Introduction
A previous study reported 85-90% of cases of primary
liver cancer were hepatocellular carcinoma (HCC); globally, the
third cause of death related to cancer (1). Recent advances in systemic
chemotherapy for advanced HCC, including molecular targeted agents
(MTAs) and immune checkpoint inhibitor (ICI) therapies, have
improved patient prognosis; however, it is important to select
agents appropriate for the personalized treatment of HCC (2-4).
Around 11-37% of HCC cases harbor mutations of
WNT/β-catenin that lead to the immune microenvironment lacking
immune cell filtration, so called ‘immune exclusion’ or
‘non-inflamed cold’, in HCC (5).
Furthermore, HCC with immune exclusion associated with mutations of
WNT/β-catenin is resistant to ICI therapies (6-8).
Therefore, it is important to identify the subclass of HCC with or
without immune exclusion induced by WNT/β-catenin mutations before
treatment. Recently, HCC treatment has focused on this subclass
classification.
Ideally, mutations of WNT/β-catenin would be
identified using a non-invasive technique rather than by liver
biopsy. Previous studies demonstrated that HCC harboring mutations
of WNT/β-catenin had iso-high intensity during the hepatobiliary
phase (HBP) by magnetic resonance imaging enhanced by gadolinium
ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA-MRI,
i.e. EOB-MRI) indicating EOB-MRI may help identify an immune
exclusion class which might not respond to treatment with immune
checkpoint inhibitors (9,10). Around 12-22% of HCCs have iso-high
intensity during the HBP of EOB-MRI (11,12).
EOB-MRI may be a non-invasive alternative to liver biopsy, which
requires a histological method to identify mutations of
Wnt/β-catenin.
Previous studies have demonstrated how MTAs affect
survival of advanced HCC patients (3). Lenvatinib (LEN) is an oral
multikinase inhibitor of vascular endothelial growth factor (VEGF)
receptors 1-3, fibroblast growth factor (FGF) receptors 1-4,
platelet-derived growth factor (PDGF) receptor α, RET, and KIT
(13,14). An international randomized,
open-label trial that was performed in multiple centers to
investigate the non-inferiority of sorafenib (REFLECT; NCT01761266)
reported LEN significantly improved progression-free survival (PFS)
compared with sorafenib in patients with previously untreated,
metastatic, or unresectable HCC. LEN has been approved for HCC
treatment (3).
The therapeutic efficacy of LEN for HCC with
mutations of WNT/β-catenin has not been reported. Here, we analyzed
LEN efficacy for HCC that may have WNT/β-catenin mutations with
iso-high intensity during the HBP of EOB-MRI.
Materials and methods
Patients
This prospective single-center study analyzed LEN
efficacy for HCC that may have WNT/β-catenin mutations with
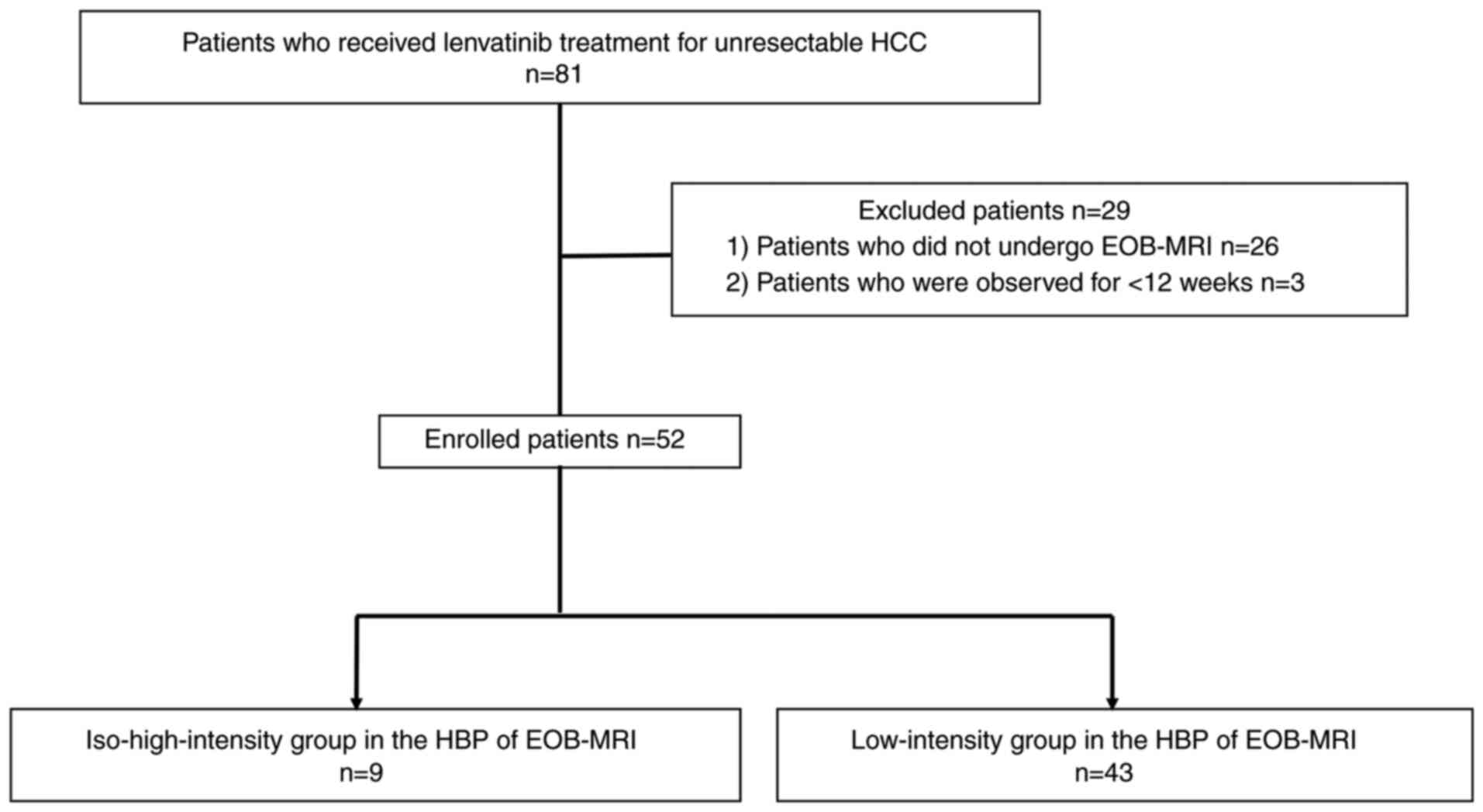
iso-high intensity during the HBP of EOB-MRI. Eighty-one patients
received LEN for non-resectable HCC at Iizuka Hospital from May
2018 to February 2021. We excluded 26 patients who did not undergo
EOB-MRI within 3 months prior to LEN administration and three
patients who were observed for <12 weeks. We evaluated 52
patients by EOB-MRI and of these, we enrolled 140 HCC (Fig. 1). This study was performed
according to the Declaration of Helsinki guidelines and was
approved by Iizuka Hospital ethics committee (approval no. 18070).
All patients gave written informed consent.
Treatment protocol
Patients received doses of LEN according to body
weight (8 mg/day for bodyweight <60 kg, or 12 mg/day for
bodyweight ≥60 kg) (Eisai Co., Ltd.). Dose interruption and
potential subsequent reduction (to 8 mg/day, 4 mg/day, or 4 mg
every other day) were permitted when patients developed LEN-related
adverse events. The protocols in the REFLECT trial were prescribed
by Eisai Co., Ltd. (3). Common
Terminology Criteria for Adverse Events, version 4.0 was used to
grade adverse events. Adverse events ≥grade 3 or any unacceptable
grade 2 adverse events were a cause for reduced drug dose or
interrupted treatment as per guidelines for LEN administration.
Following occurrence of an adverse event, LEN dose was reduced or
temporarily interrupted until symptoms were grade 1 or 2, as per
the Eisai Co., Ltd. guidelines.
Evaluation of treatment efficacy
Physicians evaluated antitumor responses using
modified RECIST v.1.1(15). The
disease control rate (DCR) was determined according to complete
response (CR), partial response (PR), or stable disease (SD)
present for ≥4 months. The objective response rate (ORR) was
defined as PR or CR. Each nodule was evaluated according to the top
three sized nodules in each case.
EOB-MRI
Characterization and pretreatment staging for HCC
was determined by Gd MRI using a 1.5-T or 3.0-T MR system (Ingeina;
Philips Healthcare) using the same protocol. For the dynamic study,
0.1 ml Primovist (0.25 mmol/ml gadoxetic acid; Bayer Schering
Pharma) was administered intravenously per kg/bodyweight. The
optimal arterial dominant phase, calculated as time of peak
enhancement in the abdominal aorta + an additional 10 sec of
imaging time (16-22 sec) was achieved using the test injection
method with 1.5 ml gadoxetic acid + 8-ml saline flush. Subsequent
to imaging during the arterial phase, portal and equilibrium phase
images were captured at 20 and 60 sec, respectively, once the
previous imaging phase was over. The HBP for all patients was
obtained 20 min post-injection.
Imaging analysis
Four hepatologists performed imaging analysis in a
blind fashion without clinicopathological information. Qualitative
analysis of HCC with iso-high intensity during the HBP of EOB-MRI
was defined by a signal intensity higher than the pre-contrast
image.
Immunohistochemical (IHC)
analyses
Liver tumor biopsy samples fixed with 10% formalin
were paraffin embedded. Serial sections (5-µm) were sectioned from
paraffin blocks of liver tissues and stained with
hematoxylin-eosin. The presence of glutamine synthetase (GS) and
β-catenin was determined in 14 HCC specimens by IHC with the
following primary antibodies: monoclonal mouse anti-human β-catenin
(#610153; BD Biosciences; 1/300 dilution) or monoclonal mouse
anti-human GS (#GS-6; Millipore; 1/500 dilution). A Bond Polymer
System was used to develop reactions (Leica Biosystems) related to
secondary antibodies. β-catenin staining in the nucleus is
indicative of an activating mutation in the catenin β-1 (CTNNB1)
gene (16,17) and strong GS diffuse staining is
indicative of the constitutive activation of WNT/β-catenin
signaling associated with β-catenin mutations (18). Therefore, the presence of β-catenin
nuclear staining in ≥5% of tumor cells (19) or strong diffuse GS staining were
considered to demonstrate the activation of WNT/β-catenin signaling
(9).
Statistical analysis
JMP Pro Version 11 statistical software was used for
all analyses (SAS Institute, Inc.). Results were shown as the
median (inter-quartile range). Significant differences between
groups were examined by χ2, Fisher's exact or
Mann-Whitney U test. The Kaplan-Meier technique was used for the
statistical analyses of overall survival (OS) and PFS; significant
differences in OS and PFS were determined by log-rank analysis.
Statistical significance was determined when P<0.05.
Results
Subjects' characteristics
Patient characteristics are shown in Table I. The iso-high-intensity group
contained patients with iso-high-intensity and low-intensity
nodules. There were nine patients (17.3%) and 14 HCC nodules (10%)
with iso-high intensity and 43 patients (82.7%) and 126 HCC nodules
(90.0%) with low intensity. Five patients (55.6%) had both
iso-high-intensity and low-intensity nodules in the
iso-high-intensity group. Tumor size was larger in the
iso-high-intensity group vs the low-intensity group [6.2 cm
(3.25-8.35) vs. 2.5 cm (1.6-3.9), P=0.004]. The levels of
antagonist-II (PIVKA-II) or vitamin K absence were higher in the
iso-high-intensity group [938 mAU/ml (26.5-31832.5) vs. 140 mAU/ml
(32-18112), P=0.0137]. There were two patients with Barcelona
Clinic Liver Cancer (BCLC) stage A, four with stage B, and three
with stage C in the iso-high-intensity group. In addition, there
were five patients with BCLC stage A, 23 with stage B and 15 with
stage C in the low-intensity group. Age, sex, etiology, Child-Pugh
grade, microvascular invasion (MVI), extrahepatic spread (HIS), and
serum α-fetoprotein levels were similar between groups.
 | Table ICharacteristics of patients in the
iso-high-intensity and low-intensity groups of HBP in EOB-MRI. |
Table I
Characteristics of patients in the
iso-high-intensity and low-intensity groups of HBP in EOB-MRI.
| Characteristic | All | Iso-high-intensity
group | Low-intensity
group | P-value |
|---|
| Number | 52 | 9 | 43 | |
| Age, years | 73 (68-79.75) | 82 (75-87) | 73 (68-78) | 0.686 |
| Sex, M/F | 36/16 | 7/2 | 29/14 | 0.53 |
| Etiology | | | | 0.3 |
|
HCV | 23 (44.2%) | 1 (11.1%) | 22 (51.2%) | |
|
HBV | 10 (19.2%) | 2 (22.2%) | 8 (18.6%) | |
|
Alcohol | 9 (17.3%) | 2 (22.2%) | 7 (16.3%) | |
|
AIH/PBC | 2 (3.8%) | 0 (0%) | 2 (4.7%) | |
|
Cryptogenic | 8 (15.5%) | 4 (44.5%) | 4 (9.2%) | |
| Max tumor size,
cm | 2.7 (1.7-4.9) | 6.2
(3.25-8.35) | 2.5 (1.6-3.9) | 0.0004 |
| Size of
intrahepatic lesion >3 cm | 24 (46.2%) | 7 (77.8%) | 17 (39.5%) | 0.0364 |
| Number of
intrahepatic lesions >5 | 31 (59.6%) | 5 (55.6%) | 26 (60.4%) | 0.7849 |
| MVI positive | 11 (21.2%) | 1 (11.1%) | 10 (23.3%) | 0.69 |
| EHS positive | 12 (23.1%) | 1 (11.1%) | 11 (25.6%) | 0.29 |
| Child-Pugh
score | | | | 0.63 |
|
5A | 29 (55.8%) | 6 (72.7%) | 23 (53.5%) | |
|
6A | 13 (25%) | 1 (11.1%) | 12 (27.9%) | |
|
>7 | 10 (19.2%) | 2 (22.2%) | 8 (18.6%) | |
| Albumin | 3.65 (3.3-4.1) | 3.7 (3.4-4.1) | 3.6 (3.3-4.2) | 0.79 |
| Total
bilirubin | 0.8 (0.6-1.2) | 1.1 (0.8-2.3) | 0.8 (0.6-1.1) | 0.0068 |
| BCLC | | | | 0.118 |
|
A | 7 (13.5%) | 2 (22.2%) | 5 (11.6%) | |
|
B | 27 (51.9%) | 4 (44.4%) | 23 (53.5%) | |
|
C D | 18 (34.6%) | 3 (33.4%) | 15 (34.9%) | |
| Tumor marker | | | | |
|
AFP
(ng/ml) | 10.1
(3.58-188.88) | 6.3
(3.3-6054.8) | 10.3
(4.1-201.8) | 0.692 |
|
PIVKA-II
(mAU/ml) | 181.5
(32-3390) | 938
(26.5-31832.5) | 140 (32-18112) | 0.0137 |
| LEN dose | | | | 0.5604 |
|
12 mg | 4 (7.7%) | 0 (0%) | 4 (9.3%) | |
|
8 mg | 27 (51.9%) | 4 (44.4%) | 23 (53.4%) | |
|
4 mg | 21 (40.4%) | 5 (54.6%) | 16 (37.2%) | |
IHC of β-catenin and GS in HCC
tissues
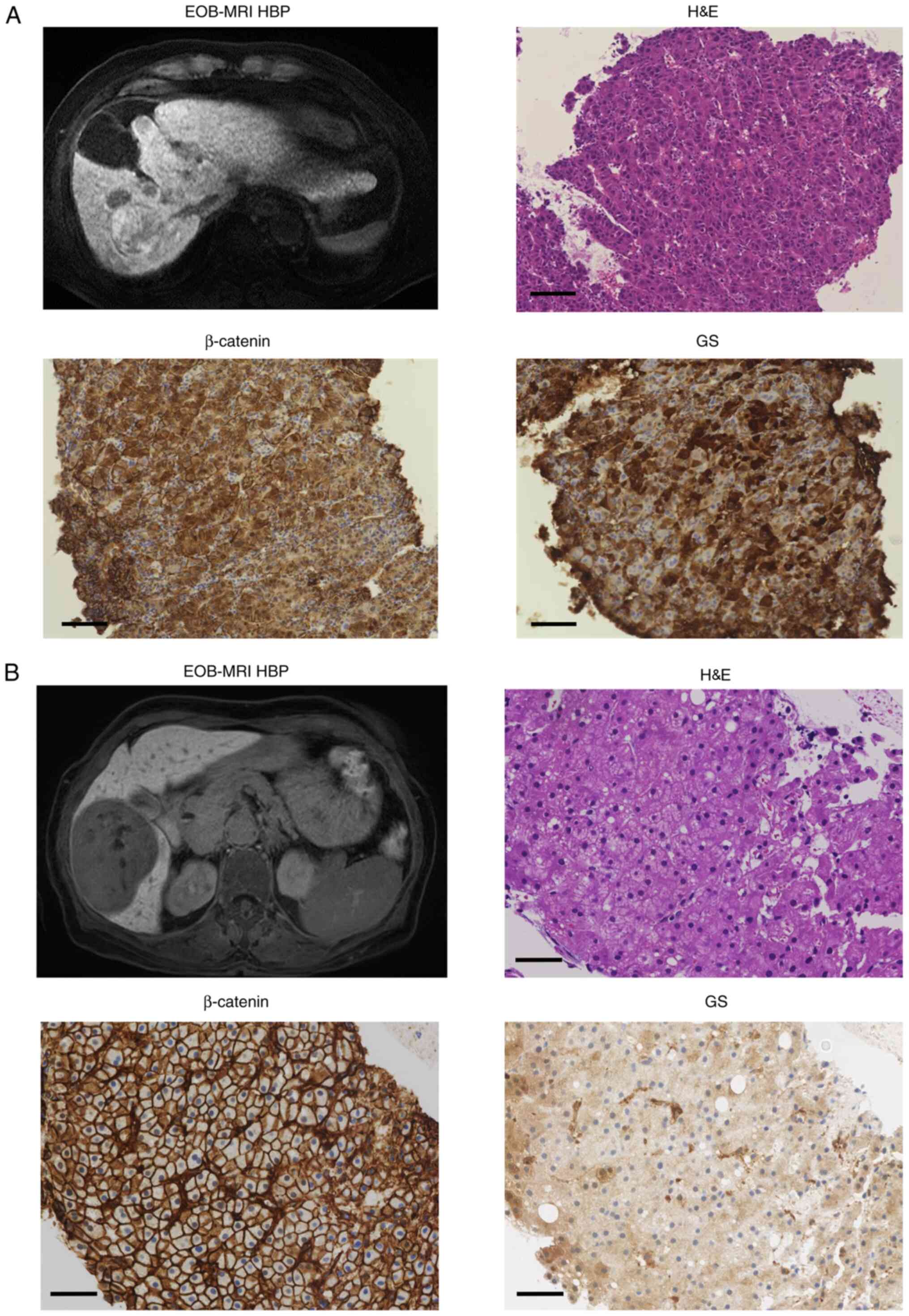
The expressions of β-catenin and GS in 13 patients
(four patients in the iso-high-intensity group and nine patients in
the low-intensity group) were assessed by IHC before LEN therapy.
Typical magnetic resonance images and immunohistochemical findings
are presented in Fig. 2A and
B. All four patients (100%) were
positive for β-catenin or GS staining in the iso-high-intensity
group, and 3/9 (33.3%) were positive for β-catenin or GS staining
in the low-intensity group (Table
II); however, there were no differences in β-catenin (P=1.00)
and GS (P=0.07) staining between the two groups because of the
small patient numbers in these groups.
 | Table IIRelationship between Wnt/β-catenin
mutations and signal intensity in the HBP of EOB-MRI in 13
patients. |
Table II
Relationship between Wnt/β-catenin
mutations and signal intensity in the HBP of EOB-MRI in 13
patients.
| Case | Group | Age, years | Sex | Etiology | β-catenin | GS |
|---|
|
1 | iso-high | 82 | M | ALC | Positive | Positive |
|
2 | iso-high | 58 | F | HBV | Negative | Positive |
|
3 | iso-high | 83 | M | NBNC | Negative | Positive |
|
4 | iso-high | 87 | M | NBNC | Positive | Positive |
|
5 | Low | 68 | M | HBV | Negative | Negative |
|
6 | Low | 80 | M | AIH | Positive | Positive |
|
7 | Low | 75 | M | HBV | Positive | Positive |
|
8 | Low | 59 | M | ALC | Negative | Negative |
|
9 | Low | 52 | M | HBV | Positive | Positive |
| 10 | Low | 76 | M | HCV | Negative | Negative |
| 11 | Low | 71 | M | HCV | Negative | Negative |
| 12 | Low | 78 | M | HCV | Negative | Negative |
| 13 | Low | 80 | M | ALC | Negative | Negative |
Efficacy of LEN in the iso-high
intensity and low-intensity groups
The OS, PFS, ORR, and DCR were similar between
groups. The ORR (CR+PR) was 3/9 (33.3%) in the iso-high-intensity
group and 13/43 (30.2%) in the low-intensity group (P=0.62). The
DCR (CR+PR+SD) was 5/9 (55.6%) in the iso-high intensity group and
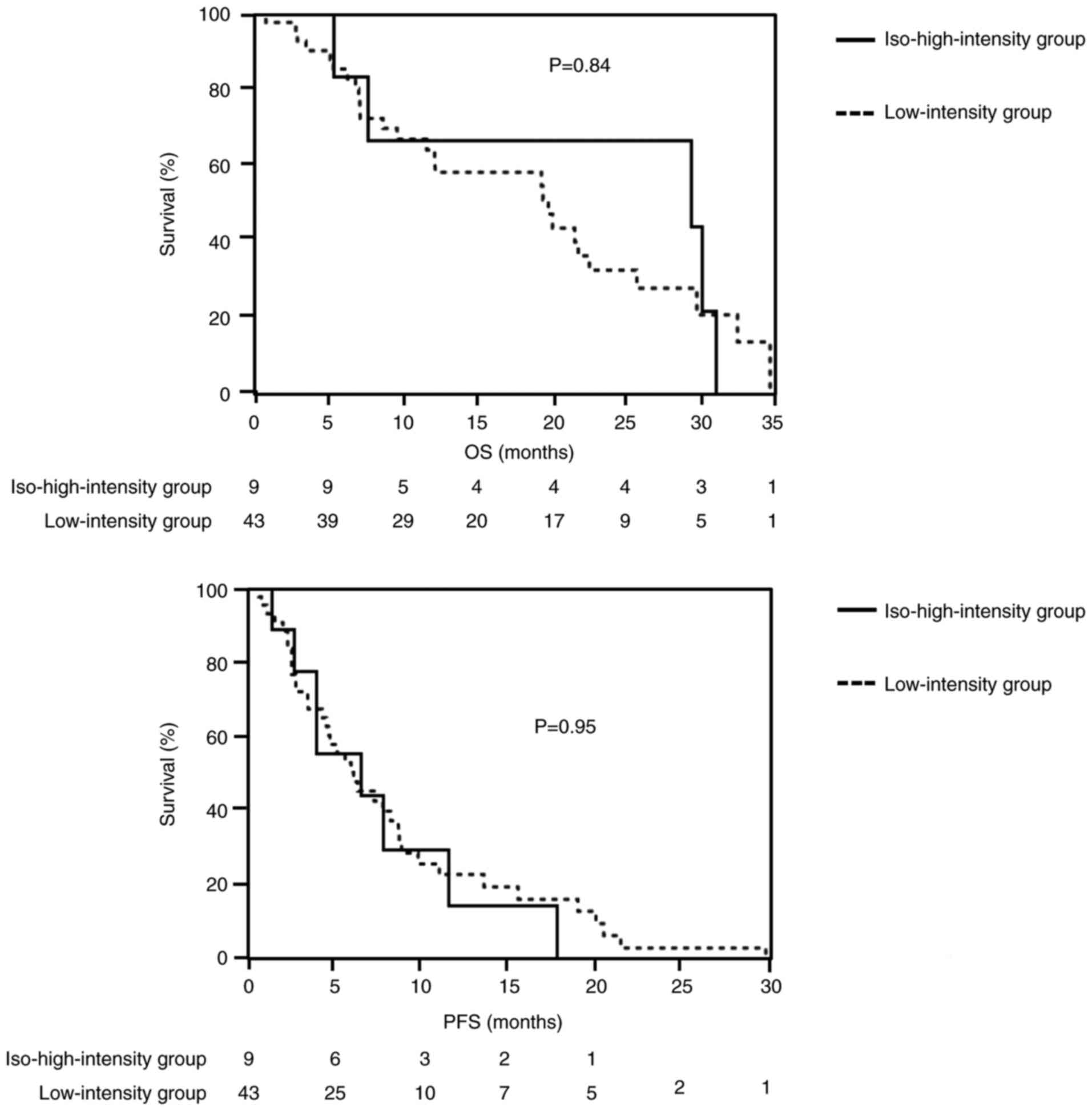
26/43 (60.5%) in the low-intensity group (P=0.45) (Table III). Median OS was 29.8 months
[95% confidence interval (CI)=7.1-31.3] in the iso-high-intensity
group versus 20.7 months (95% CI=11.2-26.3) in the low-intensity
group (P=0.84). The median PFS was 6.7 months (95% CI=2.9-11.9) in
the iso-high-intensity group compared with 5.6 months (95%
CI=3.7-7.9) in the low-intensity group (P=0.95; Fig. 3).
 | Table IIIComparison of the response to
lenvatinib between the EOB iso-high-intensity group and
low-intensity group (patient analysis). |
Table III
Comparison of the response to
lenvatinib between the EOB iso-high-intensity group and
low-intensity group (patient analysis).
| LEN
effectiveness | Iso-high-intensity
group, n=9 (%) | Low-intensity
group, n=43 (%) | P-value |
|---|
| Overall
response | | | 0.14 |
|
CR | 0 (0) | 2 (4.7) | |
|
PR | 3 (33.3) | 11 (25.6) | |
|
SD | 2 (22.3) | 13 (30.2) | |
|
PD | 4 (44.4) | 17 (39.5) | |
| ORR (CR+PR) | 3 (33.3) | 13 (30.2) | 0.62 |
| DCR (CR+PR+SD) | 5 (55.6) | 26 (60.5) | 0.45 |
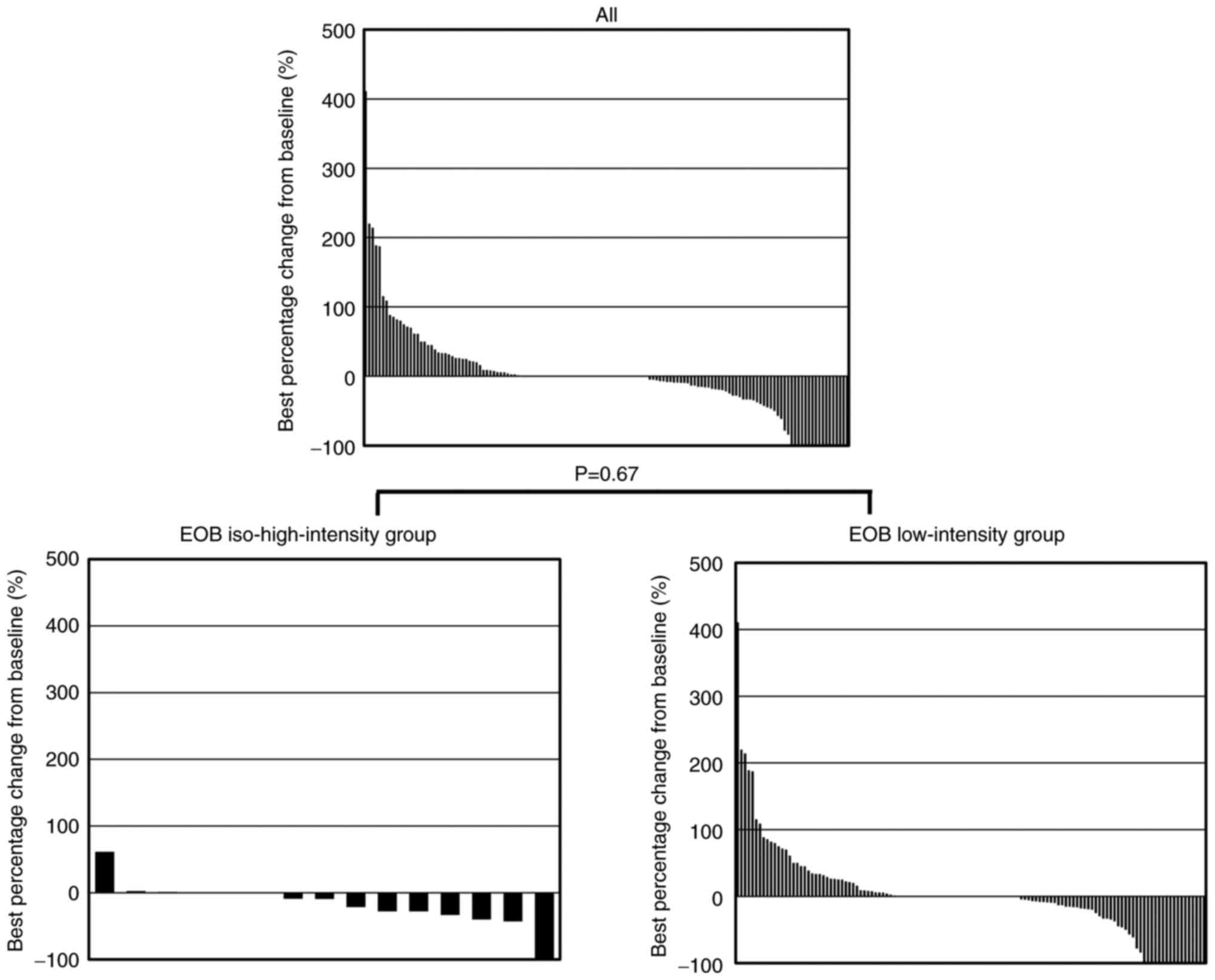
We also evaluated the change in each HCC tumor
treated with LEN. The best percentage change was -10.5% in the
iso-high-intensity group (4.99±0.44 cm to 4.79±0.54 cm) and 1.1% in
the low-intensity group (1.88±0.15 cm to 1.88±0.18 cm; Fig. 4). We compared the values of the
best percentage change between groups using Mann-Whitney U test.
The best percentage change from the baseline tumor size (P=0.67),
the ORR [28.6% (4/14) in the iso-high-intensity group and 27.8%
(35/126) in the low-intensity group (P=0.73)], and the DCR [92.9%
(13/14) in the iso-high-intensity group and 73.0% (92/126) in the
low-intensity group (P=0.39; Table
IV)] were similar between groups.
 | Table IVComparison of the response to
lenvatinib between the EOB iso-high-intensity group and
low-intensity group (nodule analysis). |
Table IV
Comparison of the response to
lenvatinib between the EOB iso-high-intensity group and
low-intensity group (nodule analysis).
| LEN
effectiveness | Iso-high-intensity
group, n=14 (%) | Low-intensity
group, n=126 (%) | P-value |
|---|
| Overall
response | | | 0.85 |
|
CR | 0 (0) | 16 (12.7) | |
|
PR | 4 (28.6) | 21(16.7) | |
|
SD | 9 (64.3) | 57 (45.2) | |
|
PD | 1 (7.1) | 32 (25.4) | |
| ORR (CR+PR) | 4 (28.6) | 35 (27.8) | 0.73 |
| DCR (CR+PR+SD) | 13 (92.9) | 92 (73.0) | 0.39 |
Discussion
Our current study suggested that the status of
mutations of WNT/β-catenin may not influence LEN effectiveness.
Approximately 30% of HCC cases were reported to show the
constitutive activation of WNT/β-catenin signaling, induced by the
relevant gene mutations (20). The
negative influence of WNT/β-catenin activation on the DCR and PFS
of patients receiving anti-PD-1 therapy (7,8,21).
However, the PFS was similar between sorafenib-treated patients
with and without mutations in WNT/β-catenin genes who had undergone
gene sequencing (10). Although
the efficacy of sorafenib and other MTAs might be independent of
WNT/β-catenin mutation status, clinical evidence demonstrating how
responses of HCC patients to LEN are affected by WNT/β-catenin
activation and how an immune cold phenotype is acquired, is still
lacking.
WNT/β-catenin pathway activation promotes β-catenin
accumulation in the cytoplasm, its nuclear translocation, and
diffuse accumulation of GS, a transcriptional target of β-catenin
(22-24).
The presence of WNT/β-catenin mutations is determined by IHC
analysis showing the nuclear expression of β-catenin or cytoplasmic
overexpression of GS in HCC tissues, indicating they might be
useful biomarkers of WNT/β-catenin mutations (22-24).
Furthermore, GS and β-catenin expressions were
reported to correlate with OATP1B3 expression (25) and HCC with WNT/β-catenin mutations
showed iso-high intensity during the HBP of EOB-MRI (9,10).
The mechanism involves the induction of OATP1B3 (an EOB
transporter) by WNT/β-catenin mutations (9,26).
Therefore, iso-high intensity during the HBP of EOB-MRI might be
useful as an imaging biomarker of HCC patients with mutations of
WNT/β-catenin. The frequency of HCCs with iso-high intensity was
12-22% during the HBP of EOB-MRI (11,12).
We investigated the immunostaining of β-catenin and GS for the
detection of mutations of WNT/β-catenin. Several HCC tissues with
iso-high intensity during the HBP of EOB-MRI showed GS or β-catenin
positive staining. Thus, we confirmed mutations of WNT/β-catenin in
HCC with iso-high intensity during the HBP of EOB-MRI as previously
reported (25).
Our findings suggested that the efficacy of LEN did
not differ between the iso-high-intensity group, in which patients
may have WNT/β-catenin mutations, and the low-intensity group.
Clinical trials or studies using pre-clinical models
to investigate immunomodulatory influences of antiangiogenic agents
on the tumor microenvironment reported enhanced maturation of
dendritic cells, trafficking and function of T cells, and reversed
immunosuppression induced by hypoxia or immunosuppressive cells
(27-29).
Other in vivo and in vitro studies reported sorafenib
enhanced antitumor immunity by promoting tumor-associated
macrophage polarization to an M1 phenotype (30-32),
enhancing the infiltration and functions of CD4+ and
CD8+ T cells (33,34),
lowering numbers of Tregs (35-37),
and reversing suppressive functions of myeloid-derived cells in
tumor microenvironments (22,38,39).
Other MTAs including LEN were shown to promote antitumor immune
activity in pre-clinical models (40-42).
Many of these immunomodulatory effects of MTAs might be associated
with the inhibition of VEGFR signaling (27). Compared with earlier tyrosine
kinase inhibitors, LEN has a greater inhibitory effect on
FGFR4(43). WNT/β-catenin
mutations are associated with FGFR4 overexpression (43) suggesting the therapeutic effects of
MTAs including LEN might not be affected by WNT/β-catenin mutations
although they might alter the ‘non-inflamed cold’ subclass to the
‘inflamed hot’ subclass in the immune microenvironment of
WNT/β-catenin-mutant HCC.
Fujita et al classified HCCs into one of the
three groups on the basis of intensity during the HBP of EOB-MRI:
i) homogeneous hypointensity; ii) heterogeneous hyperintensity; and
iii) homogeneous hyperintensity (44). During the HBP, all HCCs in the
iso-high-intensity group in our study displayed heterogeneous
hyperintensity (group 2). The tumor size was larger and PIVKA-II
levels were higher in the iso-high-intensity group compared with
the low-intensity group, similar to our previous study. Other
studies reported that GS-positive HCCs were larger although
PIVKA-II levels were not associated to tumor size. Therefore,
PIVKA-II levels in the iso-high-intensity group of GS-positive HCCs
might be higher than those in the low-intensity group (45,46).
Fujita et al recorded a disease-free survival rate in group
2 that was significantly lower compared with group 1(44), but our study revealed the OS, PFS,
ORR, and DCR were similar in all groups after LEN treatment.
Study limitations were as follows. First, this study
was performed at a single center and therefore had limited numbers
of HCC cases. Second, it was unclear whether the mutations of one
tumor reflected the mutations in other masses when considering the
heterogeneity of HCC in multiple masses. Third, we could not
evaluate the frequency of β-catenin mutations in HCC with iso-high
intensity or low-intensity during the HBP of EOB-MRI because
biopsies were not obtained from all patients. We also had to
consider that HCC with low intensity could also be positive for
β-catenin or GS.
The results suggested that the efficacy of LEN was
similar between the iso-high-intensity and low-intensity groups.
Given these limitations, further studies enrolling a larger number
of cases is needed before MTAs such as LEN can be used as a
first-line treatment for HCC with iso-high intensity instead of
ICIs alone or in combination with angiogenesis inhibitors, which
are the most common first-line regimens for advanced HCC in many
countries (2). These findings
might allow us to suggest the early therapeutic evaluation of ICIs
alone or in combination with angiogenesis inhibitors for HCC with
iso-high intensity and quickly change to MTAs if the initial
treatment is ineffective. The findings of this study might allow
the personalized treatment of patients with HCC by aiding the
selection of appropriate therapeutic agents. However, these
findings should be confirmed using a larger number of patients.
Acknowledgements
The authors would like to acknowledge Mrs. Y.
Ishibashi (Department of Hepatology, Iizuka Hospital) for aiding
preparation of the text.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AK, MY, KT, AM and KM designed the study. AK, MY,
SN, KT and YM assisted with the data analyses. AK wrote the initial
draft of the manuscript. MY and KM contributed to data analysis and
interpretation. MY and KM helped prepare the manuscript and
critically reviewed the manuscript. AK and KT confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The research and protocol were in accord with the
Declaration of Helsinki principles and the ethical guidelines of
the 1975 Declaration of Helsinki, respectively. This study received
approval from the Iizuka Hospital Ethics Committee (no. 18070).
Written informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Caldwell S and Park SH: The epidemiology
of hepatocellular cancer: From the perspectives of public health
problem to tumor biology. J Gastroenterol. 44 Suppl:S96–S101.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eso Y and Marusawa H: Novel approaches for
molecular targeted therapy against hepatocellular carcinoma.
Hepatol Res. 48:597–607. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Torbenson MS, Ng IO, Park YN, Roncalli M
and Sakamoto M: Digestive system tumours: WHO classification of
tumours. In: Hepatocellular Carcinoma. 5th edition. WHO
Classification of Tumours Editorial Board. WHO Press, Geneva,
pp229-239, 2019.
|
|
6
|
Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk
O, Villacorta-Martin C, Castro de Moura M, Putra J, Camprecios G,
Bassaganyas L, Akers N, et al: Identification of an immune-specific
class of hepatocellular carcinoma, based on molecular features.
Gastroenterology. 153:812–826. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Llovet JM, Montal R, Sia D and Finn RS:
Molecular therapies and precision medicine for hepatocellular
carcinoma. Nat Rev Clin Oncol. 15:599–616. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pinyol R, Sia D and Llovet JM: Immune
exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies
in HCC. Clin Cancer Res. 25:2021–2023. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ueno A, Masugi Y, Yamazaki K, Komuta M,
Effendi K, Tanami Y, Tsujikawa H, Tanimoto A, Okuda S, Itano O, et
al: OATP1B3 expression is strongly associated with Wnt/β-catenin
signalling and represents the transporter of gadoxetic acid in
hepatocellular carcinoma. J Hepatol. 61:1080–1087. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kudo M: Gd-EOB-DTPA-MRI could predict
WNT/β-catenin mutation and resistance to immune checkpoint
inhibitor therapy in hepatocellular carcinoma. Liver Cancer.
9:479–490. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tsuboyama T, Onishi H, Kim T, Akita H,
Hori M, Tatsumi M, Nakamoto A, Nagano H, Matsuura N, Wakasa K and
Tomoda K: Hepatocellular carcinoma: Hepatocyte-selective
enhancement at gadoxetic acid-enhanced MR imaging-correlation with
expression of sinusoidal and canalicular transporters and bile
accumulation. Radiology. 255:824–833. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kitao A, Matsui O, Yoneda N, Kozaka K,
Kobayashi S, Koda W, Gabata T, Yamashita T, Kaneko S, Nakanuma Y,
et al: Hypervascular hepatocellular carcinoma: Correlation between
biologic features and signal intensity on gadoxetic acid-enhanced
MR images. Radiology. 265:780–789. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res.
2014(638747)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6(18)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL
and Peng SY: Beta-catenin mutations are associated with a subset of
low-stage hepatocellular carcinoma negative for hepatitis B virus
and with favorable prognosis. Am J Pathol. 157:763–770.
2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Laurent-Puig P, Legoix P, Bluteau O,
Belghiti J, Franco D, Binot F, Monges G, Thomas G, Bioulac-Sage P
and Zucman-Rossi J: Genetic alterations associated with
hepatocellular carcinomas define distinct pathways of
hepatocarcinogenesis. Gastroenterology. 120:1763–1773.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zucman-Rossi J, Benhamouche S, Godard C,
Boyault S, Grimber G, Balabaud C, Cunha AS, Bioulac-Sage P and
Perret C: Differential effects of inactivated Axin1 and activated
beta-catenin mutations in human hepatocellular carcinomas.
Oncogene. 26:774–780. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tsujikawa H, Masugi Y, Yamazaki K, Itano
O, Kitagawa Y and Sakamoto M: Immunohistochemical molecular
analysis indicates hepatocellular carcinoma subgroups that reflect
tumor aggressiveness. Hum Pathol. 50:24–33. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee HC, Kim M and Wands JR: Wnt/Frizzled
signaling in hepatocellular carcinoma. Front Biosci. 11:1901–1915.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Harding JJ, Nandakumar S, Armenia J,
Khalil DN, Albano M, Ly M, Shia J, Hechtman JF, Kundra R, El Dika
I, et al: Prospective Genotyping of hepatocellular carcinoma:
Clinical implications of next-generation sequencing for matching
patients to targeted and immune therapies. Clin Can Res.
25:2116–2126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nhieu JT, Renard CA, Wei Y, Cherqui D,
Zafrani ES and Buendia MA: Nuclear accumulation of mutated
beta-catenin in hepatocellular carcinoma is associated with
increased cell proliferation. Am J Pathol. 155:703–710.
1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cadoret A, Ovejero C, Terris B, Souil E,
Lévy L, Lamers WH, Kitajewski J, Kahn A and Perret C: New targets
of beta-catenin signaling in the liver are involved in the
glutamine metabolism. Oncogene. 21:8293–8301. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Loeppen S, Schneider D, Gaunitz F,
Gebhardt R, Kurek R, Buchmann A and Schwarz M: Overexpression of
glutamine synthetase is associated with beta-catenin-mutations in
mouse liver tumors during promotion of hepatocarcinogenesis by
phenobarbital. Cancer Res. 62:5685–5688. 2002.PubMed/NCBI
|
|
25
|
Kitao A, Matsui O, Yoneda N, Kozaka K,
Kobayashi S, Sanada J, Koda W, Minami T, Inoue D, Yoshida K, et al:
Hepatocellular carcinoma with β-catenin mutation: Imaging and
pathologic characteristics. Radiology. 275:708–717. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Giles RH, van Es JH and Clevers H: Caught
up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta.
1653:1–24. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ramjiawan RR, Griffioen AW and Duda DG:
Anti-angiogenesis for cancer revisited: Is there a role for
combinations with immunotherapy? Angiogenesis. 20:185–204.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hegde PS, Wallin JJ and Mancao C:
Predictive markers of anti-VEGF and emerging role of angiogenesis
inhibitors as immunotherapeutics. Semin Cancer Biol. 52:117–124.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kwilas AR, Donahue RN, Tsang KY and Hodge
JW: Immune consequences of tyrosine kinase inhibitors that
synergize with cancer immunotherapy. Cancer Cell Microenviron.
2(e677)2015.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Wang DY, Johnson DB and Davis EJ:
Toxicities associated with PD-1/PD-L1 blockade. Cancer J. 24:36–40.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sprinzl MF, Reisinger F, Puschnik A,
Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A,
Galle PR, Schuchmann M, et al: Sorafenib perpetuates cellular
anticancer effector functions by modulating the crosstalk between
macrophages and natural killer cells. Hepatology. 57:2358–2368.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wei X, Tang C, Lu X, Liu R, Zhou M, He D,
Zheng D, Sun C and Wu Z: MiR-101 targets DUSP1 to regulate the
TGF-β secretion in sorafenib inhibits macrophage-induced growth of
hepatocarcinoma. Oncotarget. 6:18389–18405. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Farsaci B, Donahue RN, Coplin MA, Grenga
I, Lepone LM, Molinolo AA and Hodge JW: Immune consequences of
decreasing tumor vasculature with antiangiogenic tyrosine kinase
inhibitors in combination with therapeutic vaccines. Cancer Immunol
Res. 2:1090–1102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Romero AI, Chaput N, Poirier-Colame V,
Rusakiewicz S, Jacquelot N, Chaba K, Mortier E, Jacques Y,
Caillat-Zucman S, Flament C, et al: Regulation of CD4(+)NKG2D(+)
Th1 cells in patients with metastatic melanoma treated with
sorafenib: Role of IL-15Rα and NKG2D triggering. Cancer Res.
74:68–80. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sunay MM, Foote JB, Leatherman JM, Edwards
JP, Armstrong TD, Nirschl CJ, Hicks J and Emens LA: Sorafenib
combined with HER-2 targeted vaccination can promote effective T
cell immunity in vivo. Int Immunopharmacol. 46:112–123.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chuang HY, Chang YF, Liu RS and Hwang JJ:
Serial low doses of sorafenib enhance therapeutic efficacy of
adoptive T cell therapy in a murine model by improving tumor
microenvironment. PLoS One. 9(e109992)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen ML, Yan BS, Lu WC, Chen MH, Yu SL,
Yang PC and Cheng AL: Sorafenib relieves cell-intrinsic and
cell-extrinsic inhibitions of effector T cells in tumor
microenvironment to augment antitumor immunity. Int J Cancer.
134:319–331. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cabrera R, Ararat M, Xu Y, Brusko T,
Wasserfall C, Atkinson MA, Chang LJ, Liu C and Nelson DR: Immune
modulation of effector CD4+ and regulatory T cell function by
sorafenib in patients with hepatocellular carcinoma. Cancer Immunol
Immunother. 62:737–746. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chang CJ, Yang YH, Chiu CJ, Lu LC, Liao
CC, Liang CW, Hsu CH and Cheng AL: Targeting tumor-infiltrating
Ly6G+ myeloid cells improves sorafenib efficacy in mouse
orthotopic hepatocellular carcinoma. Int J Cancer. 142:1878–1889.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kwilas AR, Ardiani A, Donahue RN, Aftab DT
and Hodge JW: Dual effects of a targeted small-molecule inhibitor
(cabozantinib) on immune-mediated killing of tumor cells and immune
tumor microenvironment permissiveness when combined with a cancer
vaccine. J Transl Med. 12(294)2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tsai AK, Khan AY, Worgo CE, Wang LL, Liang
Y and Davila E: A multikinase and DNA-PK inhibitor combination
immunomodulates melanomas, suppresses tumor progression, and
enhances immunotherapies. Cancer Immunol Res. 5:790–803.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Heine A, Schilling J, Grünwald B, Krüger
A, Gevensleben H, Held SA, Garbi N, Kurts C, Brossart P, Knolle P,
et al: The induction of human myeloid derived suppressor cells
through hepatic stellate cells is dose-dependently inhibited by the
tyrosine kinase inhibitors nilotinib, dasatinib and sorafenib, but
not sunitinib. Cancer Immunol Immunother. 65:273–282.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yamauchi M, Ono A, Ishikawa A, Kodama K,
Uchikawa S, Hatooka H, Zhang P, Teraoka Y, Morio K, Fujino H, et
al: Tumor fibroblast growth factor receptor 4 level predicts the
efficacy of lenvatinib in patients with advanced hepatocellular
carcinoma. Clin Transl Gastroenterol. 11(e00179)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fujita N, Nishie A, Kubo Y, Asayama Y,
Ushijima Y, Takayama Y, Moirta K, Shirabe K, Aishima S and Honda H:
Hepatocellular carcinoma: Clinical significance of signal
heterogeneity in the hepatobiliary phase of gadoxetic acid-enhanced
MR imaging. Eur Radiol. 25:211–220. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Dal Bello B, Rosa L, Campanini N, Tinelli
C, Torello Viera F, D'Ambrosio G, Rossi S and Silini EM: Glutamine
synthetase immunostaining correlates with pathologic features of
hepatocellular carcinoma and better survival after radiofrequency
thermal ablation. Clin Cancer Res. 16:2157–2166. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Weitz IC and Liebman HA: Des-gamma-carboxy
(abnormal) prothrombin and hepatocellular carcinoma: A critical
review. Hepatology. 18:990–997. 1993.PubMed/NCBI View Article : Google Scholar
|