Introduction
Anterior resection (AR) for rectal and rectosigmoid
(RS) cancer has become a standard procedure, thus improving
oncological and surgical outcomes because of advances in surgical
strategy and perioperative management. Despite these advances,
anastomotic leakage (AL) is an important complication that occurs
during the acute phase after AR; the anastomotic leakage rate,
regardless of temporary stoma use, varies from 2% to 15% among
reports (1-3).
AL is associated with poor functional outcome,
reduced quality of life, and prolonged hospital stay, as well as
with poor oncological outcomes (e.g., morbidity, mortality, and
recurrence rate) (4-7).
Patient-related and operative factors have been reported in many
studies as risk factors for the development of AL (8). For example, male sex, body mass index
(BMI), level of anastomosis, absence of a diverting stoma, use of
neoadjuvant therapy, and absence of a trans-anal tube have been
reported as risk factors (8-13).
Many of these factors remain controversial; however, surgical
techniques related to blood flow, pressure, and tension at the
anastomosis site play important roles in prevention of AL (14-18).
Blood flow is reportedly better at the
antimesenteric border than at the end of the colon (14); moreover, blood flow at the
anastomotic site is associated with AL (14-18).
Therefore, blood flow at the side-to-end anastomotic site can be
better than that at the end-to-end anastomotic site, and a
side-to-end anastomosis can reduce the rate of AL after AR. The
principle of a side anastomosis in gastrointestinal surgery is well
recognized; it is considered a standard technique for
esophagojejunal anastomosis. However, this approach is much less
frequently used in colorectal surgery; few studies have directly
compared side-to-end anastomosis and end-to-end anastomosis
(19-21).
In our hospital, side-to-end anastomosis with a
trans-anal double-stapling technique has been performed since
January 2017 as the preferred procedure to prevent AL, along with a
trans-anal tube and intraoperative indocyanine green fluorescence
angiography (ICG-FA). The purpose of this study was to investigate
whether side-to-end anastomosis could provide better surgical
outcomes, compared to end-to-end anastomosis, following AR for
rectal and RS cancer. In addition, this study identified factors
associated with AL using univariate and multivariate analyses.
Materials and methods
Patients
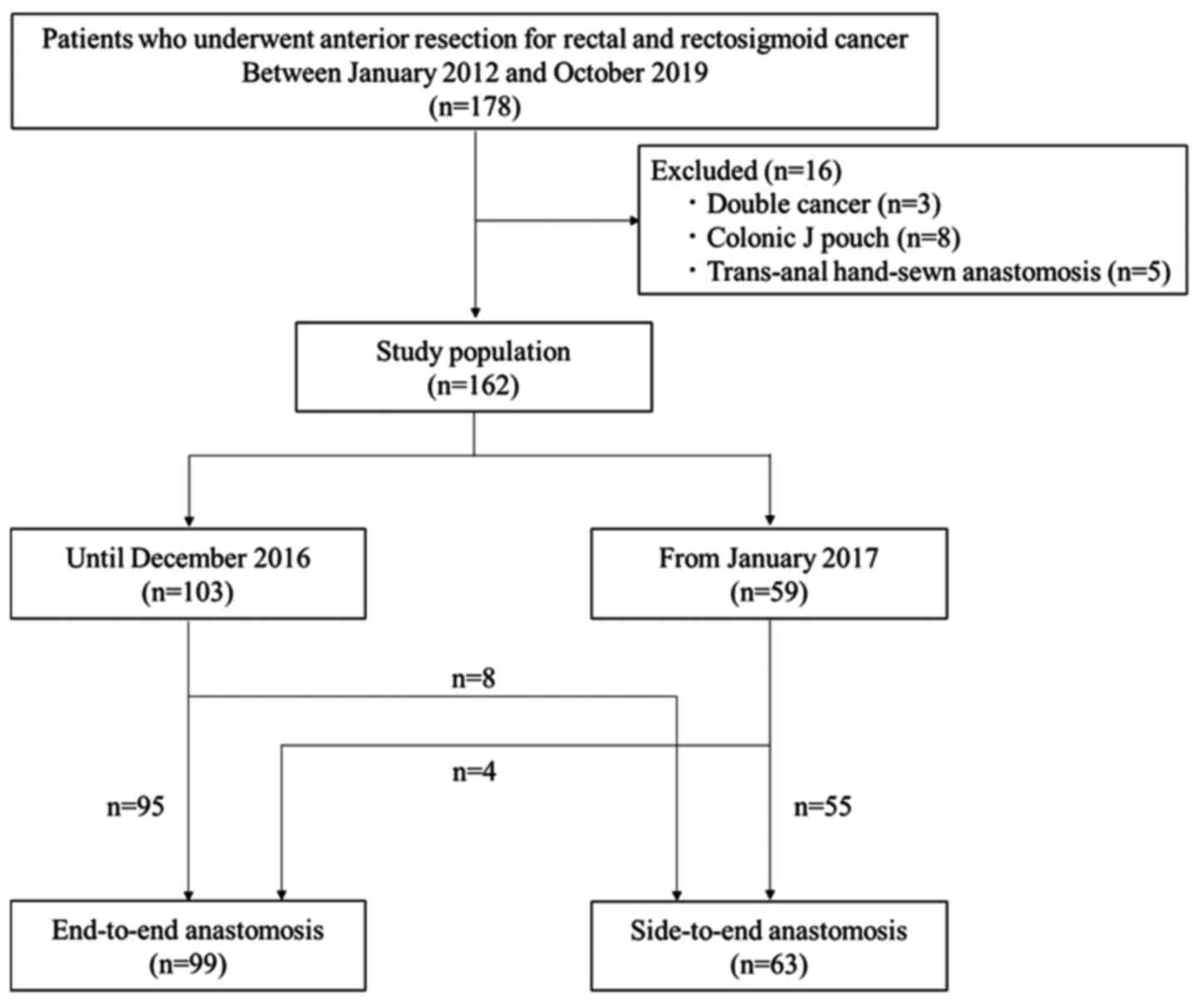
This single-center retrospective observational
clinical trial at International University of Health and Welfare
Mita Hospital enrolled 178 patients with rectal and RS cancer who
had undergone elective AR from January 2012 to October 2019. To
examine the association between anastomosis type and surgical
outcome, three patients with double cancers, eight patients with a
colonic J-pouch, and five patients with trans-anal hand-sewn
anastomoses were excluded. Therefore, 162 patients were included in
this study; all underwent anastomosis with the application of a
trans-anal double-stapling technique. Of the 162 patients, 63
underwent side-to-end anastomosis following AR. The flow chart of
patient inclusion criteria is shown in Fig. 1. Patient characteristics and
surgical outcomes were recorded. The descriptions and diagnoses of
the cancers were performed in accordance with the Japanese
classification of colorectal cancer. The treatment policy was
decided in accordance with the Japanese Society for Cancer of the
Colon and Rectum 2019 guidelines for the treatment of colorectal
cancer. This study was approved by the institutional review board
of the International University of Health and Welfare Mita Hospital
(approval no. 5-19-41).
Surgical procedure
Anterior resections were performed by a laparoscopic
or open approach. Blood vessels were ligated, lymph nodes were
dissected, and the colon and rectum were mobilized; the rectum was
then resected with a linear stapler at the anal side of the
resection range. In the laparoscopic approach, the intestine was
pulled out from an elongated umbilical incision wound; the oral
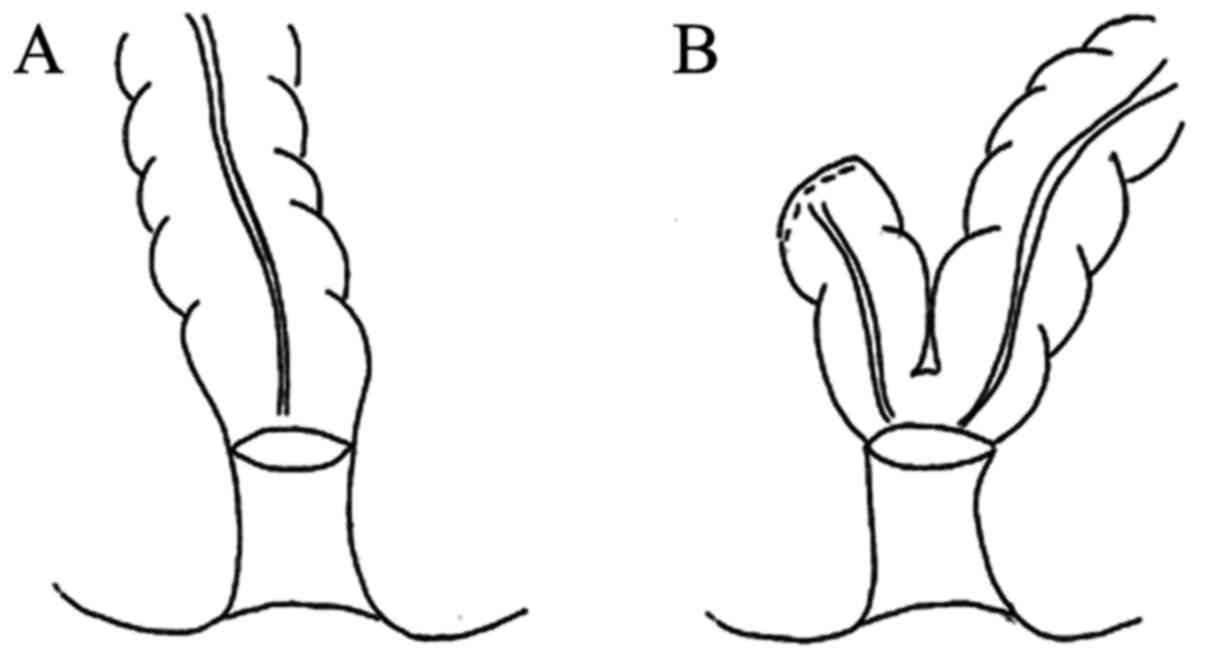
side of the resection range was then determined. Either side-to-end
or end-to-end anastomosis was performed (Fig. 2). Side-to-end anastomosis was
considered the preferred approach beginning in January 2017;
however, end-to-end anastomosis was typically applied when tension
at the anastomosis site was expected to be high, as determined by
the surgeons. In the side-to-end anastomosis group, the anvil of
the circular stapler was inserted into the lumen of the open end;
the antimesenteric wall of the intestine was then stapled
approximately 3-4 cm from the open end. The open end was stapled
with the same stapler. In the end-to-end anastomosis group, the
anvil of the circular stapler was fixed at the open end. The
splenic flexure was often mobilized to avoid tension at the
anastomosis site in both groups, as determined by the surgeons.
Following anastomosis, a drain was placed near the anastomosis
region. The need for intraoperative ICG-FA, stoma diversion, or
trans-anal tube placement was determined by the surgeons. A
charge-coupled device camera (HyperEye Medical System™; HEMS,
Mizuho) was used for ICG-FA, following dissection of the intestine.
The anesthesiologist injected 0.25 mg/kg ICG, followed by
flash-injection of 10 ml of saline. When fluorescent labeling at
the intended dissection site was poor, the site was modified to a
well-perfused proximal site, as determined by the surgeons.
Definition of postoperative
complications
Postoperative complications included AL, ileus, and
surgical site infections defined as Grade II or higher, according
to the Clavien-Dindo classification. The definition of AL was both
clinical (i.e., fever, abdominal pain, drain contents, and enhanced
inflammatory response) and radiological (i.e., computed tomography
scan and contrast enema study). AL in this study included
peritonitis due to leakage from any staple line or a pelvic abscess
near the anastomosis region, with or without a proven defect in the
intestinal wall of the anastomosis, as verified by both clinical
and radiological investigations. Patients with AL in this study
included those who were diagnosed during their initial hospital
stay or after discharge. Leakage verified by either clinical or
radiological investigations was not included.
Perioperative management
Mechanical bowel preparation was performed 1 and 2
days before surgery. Sodium picosulfate hydrate (5 ml; sodium
picosulfate solution 0.75%) was used 2 days before surgery.
Magnesium citrate (100 g; MAGCOROL P) and sodium picosulfate
hydrate (5 ml; sodium picosulfate solution 0.75%) were used 1 day
before surgery. Patients were only permitted to drink clear liquid
after mechanical bowel preparation. No chemical bowel preparation
was performed.
Patients were permitted to drink clear liquids on
the day of surgery. The trans-anal tube was removed on day 3 after
surgery, and patients were permitted to take liquid food on day 4.
Blood and abdominal X-ray examinations were performed on days 1, 3,
5, and 7 after surgery.
Statistical analysis
The Mann-Whitney U test and Fisher's exact test were
used to analyze continuous and categorical variables, respectively.
Multivariate logistic regression analysis was conducted to identify
factors related to AL at P<0.05. Covariates for multivariate
analysis that were statistically significant on univariate analysis
(P<0.05) were included in the multivariate model. All
statistical analyses were performed using R software (R Foundation
for Statistical Computing, Vienna, Austria), and a P-value <
0.05 was considered statistically significant.
Results
Patient characteristics and surgical
outcomes
In total, 162 patients (89 men and 73 women) who
underwent anterior resection with anastomosis using a
double-stapling technique were divided into the side-to-end or
end-to-end anastomosis groups between January 2012 and December
2019. Side-to-end anastomosis was performed in 63 patients (31 men
and 32 women); end-to-end anastomosis was performed in 99 patients
(58 men and 41 women). The characteristics of the patients and
tumors are shown in Table I. The
side-to-end anastomosis group tended to more frequently exhibit
tumor locations in the lower rectum, compared to the end-to-end
anastomosis group (end-to-end anastomosis group: RS/upper rectum
(n=93), lower rectum (n=6), side-to-end anastomosis group: RS/upper
rectum (n=49), lower rectum (n=14), P<0.01). No significant
differences were observed in other characteristics between the two
groups.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Overall | End-to-end
anastomosis | Side-to-end
anastomosis | P-value |
|---|
| Patients, n | 162 | 99 | 63 | |
| Age, years, median
(IQR) | 63 (55-71) | 64 (55-71) | 62 (56-71) | 0.74 |
| Sex, male/female,
n | 89/73 | 58/41 | 31/32 | 0.26 |
| BMI,
kg/m2, median (IQR) | 22.1 (20.3-24.5) | 22.5 (20.5-24.4) | 21.9 (20.3-23.9) | 0.32 |
| Smoking, n | 47 | 30 | 17 | 0.72 |
| Cardiovascular
disease, n | 34 | 33 | 21 | 1.00 |
| Lung disease, n | 18 | 10 | 8 | 0.62 |
| Liver disease, n | 3 | 1 | 2 | 0.56 |
| Renal disease, n | 3 | 2 | 1 | 1.00 |
| Diabetes mellitus,
n | 27 | 16 | 11 | 0.83 |
| Preoperative
chemoradiation therapy, n | 3 | 0 | 3 | 0.06 |
| Albumin, g/dl, median
(IQR) | 4.3 (4.1-4.6) | 4.4 (4.1-4.6) | 4.3 (4.0-4.5) | 0.40 |
| Location | | | | <0.01 |
|
RS/upper
rectum, n | 142 | 93 | 49 | |
|
Lower
rectum, n | 20 | 6 | 14 | |
|
Tumor size,
mm, median (IQR) | 40 (25-55) | 38 (25-55) | 41 (26-53) | 0.62 |
| pStagea, n | | | | 0.19 |
|
I | 56 | 31 | 25 | |
|
II | 31 | 24 | 7 | |
|
III | 61 | 35 | 26 | |
|
IV | 14 | 9 | 5 | |
Surgical outcomes are shown in Table II. No significant differences were
observed in the approach (open or laparoscopy), surgical type (high
or low anterior resection), lymphadenectomy grade, stoma diversion,
lateral lymph node dissection, or trans-anal tube placement. The
mean operating time was significantly longer in the side-to-end
anastomosis group than in the end-to-end anastomosis group (236 vs.
305 min, P<0.01). Blood loss was also significantly greater in
the side-to-end anastomosis group than in the end-to-end
anastomosis group (10 vs. 11 ml, P=0.03). The rate of ICG-FA
performance was higher in the side-to-end anastomosis group than in
the end-to-end anastomosis group. Notably, no significant
difference was observed in terms of additional colon resection
after ICG-FA. The AL rate was significantly lower in the
side-to-end anastomosis group than in the end-to-end anastomosis
group (4.8 vs. 18.2%, P=0.02). Finally, no significant differences
were observed between the groups in the incidence rates of other
complications, nor in the number of postoperative hospital
days.
 | Table IISurgical outcomes. |
Table II
Surgical outcomes.
| Surgical
outcomes | Overall
(n=162) | End-to-end
anastomosis (n=99) | Side-to-end
anastomosis (n=63) | P-value |
|---|
| Open/laparoscopy,
n | 12/150 | 8/91 | 4/59 | 0.77 |
| Operation, n | | | | 0.09 |
|
HAR | 52 | 37 | 15 | |
|
LAR | 110 | 62 | 48 | |
| D-number, n | | | | 0.38 |
|
3 | 114 | 67 | 47 | |
|
1 or 2 | 48 | 32 | 16 | |
| Diverting stoma,
n | 18 | 7 | 11 | 0.07 |
| Simultaneous
resection, n | 21 | 16 | 5 | 0.16 |
| Lateral lymph node
dissection, n | 14 | 7 | 7 | 0.40 |
| Operation time,
min, median (IQR) | 254 (208-346) | 236 (200-298) | 305 (236-395) | <0.01 |
| Bleeding volume,
ml, median (IQR) | 10 (10-23) | 10 (8-20) | 11 (10-29) | 0.03 |
| Trans-anal tube,
n | 148 | 91 | 57 | 0.78 |
| ICG-FA, n | 114 | 54 | 60 | <0.01 |
| Additional
resection after ICG-FA, n | 11 | 7 | 4 | 1 |
| Anastomotic
leakage, n | 21 | 18 | 3 | 0.02 |
| Ileus, n | 4 | 3 | 1 | 1.00 |
| Surgical site
infection, | 3 | 2 | 1 | 1.00 |
| Hospital days,
median (IQR) | 12 (10-16) | 12 (10-18) | 12 (11-16) | 0.73 |
| Mortality, n | 0 | 0 | 0 | N/A |
Furthermore, propensity score matching (PSM) was
used to minimize the effects of potential confounders. The
propensity score was calculated for each patient with variables
(age, sex, BMI, smoking, preoperative chemoradiation therapy,
location, tumor size, pStage, diverting stoma, ICG-FA, trans-anal
tube) that were not equally distributed and were thought to be
confounding factors between the two groups. In PSM, one-to-one
matching between the groups was performed using the nearest
neighbor matching method with a caliper width of 0.2. By PSM, 36
cases were selected in each group. Although not significantly
different after PSM, the rate of AL still tended to be lower in the
side-to-end anastomosis group than the end-to-end anastomosis group
(5.6 vs. 19.4%, P=0.15) (Tables
SI and SII).
Factors associated with anastomotic
leakage
Based on these data, we investigated the factors
associated with AL. The results of univariate and multivariate
analyses of these factors are shown in Table III. Univariate analysis revealed
that AL was significantly associated with a smoking habit, blood
loss (>100 ml), anastomosis type (side-to-end anastomosis), and
additional colon resection after ICG-FA. Importantly, performance
of ICG-FA was not significantly associated with AL. Multivariate
analysis showed that a smoking habit (odds ratio: 2.84, 95%
confidence interval: 1.04-7.77; P=0.04) and anastomosis
(side-to-end anastomosis, odds ratio: 0.22, 95% confidence
interval: 0.06-0.82; P=0.02) were significantly associated with
AL.
 | Table IIIUnivariate and multivariate analyses
of risk factors associated with anastomotic leakage. |
Table III
Univariate and multivariate analyses
of risk factors associated with anastomotic leakage.
| | Univariate
analysis | Multivariate
analysis |
|---|
|
Characteristics | Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value |
|---|
| Age, >65
years | 1.25 | 0.50-3.13 | 0.63 | | | |
| Sex, male | 2.26 | 0.98-6.17 | 0.11 | | | |
| BMI, ≥25
kg/m2 | 1.53 | 0.50-4.64 | 0.45 | | | |
| Smoking | 3.21 | 1.26-8.18 | 0.02 | 2.84 | 1.04-7.77 | 0.04 |
| Cardiovascular
disease | 0.78 | 0.28-2.13 | 0.62 | | | |
| Lung disease | 2.13 | 0.63-7.24 | 0.22 | | | |
| Liver disease | 0.92 | 0.05-18.44 | 1.00 | | | |
| Renal disease | 0.92 | 0.05-18.44 | 1.00 | | | |
| Diabetes
mellitus | 0.22 | 0.03-1.72 | 0.15 | | | |
| Preoperative
chemoradiation therapy | 0.92 | 0.05-18.44 | 1.00 | | | |
| Albumin, ≥4.0
mg/dl | 1.34 | 0.27-6.60 | 0.72 | | | |
| Location, lower
rectum | 1.84 | 0.55-6.15 | 0.32 | | | |
| pStage, III-IV | 1.65 | 0.65-4.17 | 0.29 | | | |
| Surgical procedure,
laparoscopy | 0.41 | 0.10-1.65 | 0.21 | | | |
| D-number, D3 | 0.91 | 0.60-1.37 | 0.65 | | | |
| Diverting
stoma | 0.37 | 0.05-2.89 | 0.34 | | | |
| Simultaneous
resection | 1.14 | 0.31-4.26 | 0.85 | | | |
| Lateral lymph node
dissection | 1.97 | 0.50-7.74 | 0.33 | | | |
| Operation time,
≥300 min | 2.13 | 0.85-5.37 | 0.11 | | | |
| Bleeding volume,
≥100 ml | 3.94 | 1.30-11.90 | 0.02 | 3.43 | 0.99-12.00 | 0.05 |
| Trans-anal
tube | 2.03 | 0.25-16.40 | 0.51 | | | |
| ICG-FA | 0.82 | 0.31-2.18 | 0.69 | | | |
| Additional
resection after ICG-FA | 4.50 | 1.19-17.00 | 0.03 | 4.46 | 0.95-20.90 | 0.06 |
| Tumor size, ≥40
mm | 1.00 | | 0.37 | | | |
| Anastomosis,
side-to-end anastomosis | 0.23 | 0.06-0.80 | 0.02 | 0.22 | 0.06-0.82 | 0.02 |
Discussion
We evaluated the surgical outcomes of trans-anal
side-to-end anastomosis, and identified patient and operative
factors associated with AL. The most important finding was that the
AL rate was significantly lower in the side-to-end than end-to-end
anastomosis group (4.8 vs. 18.2%, respectively, P=0.02). After PSM
which was used to minimize the effects of potential confounders,
the rate of AL tended to be lower in the side-to-end anastomosis
group than the end-to-end anastomosis group. Furthermore, the AL
differed significantly according to the method of anastomosis in
both univariate and multivariate analyses. Finally, the tumors
tended to be located lower down in the side-to-end anastomosis
group than in the end-to-end anastomosis group. We suggest that
side-to-end anastomosis using a double-stapling technique after
anterior resection of rectal cancer may prevent AL. Our findings
are important because few similar studies have been reported in the
literature.
The mechanism by which the anastomosis method
affects the AL rate remains unclear. However, one possible
explanation is poorer vascular perfusion at the distal end of the
colon, as suggested in a previous randomized study (14). Adequate blood flow at the site of
anastomosis is important for the prevention of AL (14-18).
Blood flow at the anastomotic site of side-to-end anastomosis may
be better than at the anastomotic site of end-to-end anastomosis.
In principle, we perform ligation of the inferior mesenteric artery
for D3 lymph node dissection. It has been reported that a left
colic artery-sparing procedure provides better blood flow to the
distal end of the colon but does not contribute to AL (22,23).
Further studies are required to determine the impacts of inferior
mesenteric artery ligation.
Smoking was a risk factor for AL in the present
study. An association between smoking and AL has been reported
previously, with reduced mucosal blood flow cited as a possible
contributing factor (24-30).
In this study, selection bias might have significantly impacted the
results because smoking histories were not available. A smoking
index would have been useful to discriminate between current and
ex-smokers.
In the present study, operating time was
significantly longer in the side-to-end anastomosis group than the
end-to-end anastomosis group. There were several reasons for this
difference. First, tumors were located more frequently at the lower
rectum in the side-to-end anastomosis group. Second, ICG-FA tended
to be performed in more patients in the side-to-end anastomosis
group. As side-to-end anastomosis was introduced in January 2017 in
our hospital, ICG-FA was not routinely performed prior to that
time. Third, side-to-end anastomosis generally requires slightly
longer than end-to-end anastomosis. However, the location of the
tumor and performance of ICG-FA, rather than the type of
anastomosis, may have led to the longer operating time. Notably,
significantly more blood was lost in the side-to-end anastomosis
group than in the end-to-end anastomosis group. Tumor location,
rather than the type of anastomosis, may have been the main reason
for the blood loss.
This study had some limitations. First, the criteria
to determine side-to-end anastomosis or end-to-end anastomosis were
partially subjective. Tension at the anastomosis site was
determined by the surgeon, and it is sometimes difficult to
determine whether high tension is present. An objective evaluation
of tension at the anastomosis site and objective criteria for
selection of anastomosis type are needed. Second, this was a
small-scale, retrospective, single-center study and the number of
cases of AL was small, so the results might have been subject to
various biases, although biases were reduced by PSM. Third, smoking
history data were not available and a detailed review was not
conducted, which might have enabled the use of a smoking index and
discrimination between current smokers and ex-smokers. Fourth, it
has been reported that side-to-end anastomosis may be superior to
end-to-end anastomosis in terms of postoperative bowel function;
however, no comparison of functional outcome was performed between
side-to-end anastomosis and end-to-end anastomosis in the present
study (19, 20, 31-35). Fifth, data are not available about the
intactness of the mesorectum and the CRM status of the resected
specimens in the two groups. It is difficult to demonstrate the
usefulness of a single factor for preventing AL, because AL is
multifactorial. Prospective, randomized controlled, and
multi-institutional studies are required to validate these
findings.
In conclusion, side-to-end anastomosis with a
double-stapling technique might be useful for prevention of AL,
following AR. Further large-scale randomized controlled trials are
required to validate the usefulness of side-to-end anastomosis for
reducing the rate of AL in patients who undergo AR.
Supplementary Material
Patient characteristics after
propensity score matching.
Surgical outcomes after propensity
score matching.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HK, TI, NN, AK, TT, SI, TO, AK, TH, JN, SM, MM, MT
and OI conceived the study concept and design, and were involved in
data interpretation. HK, TI, NN, AK, JN and SM were involved in
data collection. TI and NN confirm the authenticity of all the raw
data. HK, TI, SM and MT performed the data analysis. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the institutional review
board of the International University of Health and Welfare Mita
Hospital (Minato-ku, Japan; approval no. 5-19-41), and disclosed in
the form of opt-out. The informed consent forms for treatment
included consent for the use of patient data and materials for
research purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ortiz H, Wibe A, Ciga MA, Kreisler E,
Garcia-Granero E, Roig JV and Biondo S: Spanish Rectal Cancer
Project. Multicenter study of outcome in relation to the type of
resection in rectal cancer. Dis Colon Rectum. 57:811–822.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
van der Pas MH, Haglind E, Cuesta MA,
Fürst A, Lacy AM, Hop WC and Bonjer HJ: COlorectal cancer
Laparoscopic or Open Resection II (COLOR II) Study Group.
Laparoscopic versus open surgery for rectal cancer (COLOR II):
Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol.
14:210–218. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Park JS, Choi GS, Kim SH, Kim HR, Kim NK,
Lee KY, Kang SB, Kim JY, Lee KY, Kim BC, et al: Multicenter
analysis of risk factors for anastomotic leakage after laparoscopic
rectal cancer excision: The Korean laparoscopic colorectal surgery
study group. Ann Surg. 257:665–671. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rahbari NN, Weitz J, Hohenberger W, Heald
RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, et al:
Definition and grading of anastomotic leakage following anterior
resection of the rectum: A proposal by the International Study
Group of Rectal Cancer. Surgery. 147:339–351. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rullier E, Laurent C, Garrelon JL, Michel
P, Saric J and Parneix M: Risk factors for anastomotic leakage
after resection of rectal cancer. Br J Surg. 85:355–358.
1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chang SC, Lin JK, Yang SH, Jiang JK, Chen
WC and Lin TC: Long-term outcome of anastomosis leakage after
curative resection for mid and low rectal cancer.
Hepatogastroenterology. 50:1898–1902. 2003.PubMed/NCBI
|
|
7
|
Dehni N, Parc R and Church JM: Colonic
J-pouch-anal anastomosis for rectal cancer. Dis Colon Rectum.
46:667–675. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sciuto A, Merola G, De Palma GD, Sodo M,
Pirozzi F, Bracale UM and Bracale U: Predictive factors for
anastomotic leakage after laparoscopic colorectal surgery. World J
Gastroenterol. 24:2247–2260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Akiyoshi T, Ueno M, Fukunaga Y, Nagayama
S, Fujimoto Y, Konishi T, Kuroyanagi H and Yamaguchi T: Effect of
body mass index on short-term outcomes of patients undergoing
laparoscopic resection for colorectal cancer: A single institution
experience in Japan. Surg Laparosc Endosc Percutan Tech.
21:409–414. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hamabe A, Ito M, Nishigori H, Nishizawa Y
and Sasaki T: Preventive effect of diverting stoma on anastomotic
leakage after laparoscopic low anterior resection with double
stapling technique reconstruction applied based on risk
stratification. Asian J Endosc Surg. 11:220–226. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tanaka K, Okuda J, Yamamoto S, Ito M,
Sakamoto K, Kokuba Y, Yoshimura K and Watanabe M: Risk factors for
anastomotic leakage after laparoscopic surgery with the double
stapling technique for stage 0/I rectal carcinoma: A subgroup
analysis of a multicenter, single-arm phase II trial. Surg Today.
47:1215–1222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Moran BJ: Predicting the risk and
diminishing the consequences of anastomotic leakage after anterior
resection for rectal cancer. Acta Chir Iugosl. 57:47–50.
2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Testini M, Margari A, Amoruso M, Lissidini
G and Bonomo GM: The dehiscence of colorectal anastomoses: The risk
factors. Ann Ital Chir. 71:433–440. 2000.PubMed/NCBI(In Italian).
|
|
14
|
Hallböök O, Johansson K and Sjödahl R:
Laser Doppler blood flow measurement in rectal resection for
carcinoma - comparison between the straight and colonic J pouch
reconstruction. Br J Surg. 83:389–392. 1996.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dennett ER and Parry BR: Misconceptions
about the colonic J-pouch: What the accumulating data show. Dis
Colon Rectum. 42:804–811. 1999.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Floodeen H, Hallböök O, Rutegård J,
Sjödahl R and Matthiessen P: Early and late symptomatic anastomotic
leakage following low anterior resection of the rectum for cancer:
Are they different entities? Colorectal Dis. 15:334–340.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chung RS: Blood flow in colonic
anastomoses. Effect of stapling and suturing. Ann Surg.
206:335–339. 1987.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qu H, Liu Y and Bi DS: Clinical risk
factors for anastomotic leakage after laparoscopic anterior
resection for rectal cancer: A systematic review and meta-analysis.
Surg Endosc. 29:3608–3617. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rubin F, Douard R and Wind P: The
functional outcomes of coloanal and low colorectal anastomoses with
reservoirs after low rectal cancer resections. Am Surg.
80:1222–1229. 2014.PubMed/NCBI
|
|
20
|
Hüttner FJ, Tenckhoff S, Jensen K, Uhlmann
L, Kulu Y, Büchler MW, Diener MK and Ulrich A: Meta-analysis of
reconstruction techniques after low anterior resection for rectal
cancer. Br J Surg. 102:735–745. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Rybakov EG, Pikunov DY, Fomenko OY,
Chernyshov SV and Shelygin YA: Side-to-end vs. straight stapled
colorectal anastomosis after low anterior resection: Results of
randomized clinical trial. Int J Colorectal Dis. 31:1419–1426.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Seike K, Koda K, Saito N, Oda K, Kosugi C,
Shimizu K and Miyazaki M: Laser Doppler assessment of the influence
of division at the root of the inferior mesenteric artery on
anastomotic blood flow in rectosigmoid cancer surgery. Int J
Colorectal Dis. 22:689–697. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Akagi T, Inomata M, Hara T, Mizusawa J,
Katayama H, Shida D, Ohue M, Ito M, Kinugasa Y, Saida Y, et al:
Clinical impact of D3 lymph node dissection with left colic artery
(LCA) preservation compared to D3 without LCA preservation:
Exploratory subgroup analysis of data from JCOG0404. Ann
Gastroenterol Surg. 4:163–169. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bertelsen CA, Andreasen AH, Jørgensen T
and Harling H: Danish Colorectal Cancer Group. Anastomotic leakage
after anterior resection for rectal cancer: Risk factors.
Colorectal Dis. 12:37–43. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Richards CH, Campbell V, Ho C, Hayes J,
Elliott T and Thompson-Fawcett M: Smoking is a major risk factor
for anastomotic leak in patients undergoing low anterior resection.
Colorectal Dis. 14:628–633. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kim MJ, Shin R, Oh HK, Park JW, Jeong SY
and Park JG: The impact of heavy smoking on anastomotic leakage and
stricture after low anterior resection in rectal cancer patients.
World J Surg. 35:2806–2810. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kruschewski M, Rieger H, Pohlen U, Hotz HG
and Buhr HJ: Risk factors for clinical anastomotic leakage and
postoperative mortality in elective surgery for rectal cancer. Int
J Colorectal Dis. 22:919–927. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vignali A, Gianotti L, Braga M, Radaelli
G, Malvezzi L and Di Carlo V: Altered microperfusion at the rectal
stump is predictive for rectal anastomotic leak. Dis Colon Rectum.
43:76–82. 2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fawcett A, Shembekar M, Church JS,
Vashisht R, Springall RG and Nott DM: Smoking, hypertension, and
colonic anastomotic healing; a combined clinical and
histopathological study. Gut. 38:714–718. 1996.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Emmanuel AV and Kamm MA: Laser Doppler
measurement of rectal mucosal blood flow. Gut. 45:64–69.
1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Parc Y, Ruppert R, Fuerst A, Golcher H,
Zutshi M, Hull T, Tiret E, Hemminger F, Galandiuk S, Fender S, et
al: Better Function With a Colonic J-Pouch or a Side-to-end
Anastomosis?: A randomized controlled trial to compare the
complications, functional outcome, and quality of life in patients
with low rectal cancer after a J-Pouch or a side-to-end
anastomosis. Ann Surg. 269:815–826. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Machado M, Nygren J, Goldman S and
Ljungqvist O: Similar outcome after colonic pouch and side-to-end
anastomosis in low anterior resection for rectal cancer: A
prospective randomized trial. Ann Surg. 238:214–220.
2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Machado M, Nygren J, Goldman S and
Ljungqvist O: Functional and physiologic assessment of the colonic
reservoir or side-to-end anastomosis after low anterior resection
for rectal cancer: A two-year follow-up. Dis Colon Rectum.
48:29–36. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Siddiqui MR, Sajid MS, Woods WG, Cheek E
and Baig MK: A meta-analysis comparing side to end with colonic
J-pouch formation after anterior resection for rectal cancer. Tech
Coloproctol. 14:113–123. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fazio VW, Zutshi M, Remzi FH, Parc Y,
Ruppert R, Fürst A, Celebrezze J Jr, Galanduik S, Orangio G, Hyman
N, et al: A randomized multicenter trial to compare long-term
functional outcome, quality of life, and complications of surgical
procedures for low rectal cancers. Ann Surg. 246:481–490.
2007.PubMed/NCBI View Article : Google Scholar
|