate of RT2 Profiler PCR Array. Each plate was sealed
and centrifuged for 1 min at 1,000 x g at room temperature and
loaded into the ViiA 7 Real-Time PCR System (Thermo Fisher
Scientific, Inc.). Amplification of cDNA was initiated by
denaturation at 95˚C for 10 min followed by 40 PCR stage (95˚C, 15
min; 60˚C, 1 min) and melting curve stage (95˚C, 15 min; 60˚C, 1
min; 95˚C, 15 min). The relative changes in gene expression were
analysed by using the 2-ΔΔCq method (28), where ΔΔCt =
(CtGOI-CtHKG)TESTING GROUP -
(CtGOI-CtHKG)CONTROL GROUP.
Commercially available total RNAs from normal rectum tissue were
used as a control group for quantitative PCR (Ambsbio;
R1234206-50_Human Adult Normal Rectum Tissue and HR-312_Normal
Human Rectum Tissue).
Statistical analysis
The relative mRNA levels of ART (both with/without
pre-therapy) were compared with normal rectal mRNA by the Welch's
ANOVA and Tukey's HSD post hoc test. Relations between gene
expression and clinicobiochemical parameters were modelled by
multivariate linear regression with all continuous predictors,
except for age, and were log-transformed to bring the distributions
closer to a Gaussian one. The Akaike Information Criterion was used
to reduce the full regression model. The correlation between the
log-transformed gene expressions was assessed by the Pearson
correlation coefficient. The data were analysed in R (R Development
Core Team, R: A Language and environment for statistical computing,
R Foundation for Statistical Computing, Vienna, Austria, URL
http://www.R-project.org, 2006). Data are
represented as means ± standard error of mean. All statistical
comparisons were performed two-sided in the sense of an exploratory
data analysis using *P<0.05, **P<0.01
and ***P<0.001 as levels of significance.
Gene expression and fold-change calculations were
performed using RT2 Profiler PCR Arrays & Assays Data Analysis
Webportal (https://geneglobe.qiagen.com/us/analyze, Qiagen,
Inc.). The genes with a significant difference in expression were
those with an average fold-change of ≤-2.0 or ≥2.0. P<0.05 was
considered to indicate a statistically significant difference.
Results
Absolute level of mRNA and correlation
with clinical data
The absolute pattern of relevant MMPs,
TIMPs and COLs was analysed through mRNA expression
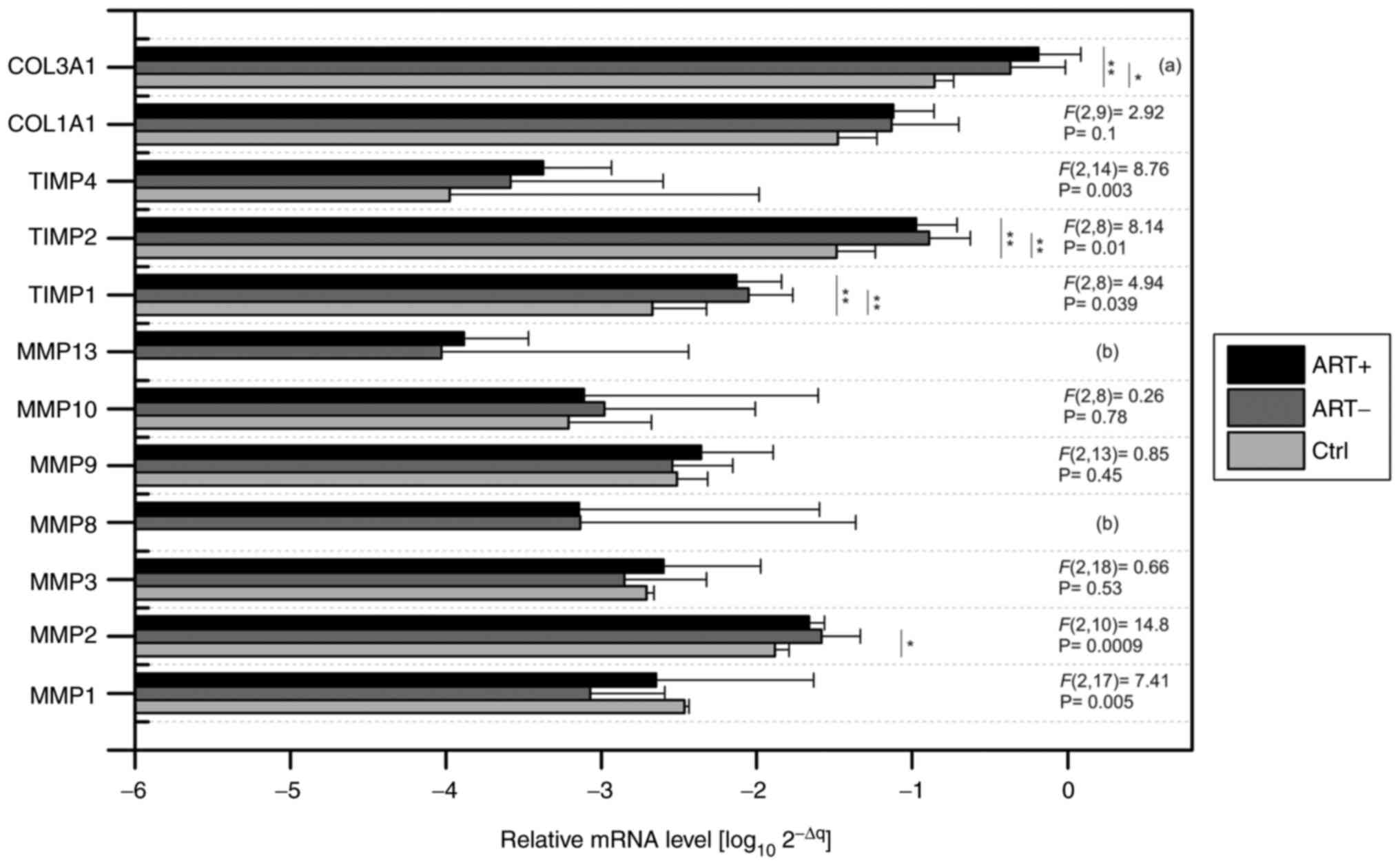
(Fig. 1). When comparing the
absolute levels of gene expression, we decided to represent them in
log(10) of 2-ΔΔCq
values. It should be added that the average Ct of the control genes
(ACTB, GAPDH and B2M) was subtracted from the
expression value of the determined gene for each sample separately.
Fig. 1 shows the transcriptomic
levels of the genes of interest in all sample types [control mRNA
from normal human rectum (Ctrl); adjacent rectal tissue of patients
with pre-therapy (ART+) and adjacent rectal tissue of patients
without pre-therapy (ART-)]. It can be clearly stated that the
highest amplification was recorded for COL3A1, TIMP2 and
COL1A1. Among MMPs, the highest levels in MMP2 were
detected in both the control and patient samples. An absolute lack
of amplification signal was accompanied by control of the human
rectum for the MMP8 and MMP13 genes. In patient
samples, amplification of MMP8 was detected in 65.5% of
cases and MMP13 in 89% of cases. No statistical differences
were observed when comparing gene levels between both patient
groups. On the contrary, significant differences in the
TIMP1, TIMP2, TIMP4 and COL3A1 genes were
demonstrated when comparing patient samples with the control group
(Fig. 1). Significantly increased
MMP2 levels were also detected in patients without
pre-therapy compared with controls (-3.07 vs. -2.48, P=0.025).
The comparison between gene levels and
clinicobiochemical parameters of all patients is summarised in
Table III, where only
significant differences are shown.
 | Table IIIRelationship between the expression
of mRNAs and clinicobiochemical parameters in adjacent rectal
tissues of patients with colorectal carcinoma. |
Table III
Relationship between the expression
of mRNAs and clinicobiochemical parameters in adjacent rectal
tissues of patients with colorectal carcinoma.
| | Clinical and
biochemical parameters (estimate; P-value) | |
|---|
| Gene symbol | Albumin | Age | BMI | Collagen | CRP | Therapy | Regression
P-value | Adjusted
R2 |
|---|
| MMP1 | -11.66 | | | | -0.688 | 9.58 | 0.074 | 0.232 |
| | 0.009 | | | | 0.024 | 0.034 | | |
| MMP2 | | 0.015 | | | | 9.11 | 0.044 | 0.14 |
| | | 0.044 | | | | 0.043 | | |
| MMP8 | -13.8 | -0.09 | 0.288 | -3.30 | | | 0.005 | 0.74 |
| | 0.011 | 0.024 | 0.008 | 0.001 | | | | |
| MMP10 | -15.69 | | | | -1.04 | 21.3 | 0.05 | 0.38 |
| | 0.003 | | | | 0.024 | 0.002 | | |
| COL1A1 | | | -0.144 | 0.92 | | | 0.121 | 0.26 |
| | | | 0.010 | 0.009 | | | | |
| COL3A1 | 2.34 | | | 1.47 | | |
<0.000 | 0.81 |
| | 0.038 | | | 0.000 | | | | |
It should be noted at the outset that none of the
TIMP genes showed a relationship with the clinical
parameters. MMP1, MMP8 and MMP10 gene
expression levels in all samples of patients were negatively
related to albumin concentration. In contrast, a positive
significant association of albumin vs. COL3A1 mRNA level was
detected. Moreover, MMP1 and MMP10 were negative
trends to C-reactive protein values (Table III). The expression of
MMP2 and COL3A1 correlated with increased age, while
high levels of MMP8 were typical for younger patients. In
addition, it was demonstrated that the level of MMP8
increased with body mass index (BMI) value and decreased with the
collagen concentration in the examined biopsies. The high
expression of the COL1A1 gene was significantly related to
collagen concentration and was in overweight individuals (Table III). The weak effect of
neoadjuvant therapy in association with MMP1, MMP2
and MMP10 genes was observed.
Correlation between individual gene
expression in ART samples
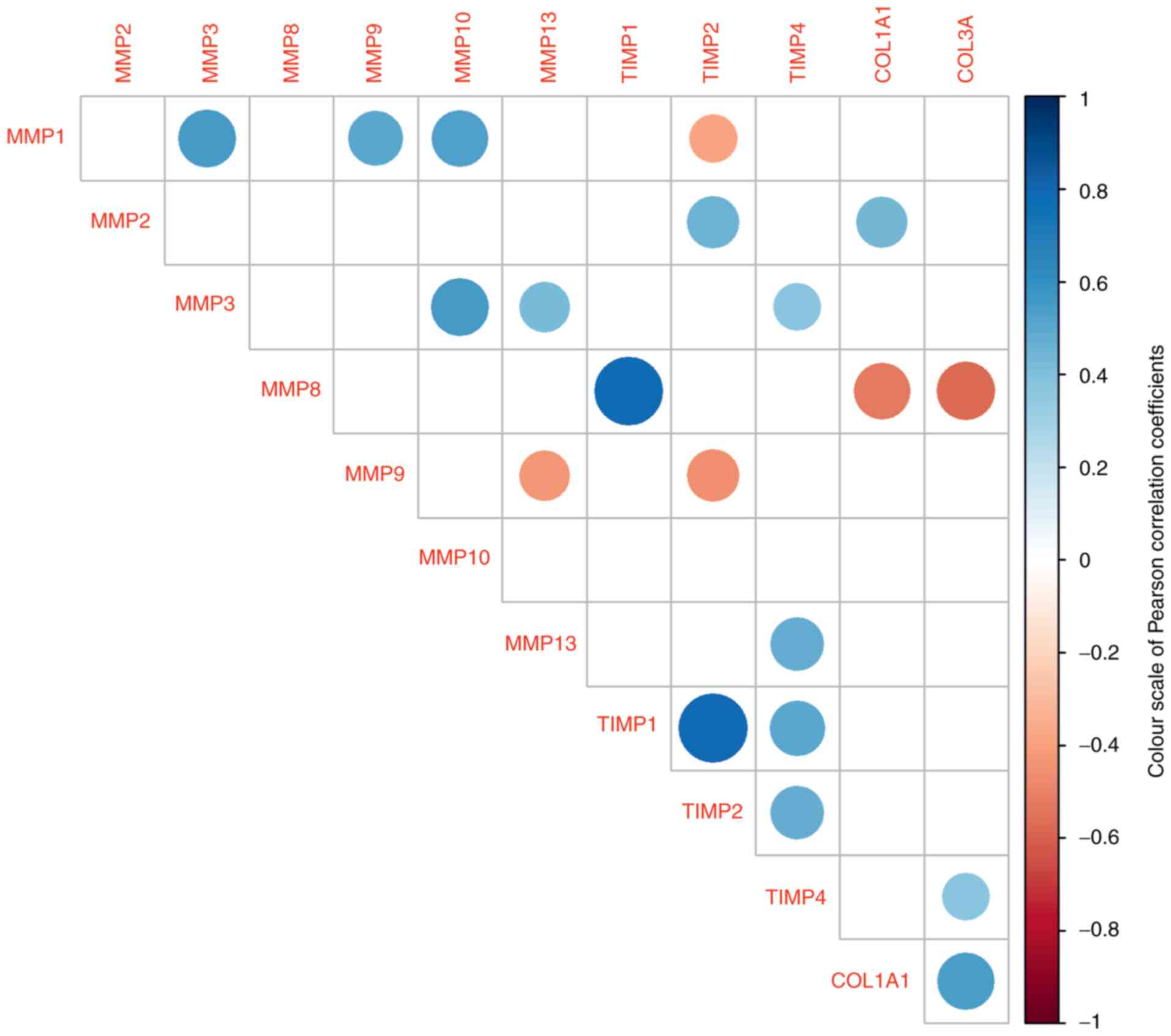
The present study investigated the correlations
between individual levels of mRNA obtained from patient samples.
The power of correlations (positive or negative) were categorised
as low, medium and high according to their correlation coefficient
and P-values (Fig. 2). The highest
positive association of the MMP8 gene was significantly
related to the TIMP1 gene (0.78; P=0.0001). The same strong
effect was observed between TIMP1 and TIMP2 (0.7;
P=0.00001). Among the significant positive correlations, the
relationship between the two genes for collagen was considered. The
remaining positive relationships are indicated by blue spots. A
negative relationship (red spots) was detected between MMP1
and TIMP2 (-0.38; P=0.043), between MMP8 and both
collagen genes (COL1A1: -0.52; P=0.020; COL3A1:
-0.57, P=0.01) and between MMP9 and MMP13 (-0.42;
P=0.032).
Fold change in gene regulation
In this section, the present study focused on the
regulatory changes of genes measured in the adjacent rectum tissue
from carcinoma patients compared with control biopsies (RNAs from
Human Adult Normal Rectum Tissue; Amsbio), where cancer status was
not present. In addition, the patient samples were divided into two
groups, with or without applied therapy. Table IV represents changes in fold
regulation of individual genes. The data themselves were calculated
as the difference of log10 2-ΔCq between the
groups of samples that are illustrated in Fig. 1. It was found that the present of
therapy did not serve a significant role in the regulation of genes
(Table IV). This was reflected in
a parallel comparison of patient samples vs. the control samples.
MMP2 (2.0 fold; P=0.025), TIMP1 (4.2; P=0.0022) and
TIMP2 (3.9; P=0.0012) were significantly increased in
patients without therapy (ART-), compared with the control.
Similarly, the mRNA of COL1A1 (2.2; P=0.22) and
COL3A1 (3.1; P=0.023) increased in the ART-group.
TIMP1 showed the highest fold difference, while in the MMP1
gene higher downregulation was detected, but without statistical
significance. The present study did not find fold-change
differences in MMP regulation between patient samples with
therapy compared with the control. In contrast, fold regulation for
TIMPs and COLs had a similar trend as in patients
without therapy.
 | Table IVComparison of fold regulation of gene
expression changes in different rectal tissues. |
Table IV
Comparison of fold regulation of gene
expression changes in different rectal tissues.
| | Fold of
regulation |
|---|
| | ART- | ART+ | ART | ART+ |
|---|
| Gene symbol | Ctrl | Ctrl | Ctrl | ART- |
|---|
| MMP1 | -4.04 | -1.52 | -2.48 | 2.65 |
| MMP2 |
2.00a | 1.66 | 1.82 | -1.21 |
| MMP3 | -1.39 | 1.29 | -1.04 | 1.79 |
| MMP8 | ND | ND | ND | -1.02 |
| MMP9 | -1.07 | 1.43 | 1.16 | 1.53 |
| MMP10 | 1.70 | 1.26 | 1.46 | -1.35 |
| MMP13 | ND | ND | ND | 1.40 |
| TIMP1 |
4.21b |
3.51b |
3.84b | -1.20 |
| TIMP2 |
3.94b |
3.26b |
3.58b | -1.21 |
| TIMP4 | 2.47 | 3.98 | 3.13 | 1.61 |
| COL1A1 | 2.22 | 2.27 | 2.25 | 1.02 |
| COL3A1 |
3.08a |
4.64b |
3.78a | 1.51 |
The present study confirmed the similar profile of
fold regulation when comparing all patient samples versus the
control (ART/Ctrl; Table IV). As
mentioned earlier, the therapy did not affect the change in gene
regulation; although, the mRNA of MMP1 showed a
non-significant upregulation in patients after therapy compared
with patients without therapy (ART+/ART-).
Association between collagen
concentration and clinicobiochemical parameters
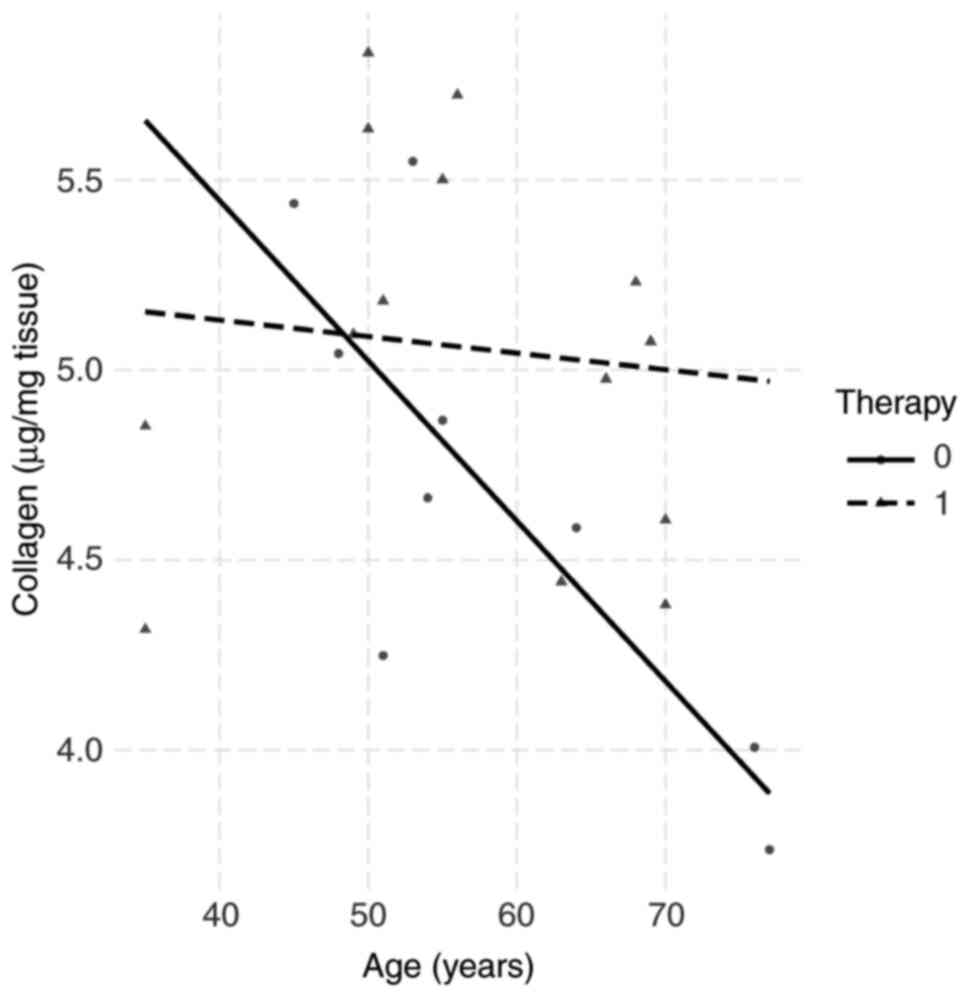
The biopsies for collagen determination after
storage at -80˚C weighed 75±5.2 mg wet tissue. The relationships
between the collagen concentrations of each patient and the
clinicobiochemical parameters were then examined. The collagen
amounts in the biopsies were unrelated to gender, BMI, proteins in
plasma, leukocytes and plasma iron. In 24 biopsy specimens of
rectal tissue, a decrease in collagen concentration with the
patient's age was observed. Combined with the third parameter
(neoadjuvant therapy), it was found that 13 patients who underwent
preoperative therapy almost doubled their collagen content in
rectal tissue (Fig. 3).
Discussion
It is apparent that the formation of CRC results in
pathomolecular changes in the ECM of the colon tissue. Defective
ECM remodelling is associated with the accumulation of inflammatory
cells, the presence of apoptotic cells, neovascularisation, hypoxia
and loss of elastic fibres (29,30).
Previous studies based on proteomic or transcriptomic analyses
point to specific molecules that are dysregulated in the progress
of colorectal carcinoma (31,32).
Collagen is a key factor among ECM adhesive components that
regulate tumorigenesis and stages of cancer (29,33).
In general, it is known that the collagen content in rectal tissue
is at a low level compared with other tissues of the digestive
tract (34). Moreover, the
decrease in collagen content is exacerbated by age, which was also
confirmed in the present study. By contrast, patients who underwent
standard neoadjuvant therapy showed increased concentrations of
collagen in the ART. Previous studies have shown an increase in
collagen in tumour tissues compared with normal tissue (35,36).
From the monitored clinicobiochemical parameters, a
positive relationship between collagen content and body mass index
and a negative relationship to age, respectively, was observed. An
increase in interstitial collagen content, the main hallmark of
fibrosis, is correlated with an increase in BMI in the ECM of
adipose stromal cells from breast cancer patients (37).
It was notable that by applying neoadjuvant therapy
before surgical removal of the tumour a positive trend in the
content of collagen in the rectal tissue after resection was
attained. Impaired content or the synthesis of collagen can cause
anastomotic leakage after tumour resection (34). It was hypothesized that the
composition of collagens in normal tissue may be most affected by
the presence and aggression of a CRC.
Collagen-rich stroma in aggressive colon tumours
induces mesenchymal gene expression and tumour cell invasion based
on different biochemical processes (38). Liang et al (19) observed collagen remodelling in CRC,
demonstrating that the collagen fibres in normal intestinal mucosa
were thin, wavy and dispersed. However, in the CRC tissues, the
collagen fibres were more linearised and denser than in normal
samples.
In CRC, special proteolytic enzymes (MMPs and TIMPs)
serve critical roles in the metabolism of collagen. It was decided
to draw attention to the relationship between individual mRNAs
(COL vs. MMP or TIMP) in adjacent normal
tissue of patients with CRC. In the present study, the main
methodological difference compared with other publications was the
use of control RNA for transcriptomic analysis. Several previous
studies have compared MMP or TIMP gene expression
levels between colon carcinoma tissue and adjacent normal mucosa
(19,34,39).
Yamada et al (39) showed
that the expressions of MMP9, MMP13 and TIMP1
were higher in cancer tissue than in the adjacent normal biopsies
of 202 patients.
The present study detected the absolute absence of
the MMP8 and MMP13 genes in normal human rectal
tissue, which was almost identical to the data from Buch et
al (34). That study
demonstrated that MMP8 is not detected in any part of the
rectum tissue and MMP13 was only detected in 9 of 34
analysed samples. By contrast, the present study showed the highest
mRNA levels for COL1A1, COL3A1, TIMP2 and
MMP2 in control or patient samples.
In addition, there is still a lack of information
about the relationship between the mRNA of collagen, MMPs and
tissue inhibitors of MMPs. It was thought-provoking to monitor the
correlation between log-transformed gene expressions in the ART of
patients with CRC. In the correlation analysis, it is necessary to
emphasise the positive relationship between collagen mRNAs,
COL1A1 and COL3A1. Collagenase (MMP8 protein) is a
protein that has functions in the degradation of type I, II and III
collagens. This protein is encoded by the MMP8 gene
(40), which was the only one to
show a negatively significant relationship with genes encoding
collagens, both COL1A1 and COL3A1. Therefore, it was
hypothesized that MMP8 was a key gene in the degradation of
collagen due to the fact that MMP8 was detected in only
65.5% of patient cases. In addition, a negative correlation with
TIMP1 may be another regulatory effect on the said gene.
Although the increase in serum MMP8 and TIMP2 levels is related to
the presence of an inflammatory process (41,42),
the present study was unable to correlate these markers with
C-reactive protein, which is another sign of inflammation.
The mainstay of the present study was the results
regarding changes in gene regulation between groups of samples.
Based on the changes in fold regulation, it can be concluded that
mRNA levels of TIMPs were significantly higher in ARTs
compared with the control. A similar result was obtained for genes
encoding collagens. It was therefore considered that the regulatory
effect on the synthesis of collagen will be affected by
TIMPs rather than MMPs. The present study
hypothesized that higher levels of TIMPs in the adjacent
tumour tissue had an inhibitory effect on MMPs and thus
promoted collagen production. Several studies in CRC confirm the
role of TIMPs as inhibitors of MMPs. In particular,
TIMP1 inhibits most MMPs and its expression
correlates with poor survival of CRC patients (43). Park et al (44) showed the inhibition of MMP2
and MMP9 by TIMP2 with CRC invasion and worse
prognosis. Finally, TIMP4 suppresses MMP2 and its
expression in rectal cancer correlates with longer patient survival
(45).
Although there was a small number of samples, the
present study provides unique information on the transcriptomic
relationship between the mRNA of collagens, MMPs and tissue
inhibitors of MMPs. Unlike other studies, it did not compare levels
of genes in normal tissue of the same patient with CRC, which is a
limitation of the present study.
The main outcome of the present study was the
determination of higher levels of COL1A1, COL3A1 and
selected TIMPs in the ART of CRC patients compared with the
normal control group. This finding suggested that TIMPs
served an important role in the regulation of MMPs and, hence, the
remodelling of collagen in the ECM.
MMP8 may serve an important role in collagen
metabolism since it was not amplified in the control samples
compared with the tissue adjacent to the tumour. In addition, an
increase in collagen concentration after the application of
preoperative neoadjuvant therapy in patients with CRC was
demonstrated.
The present study confirmed the influence of CRC on
the expression of TIMPs and MMPs in the vicinity of
the tumour tissue, but, at the same time, it is necessary to point
out other factors associated with the process of tumorigenesis.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Ministry of Health of
the Slovak Republic (grant nos. 2018/16-UKMT-12 and 2019/64-UKMT-3)
and by the Slovak Research and Development Agency under the
contract no. APVV-18-0088.
Availability of data and materials
The datasets used and analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KD conceived and designed the experiments. KD and ZM
performed the experiments and contributed to the acquisition of the
data. AS and JV contributed to the acquisition of the clinical
data. AS, MA and JV made substantial contribution to the collection
of tissue samples. MG and JH were responsible for statistical
analysis and data correlation. KD and JH wrote the manuscript. AF
made substantial contributions to conception and design. JH, as the
corresponding author, was responsible for final correction. KD and
JH confirm the authenticity of all the raw data. All authors have
examined the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The experiment was approved by the Jessenius Faculty
of Medicine CU in Martin and The Faculty Hospital in Zilina,
Slovakia. This study was approved by the Ethics Committee of the
Jessenius Faculty of Medicine CU in Martin (approval no. 1/2019)
and written informed consent was obtained from all patients. The
present study was performed in accordance with the Declaration of
Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics. CA Cancer J Clin. 65:5–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kverneng Hultberg D, Svensson J, Jutesten
H, Rutegård J, Matthiessen P, Lydrup ML and Rutegård M: The impact
of anastomotic leakage on long-term function after anterior
resection for rectal cancer. Dis Colon Rectum. 63:619–628.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Smith JD, Butte JM, Weiser MR, D'Angelica
MI, Paty PB, Temple LK, Guillem JG, Jarnagin WR and Nash GM:
Anastomotic leak following low anterior resection in stage IV
rectal cancer is associated with poor survival. Ann Surg Oncol.
20:2641–2646. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hain E, Maggiori L, Manceau G, Mongin C,
Prost À la Denise J and Panis Y: Oncological impact of anastomotic
leakage after laparoscopic mesorectal excision. Br J Surg.
104:288–295. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Karim A, Cubas V, Zaman S, Khan S, Patel H
and Waterland P: Anastomotic leak and cancer-specific outcomes
after curative rectal cancer surgery: A systematic review and
meta-analysis. Tech Coloproctol. 24:513–525. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou X, Wang B, Li F, Wang J and Fu W:
Risk factors associated with nonclosure of defunctioning stomas
after sphincter-preserving low anterior resection of rectal cancer:
A meta-analysis. Dis Colon Rectum. 60:544–554. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ashraf SQ, Burns EM, Jani A, Altman S,
Young JD, Cunningham C, Faiz O and Mortensen NJ: The economic
impact of anastomotic leakage after anterior resections in English
NHS hospitals: Are we adequately remunerating them? Colorectal Dis.
15:e190–e198. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hammond J, Lim S, Wan Y, Gao X and Patkar
A: The burden of gastrointestinal anastomotic leaks: An evaluation
of clinical and economic outcomes. J Gastrointest Surg.
18:1176–1185. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Meyer J, Naiken S, Christou N, Liot E,
Toso C, Buchs NC and Ris F: Reducing anastomotic leak in colorectal
surgery: The old dogmas and the new challenges. World J
Gastroenterol. 25:5017–5025. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kawada K and Sakai Y: Preoperative,
intraoperative and postoperative risk factors for anastomotic
leakage after laparoscopic low anterior resection with double
stapling technique anastomosis. World J Gastroenterol.
22:5718–5727. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Qu H, Liu Y and Bi DS: Clinical risk
factors for anastomotic leakage after laparoscopic anterior
resection for rectal cancer: A systematic review and meta-analysis.
Surg Endosc. 29:3608–3617. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rink AD, Kienle P, Aigner F and Ulrich A:
How to reduce anastomotic leakage in colorectal surgery-report from
German expert meeting. Langenbecks Arch Surg. 405:223–232.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu R, Won JY, Kim CH, Kim DE and Yim H:
Roles of the phosphorylation of transcriptional factors in
epithelial-mesenchymal transition. J Oncol.
2019(5810465)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ricard-Blum S: The collagen family. Cold
Spring Harb Perspect Biol. 3(a004978)2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ohlund D, Lundin C, Ardnor B, Oman M,
Naredi P and Sund M: Type IV collagen is a tumour stroma-derived
biomarker for pancreas cancer. Br J Cancer. 101:91–97.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qiu S, Deng L, Liao X, Nie L, Qi F, Jin K,
Tu X, Zheng X, Li J, Liu L, et al: Tumor-associated macrophages
promote bladder tumor growth through PI3K/AKT signal induced by
collagen. Cancer Sci. 110:2110–2118. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liang Y, Lv Z, Huang G, Qin J, Li H, Nong
F and Wen B: Prognostic significance of abnormal matrix collagen
remodeling in colorectal cancer based on histologic and
bioinformatics analysis. Oncol Rep. 44:1671–1685. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu S, Xu H, Wang W, Li S, Li H, Li T,
Zhang W, Yu X and Liu L: The role of collagen in cancer: From bench
to bedside. J Transl Med. 17(309)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Libra M, Scalisi A, Vella N, Clementi S,
Sorio R, Stivala F, Spandidos DA and Mazzarino C: Uterine cervical
carcinoma: Role of matrix metalloproteinases (review). Int J Oncol.
34:897–903. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Murphy G: Tissue inhibitors of
metalloproteinases. Genome Biol. 12(233)2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Medina C and Radomski MW: Role of matrix
metalloproteinases in intestinal inflammation. J Pharmacol Exp
Ther. 318:933–938. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
DI Carlo A: Matrix metalloproteinase-2 and
-9 and tissue inhibitor of metalloproteinase-1 and -2 in sera and
urine of patients with renal carcinoma. Oncol Lett. 7:621–626.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Herszényi L, Hritz I, Lakatos G, Varga MZ
and Tulassay Z: The behavior of matrix metalloproteinases and their
inhibitors in colorectal cancer. Int J Mol Sci. 13:13240–13263.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Knight CD and Griffen FD: An improved
technique for low anterior resection of the rectum using the EEA
stapler. Surgery. 88:710–714. 1980.PubMed/NCBI
|
|
28
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Le CC, Bennasroune A, Langlois B, Salesse
S, Boulagnon-Rombi C, Morjani H, Dedieu S and Appert-Collin A:
Functional interplay between collagen network and cell behavior
within tumor microenvironment in colorectal cancer. Front Oncol.
10(527)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yuzhalin AE, Lim SY, Kutikhin AG and
Gordon-Weeks AN: Dynamic matrisome: ECM remodeling factors
licensing cancer progression and metastasis. Biochim Biophys Acta
Rev Cancer. 1870:207–228. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yuzhalin AE, Urbonas T, Silva MA, Muschel
RJ and Gordon-Weeks AN: A core matrisome gene signature predicts
cancer outcome. Br J Cancer. 118:435–440. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Naba A, Clauser KR, Whittaker CA, Carr SA,
Tanabe KK and Hynes RO: Extracellular matrix signatures of human
primary metastatic colon cancers and their metastases to liver. BMC
Cancer. 14(518)2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Skovbjerg H, Anthonsen D, Lothe IM, Tveit
KM, Kure EH and Vogel LK: Collagen mRNA levels changes during
colorectal cancer carcinogenesis. BMC Cancer. 9(136)2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Buch AS, Schjerling P, Kjaer M, Jorgensen
LN, Krarup PM and Ågren MS: Impaired collagen synthesis in the
rectum may be a molecular target in anastomotic leakage
prophylaxis. Wound Repair Regen. 25:532–535. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zou X, Feng B, Dong T, Yan G, Tan B, Shen
H, Huang A, Zhang X, Zhang M, Yang P, et al: Up-regulation of type
I collagen during tumorigenesis of colorectal cancer revealed by
quantitative proteomic analysis. J Proteomics. 94:473–485.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang Q, Feng M, Ma X, Li H and Xie W: Gene
expression profile comparison between colorectal cancer and
adjacent normal tissues. Oncol Lett. 14:6071–6078. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Springer NL, Iyengar NM, Bareja R, Verma
A, Jochelson MS, Giri DD, Zhou XK, Elemento O, Dannenberg AJ and
Fischbach C: Obesity-Associated extracellular matrix remodeling
promotes a macrophage phenotype similar to tumor-associated
macrophages. Am J Pathol. 189:2019–2035. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Vellinga TT, den Uil S, Rinkes IH, Marvin
D, Ponsioen B, Alvarez-Varela A, Fatrai S, Scheele C, Zwijnenburg
DA, Snippert H, et al: Collagen-rich stroma in aggressive colon
tumors induces mesenchymal gene expression and tumor cell invasion.
Oncogene. 35:5263–5271. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yamada T, Oshima T, Yoshihara K, Tamura S,
Kanazawa A, Inagaki D, Yamamoto N, Sato T, Fujii S, Numata K, et
al: Overexpression of MMP-13 gene in colorectal cancer with liver
metastasis. Anticancer Res. 30:2693–2699. 2010.PubMed/NCBI
|
|
40
|
Hasty KA, Pourmotabbed TF, Goldberg GI,
Thompson JP, Spinella DG, Stevens RM and Mainardi CL: Human
neutrophil collagenase. A distinct gene product with homology to
other matrix metalloproteinases. J Biol Chem. 265:11421–11424.
1990.PubMed/NCBI
|
|
41
|
Böckelman C, Beilmann-Lehtonen I, Kaprio
T, Koskensalo S, Tervahartiala T, Mustonen H, Stenman UH, Sorsa T
and Haglund C: Serum MMP-8 and TIMP-1 predict prognosis in
colorectal cancer. BMC Cancer. 18(679)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Schmalz G, Davarpanah I, Jäger J, Mausberg
RF, Krohn-Grimberghe B, Schmidt J, Haak R, Sack U and Ziebolz D:
MMP-8 and TIMP-1 are associated to periodontal inflammation in
patients with rheumatoid arthritis under methotrexate
immunosuppression-First results of a cross-sectional study. J
Microbiol Immunol Infect. 52:386–394. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lee JH, Choi JW and Kim YS: Plasma or
serum TIMP-1 is a predictor of survival outcomes in colorectal
cancer: A meta-analysis. J Gastrointestin Liver Dis. 20:287–291.
2011.PubMed/NCBI
|
|
44
|
Park KS, Kim SJ, Kim KH and Kim JC:
Clinical characteristics of TIMP2, MMP2 and MMP9 gene polymorphisms
in colorectal cancer. J Gastroenterol Hepatol. 26:391–397.
2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hilska M, Roberts PJ, Collan YU, Laine VJ,
Kössi J, Hirsimäki P, Rahkonen O and Laato M: Prognostic
significance of matrix metalloproteinases-1, -2, -7 and -13 and
tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in
colorectal cancer. Int J Cancer. 121:714–723. 2007.PubMed/NCBI View Article : Google Scholar
|