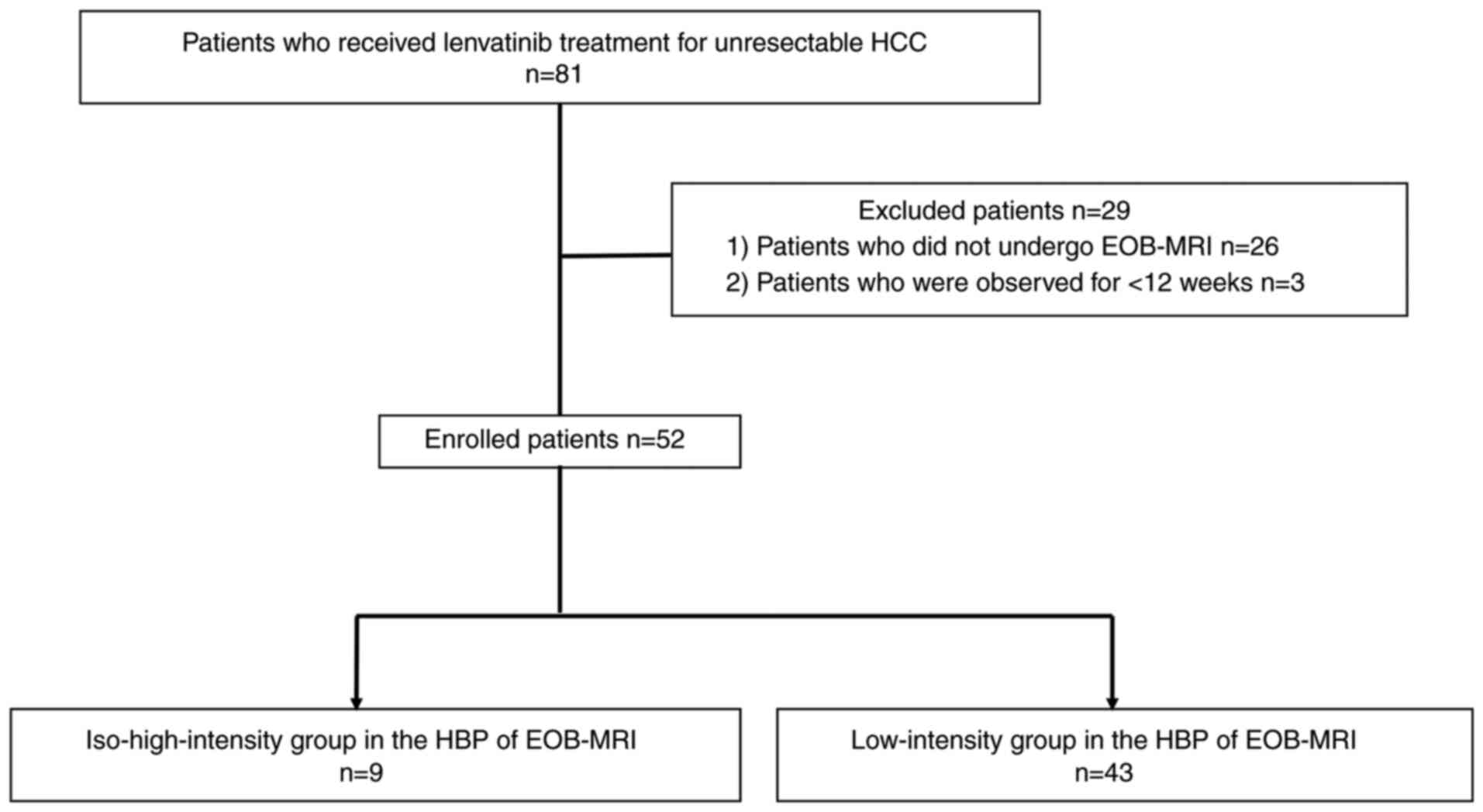
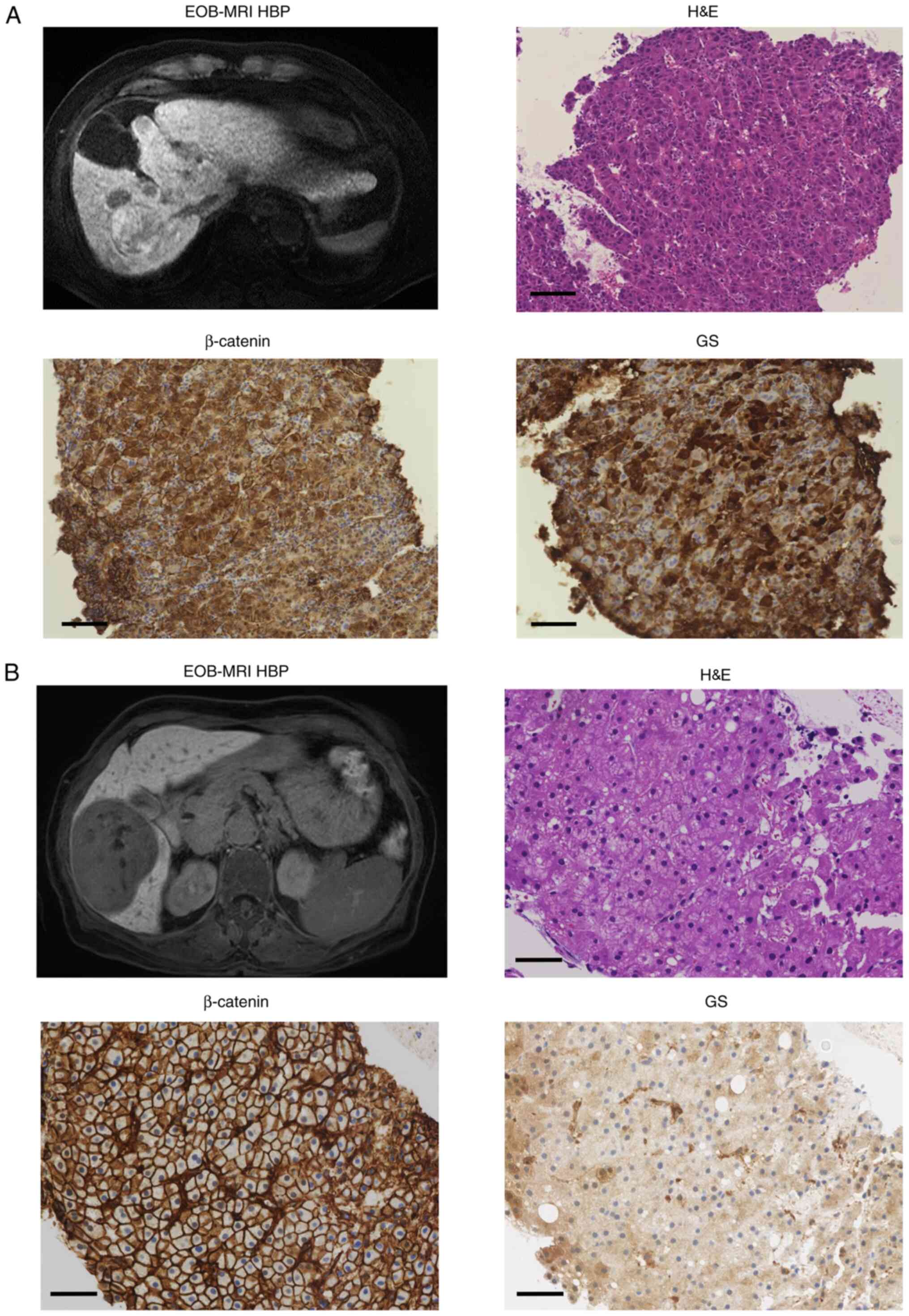
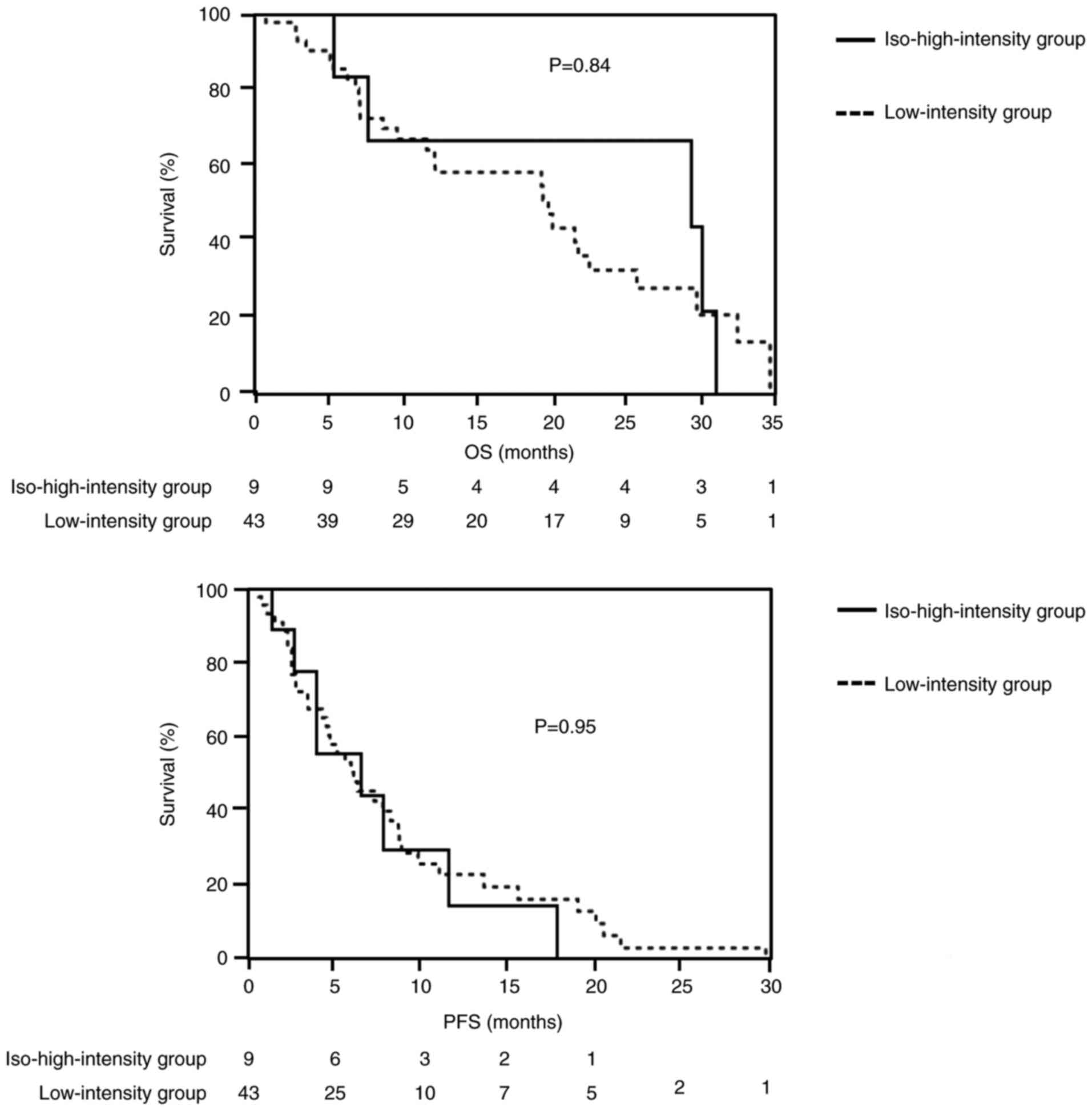
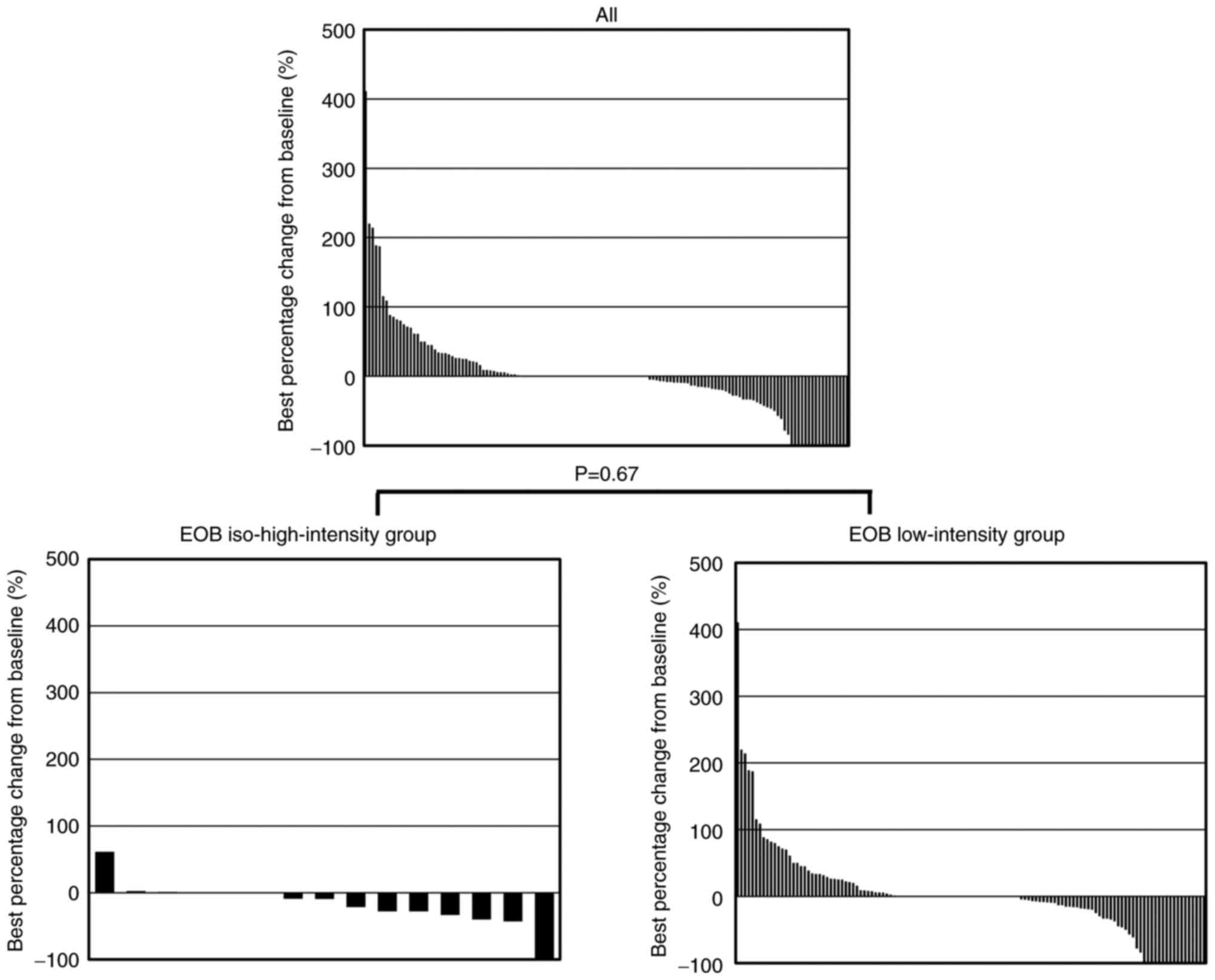
|
1
|
Caldwell S and Park SH: The epidemiology
of hepatocellular cancer: From the perspectives of public health
problem to tumor biology. J Gastroenterol. 44 Suppl:S96–S101.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eso Y and Marusawa H: Novel approaches for
molecular targeted therapy against hepatocellular carcinoma.
Hepatol Res. 48:597–607. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Torbenson MS, Ng IO, Park YN, Roncalli M
and Sakamoto M: Digestive system tumours: WHO classification of
tumours. In: Hepatocellular Carcinoma. 5th edition. WHO
Classification of Tumours Editorial Board. WHO Press, Geneva,
pp229-239, 2019.
|
|
6
|
Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk
O, Villacorta-Martin C, Castro de Moura M, Putra J, Camprecios G,
Bassaganyas L, Akers N, et al: Identification of an immune-specific
class of hepatocellular carcinoma, based on molecular features.
Gastroenterology. 153:812–826. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Llovet JM, Montal R, Sia D and Finn RS:
Molecular therapies and precision medicine for hepatocellular
carcinoma. Nat Rev Clin Oncol. 15:599–616. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pinyol R, Sia D and Llovet JM: Immune
exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies
in HCC. Clin Cancer Res. 25:2021–2023. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ueno A, Masugi Y, Yamazaki K, Komuta M,
Effendi K, Tanami Y, Tsujikawa H, Tanimoto A, Okuda S, Itano O, et
al: OATP1B3 expression is strongly associated with Wnt/β-catenin
signalling and represents the transporter of gadoxetic acid in
hepatocellular carcinoma. J Hepatol. 61:1080–1087. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kudo M: Gd-EOB-DTPA-MRI could predict
WNT/β-catenin mutation and resistance to immune checkpoint
inhibitor therapy in hepatocellular carcinoma. Liver Cancer.
9:479–490. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tsuboyama T, Onishi H, Kim T, Akita H,
Hori M, Tatsumi M, Nakamoto A, Nagano H, Matsuura N, Wakasa K and
Tomoda K: Hepatocellular carcinoma: Hepatocyte-selective
enhancement at gadoxetic acid-enhanced MR imaging-correlation with
expression of sinusoidal and canalicular transporters and bile
accumulation. Radiology. 255:824–833. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kitao A, Matsui O, Yoneda N, Kozaka K,
Kobayashi S, Koda W, Gabata T, Yamashita T, Kaneko S, Nakanuma Y,
et al: Hypervascular hepatocellular carcinoma: Correlation between
biologic features and signal intensity on gadoxetic acid-enhanced
MR images. Radiology. 265:780–789. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res.
2014(638747)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6(18)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL
and Peng SY: Beta-catenin mutations are associated with a subset of
low-stage hepatocellular carcinoma negative for hepatitis B virus
and with favorable prognosis. Am J Pathol. 157:763–770.
2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Laurent-Puig P, Legoix P, Bluteau O,
Belghiti J, Franco D, Binot F, Monges G, Thomas G, Bioulac-Sage P
and Zucman-Rossi J: Genetic alterations associated with
hepatocellular carcinomas define distinct pathways of
hepatocarcinogenesis. Gastroenterology. 120:1763–1773.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zucman-Rossi J, Benhamouche S, Godard C,
Boyault S, Grimber G, Balabaud C, Cunha AS, Bioulac-Sage P and
Perret C: Differential effects of inactivated Axin1 and activated
beta-catenin mutations in human hepatocellular carcinomas.
Oncogene. 26:774–780. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tsujikawa H, Masugi Y, Yamazaki K, Itano
O, Kitagawa Y and Sakamoto M: Immunohistochemical molecular
analysis indicates hepatocellular carcinoma subgroups that reflect
tumor aggressiveness. Hum Pathol. 50:24–33. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee HC, Kim M and Wands JR: Wnt/Frizzled
signaling in hepatocellular carcinoma. Front Biosci. 11:1901–1915.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Harding JJ, Nandakumar S, Armenia J,
Khalil DN, Albano M, Ly M, Shia J, Hechtman JF, Kundra R, El Dika
I, et al: Prospective Genotyping of hepatocellular carcinoma:
Clinical implications of next-generation sequencing for matching
patients to targeted and immune therapies. Clin Can Res.
25:2116–2126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nhieu JT, Renard CA, Wei Y, Cherqui D,
Zafrani ES and Buendia MA: Nuclear accumulation of mutated
beta-catenin in hepatocellular carcinoma is associated with
increased cell proliferation. Am J Pathol. 155:703–710.
1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cadoret A, Ovejero C, Terris B, Souil E,
Lévy L, Lamers WH, Kitajewski J, Kahn A and Perret C: New targets
of beta-catenin signaling in the liver are involved in the
glutamine metabolism. Oncogene. 21:8293–8301. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Loeppen S, Schneider D, Gaunitz F,
Gebhardt R, Kurek R, Buchmann A and Schwarz M: Overexpression of
glutamine synthetase is associated with beta-catenin-mutations in
mouse liver tumors during promotion of hepatocarcinogenesis by
phenobarbital. Cancer Res. 62:5685–5688. 2002.PubMed/NCBI
|
|
25
|
Kitao A, Matsui O, Yoneda N, Kozaka K,
Kobayashi S, Sanada J, Koda W, Minami T, Inoue D, Yoshida K, et al:
Hepatocellular carcinoma with β-catenin mutation: Imaging and
pathologic characteristics. Radiology. 275:708–717. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Giles RH, van Es JH and Clevers H: Caught
up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ramjiawan RR, Griffioen AW and Duda DG:
Anti-angiogenesis for cancer revisited: Is there a role for
combinations with immunotherapy? Angiogenesis. 20:185–204.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hegde PS, Wallin JJ and Mancao C:
Predictive markers of anti-VEGF and emerging role of angiogenesis
inhibitors as immunotherapeutics. Semin Cancer Biol. 52:117–124.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kwilas AR, Donahue RN, Tsang KY and Hodge
JW: Immune consequences of tyrosine kinase inhibitors that
synergize with cancer immunotherapy. Cancer Cell Microenviron.
2(e677)2015.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Wang DY, Johnson DB and Davis EJ:
Toxicities associated with PD-1/PD-L1 blockade. Cancer J. 24:36–40.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sprinzl MF, Reisinger F, Puschnik A,
Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A,
Galle PR, Schuchmann M, et al: Sorafenib perpetuates cellular
anticancer effector functions by modulating the crosstalk between
macrophages and natural killer cells. Hepatology. 57:2358–2368.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wei X, Tang C, Lu X, Liu R, Zhou M, He D,
Zheng D, Sun C and Wu Z: MiR-101 targets DUSP1 to regulate the
TGF-β secretion in sorafenib inhibits macrophage-induced growth of
hepatocarcinoma. Oncotarget. 6:18389–18405. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Farsaci B, Donahue RN, Coplin MA, Grenga
I, Lepone LM, Molinolo AA and Hodge JW: Immune consequences of
decreasing tumor vasculature with antiangiogenic tyrosine kinase
inhibitors in combination with therapeutic vaccines. Cancer Immunol
Res. 2:1090–1102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Romero AI, Chaput N, Poirier-Colame V,
Rusakiewicz S, Jacquelot N, Chaba K, Mortier E, Jacques Y,
Caillat-Zucman S, Flament C, et al: Regulation of CD4(+)NKG2D(+)
Th1 cells in patients with metastatic melanoma treated with
sorafenib: Role of IL-15Rα and NKG2D triggering. Cancer Res.
74:68–80. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sunay MM, Foote JB, Leatherman JM, Edwards
JP, Armstrong TD, Nirschl CJ, Hicks J and Emens LA: Sorafenib
combined with HER-2 targeted vaccination can promote effective T
cell immunity in vivo. Int Immunopharmacol. 46:112–123.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chuang HY, Chang YF, Liu RS and Hwang JJ:
Serial low doses of sorafenib enhance therapeutic efficacy of
adoptive T cell therapy in a murine model by improving tumor
microenvironment. PLoS One. 9(e109992)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen ML, Yan BS, Lu WC, Chen MH, Yu SL,
Yang PC and Cheng AL: Sorafenib relieves cell-intrinsic and
cell-extrinsic inhibitions of effector T cells in tumor
microenvironment to augment antitumor immunity. Int J Cancer.
134:319–331. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cabrera R, Ararat M, Xu Y, Brusko T,
Wasserfall C, Atkinson MA, Chang LJ, Liu C and Nelson DR: Immune
modulation of effector CD4+ and regulatory T cell function by
sorafenib in patients with hepatocellular carcinoma. Cancer Immunol
Immunother. 62:737–746. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chang CJ, Yang YH, Chiu CJ, Lu LC, Liao
CC, Liang CW, Hsu CH and Cheng AL: Targeting tumor-infiltrating
Ly6G+ myeloid cells improves sorafenib efficacy in mouse
orthotopic hepatocellular carcinoma. Int J Cancer. 142:1878–1889.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kwilas AR, Ardiani A, Donahue RN, Aftab DT
and Hodge JW: Dual effects of a targeted small-molecule inhibitor
(cabozantinib) on immune-mediated killing of tumor cells and immune
tumor microenvironment permissiveness when combined with a cancer
vaccine. J Transl Med. 12(294)2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tsai AK, Khan AY, Worgo CE, Wang LL, Liang
Y and Davila E: A multikinase and DNA-PK inhibitor combination
immunomodulates melanomas, suppresses tumor progression, and
enhances immunotherapies. Cancer Immunol Res. 5:790–803.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Heine A, Schilling J, Grünwald B, Krüger
A, Gevensleben H, Held SA, Garbi N, Kurts C, Brossart P, Knolle P,
et al: The induction of human myeloid derived suppressor cells
through hepatic stellate cells is dose-dependently inhibited by the
tyrosine kinase inhibitors nilotinib, dasatinib and sorafenib, but
not sunitinib. Cancer Immunol Immunother. 65:273–282.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yamauchi M, Ono A, Ishikawa A, Kodama K,
Uchikawa S, Hatooka H, Zhang P, Teraoka Y, Morio K, Fujino H, et
al: Tumor fibroblast growth factor receptor 4 level predicts the
efficacy of lenvatinib in patients with advanced hepatocellular
carcinoma. Clin Transl Gastroenterol. 11(e00179)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fujita N, Nishie A, Kubo Y, Asayama Y,
Ushijima Y, Takayama Y, Moirta K, Shirabe K, Aishima S and Honda H:
Hepatocellular carcinoma: Clinical significance of signal
heterogeneity in the hepatobiliary phase of gadoxetic acid-enhanced
MR imaging. Eur Radiol. 25:211–220. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Dal Bello B, Rosa L, Campanini N, Tinelli
C, Torello Viera F, D'Ambrosio G, Rossi S and Silini EM: Glutamine
synthetase immunostaining correlates with pathologic features of
hepatocellular carcinoma and better survival after radiofrequency
thermal ablation. Clin Cancer Res. 16:2157–2166. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Weitz IC and Liebman HA: Des-gamma-carboxy
(abnormal) prothrombin and hepatocellular carcinoma: A critical
review. Hepatology. 18:990–997. 1993.PubMed/NCBI View Article : Google Scholar
|