Introduction
Kochi oxydol radiation therapy for unresectable
carcinomas (KORTUC) is a novel cancer treatment method developed in
Japan (1). Although low-oxygen
environments and excessive antioxidant enzymes are known to promote
resistance to cancer therapy, elimination of these factors is
difficult and has been rarely reported. If these treatment
resistance factors can be removed, it is expected that the effects
of conventional therapies, such as radiation therapy and
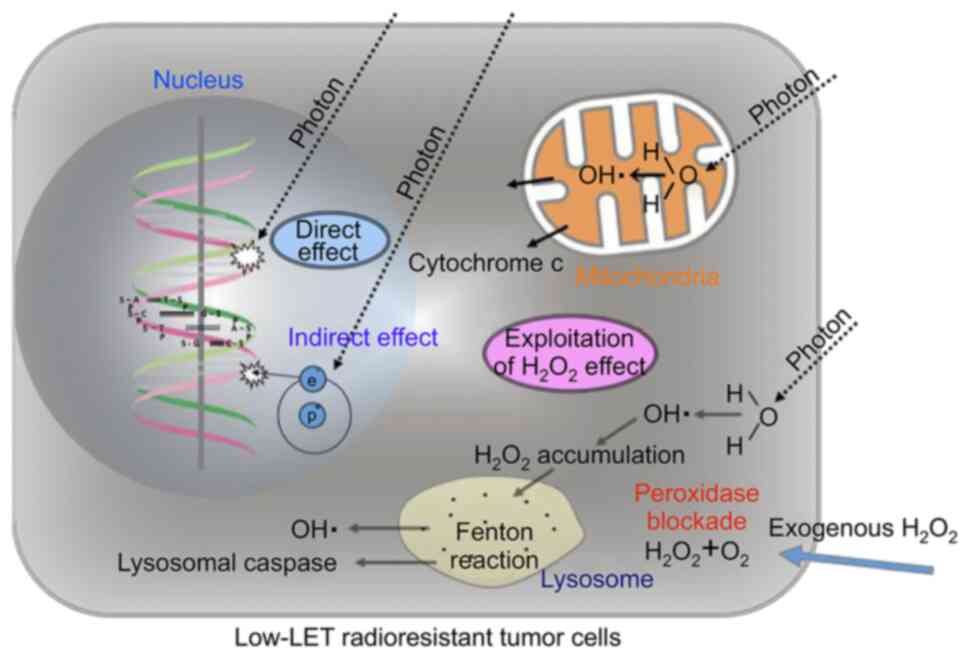
chemotherapy, will be enhanced. Tumor cells are resistant to X-ray
irradiation due to their high peroxidase and catalase activities.
Therefore, by providing exogenous hydrogen peroxide before
irradiation, the activities of anti-oxidative enzymes can be
blocked and oxygen molecules can be produced simultaneously,
leading to oxidative damage to low-linear energy transfer (LET)
radioresistant tumor cells. Through this mechanism, low-LET
radioresistant tumor cells can be converted into highly
radiosensitive cells (1-5)
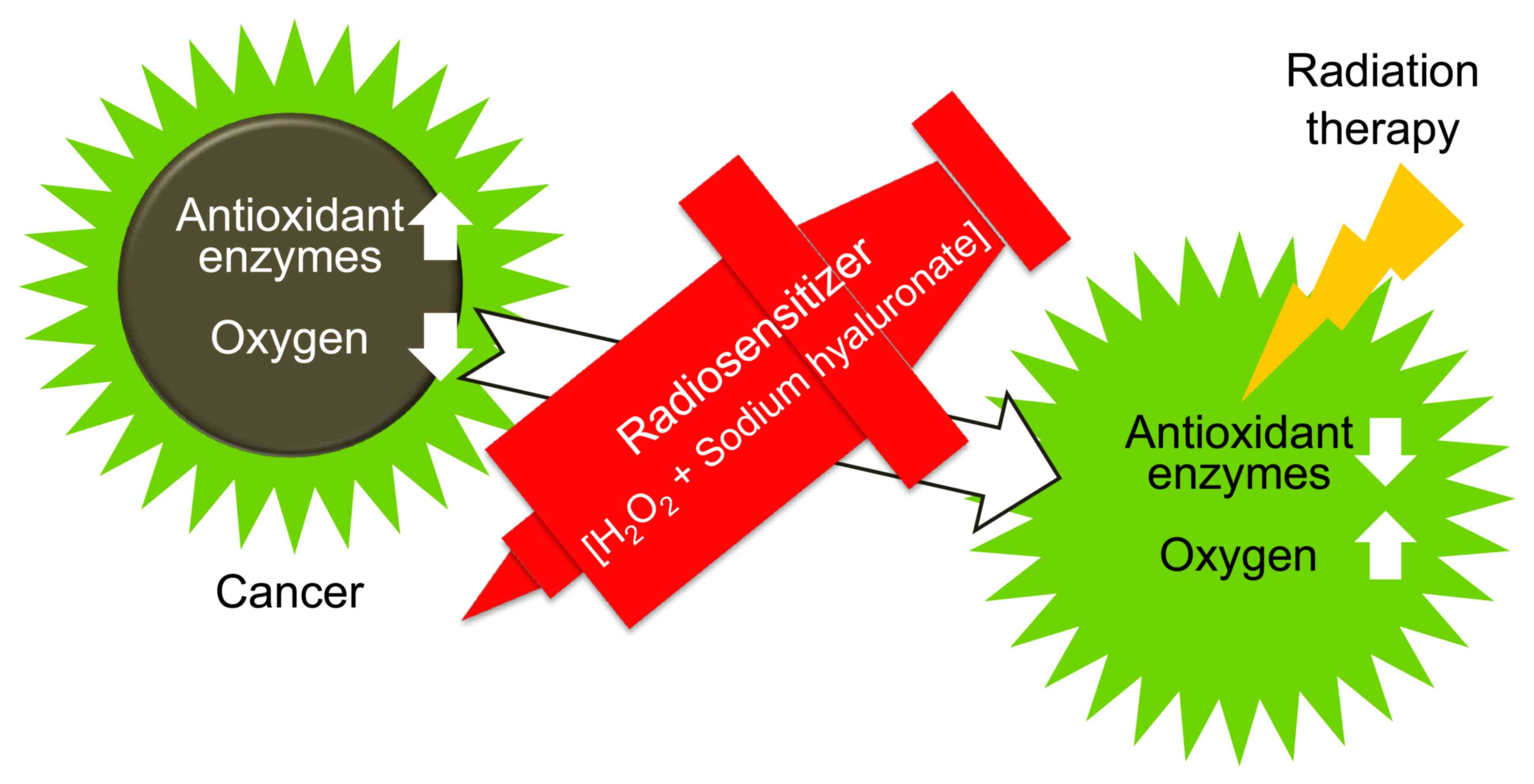
(Figs. 1 and 2). KORTUC was designed to accomplish this
goal.
When a mixture of a dilute hydrogen peroxide
solution and sodium hyaluronate is administered directly to a
cancer lesion, antioxidant enzymes are neutralized and degraded,
resulting in increased intratumor oxygen tension, thereby enhancing
the cytotoxic effect of radiation (1,6).
To date, a Japanese clinical study of KORTUC in
patients with breast cancer who refused surgery (6) and a phase I study of KORTUC in
patients with locally advanced breast cancer in the United Kingdom
(7) have been conducted, both of
which demonstrated safety and showed indications of marked
treatment effects.
The present study summarizes the outcomes of KORTUC
therapy for a case series of 210 patients and provides the case
reports of 4 patients who failed standard treatment and were
successfully treated with KORTUC, including a case of advanced,
inoperable breast cancer, a refractory patient with recurrence a
decade after postoperative irradiation, a patient with advanced,
inoperable rectal cancer and a patient with lymph node
metastases.
Materials and methods
Target patients
In the hope that KORTUC would enhance the effects of
existing treatments, from January 2010, following approval of the
Nagasaki Prefecture Shimabara Hospital Ethics Committee (Shimabara,
Japan; approval no. 21SH103 from January 14, 2010), Nagasaki
Prefecture Shimabara Hospital included the following patients with
difficult-to-control tumors as patients eligible for KORTUC: i)
Patients resistant to radiotherapy; ii) patients with repeated
recurrences; and iii) patients resistant to standard therapy. In
September 2014, after confirmation of its efficacy and safety in 62
patients, the following further patient groups were added with
approval from the Ethics Committee (additional approval no. 26SH185
from September 4, 2014): iv) Patients who refused surgery and v)
patients requiring multidisciplinary therapy. The present study
targets solid tumors of all cancer types in which a sensitizer can
be administered intratumorally. Considering the desire of each
patient for KORTUC and the need for the treatment, all patients
provided written informed consent before being included in the
study. The study was conducted following the Declaration of
Helsinki of the World Medical Association.
Treatment method
The radiosensitizer consisted of 1 unit (0.5 ml) of
a 3% hydrogen peroxide solution mixed with 2.5 ml of 0.83% sodium
hyaluronate, resulting in a 0.5% hydrogen peroxide concentration.
The actual injected dose was determined so that a reaction would
occur as evenly as possible over the entire lesion. For example,
for a lesion of 1 cm in diameter, the injected dose of the mixture
was ~1 ml, and for a lesion 3 cm in diameter, the injected dose of
the mixture was ~3 ml.
The addition of sodium hyaluronate stabilized the
hydrogen peroxide and resulted in elevated intratumor oxygen
tension even 48 h after injection (8). Thus, the radiosensitizer was
administered twice per week. To maximize its sensitizing effect,
the radiosensitizer was injected intratumorally immediately prior
to radiation whenever possible; for example, on Monday and
Wednesday or on Tuesday and Thursday, depending upon the radiation
schedule (this injected form of KORTUC is known as KORTUC II). For
a lesion located on the skin surface, the radiosensitizer was
sprayed over the lesion, or a gauze pad soaked in the
radiosensitizer was applied to the lesion just before radiation
(this form of KORTUC is known as KORTUC I). For a deep-seated
lesion, the radiosensitizer was injected slowly into the tumor with
an 18- to 26-G needle under ultrasound or computed tomography (CT)
guidance to avoid an accidental puncture of adjacent vessels or
organs. As KORTUC II is a novel treatment, there have been no data
on the risk of tumor cell dissemination. For this reason, a needle
with the smallest possible diameter, depending on the conditions,
was selected based on reports suggesting that the dissemination
risk was 0.04% [10/25,000 cases (unclarified cancer type) in a
systematic review of data up until the end of 1976] for a lung
biopsy (9) under CT guidance and
that a smaller puncture needle was associated with less frequent
dissemination (10). The needle
used most frequently was a 22-G spinal needle that was readily
visible under imaging guidance and relatively flexible and hard to
break. If a patient complained of severe pain, 1 ml of 1% lidocaine
was mixed with 1 unit of the radiosensitizer after confirming that
the patient had no Xylocaine allergy.
Treatment responses were assessed according to the
Response Evaluation Criteria In Solid Tumors (RECIST), version
1.1(11). RECIST is a standard way
to measure how well a patient with cancer responds to treatment; it
is based on whether tumors shrink, stay the same size or increase
in size. To use RECIST, there must be at least one tumor that can
be measured on X-ray, CT or magnetic resonance imaging scans. The
types of response a patient can have are a complete response (CR),
a partial response (PR), progressive disease (PD) and stable
disease (SD).
The survival period was calculated using the
Kaplan-Meier method from the planned radiation therapy date to the
date of death due to all causes or the date of the final follow-up
of surviving patients (up to June 2019).
Adverse events were assessed according to the Common
Terminology Criteria for Adverse Events (CTCAE), version 4(12). CTCAE is widely accepted as the
standard classification and severity grading scale for adverse
events in cancer therapy, clinical trials, and other oncology
settings.
Results
Treatment results
Between January 2010 and June 2019, KORTUC was
administered to 210 patients. Table
I shows the responses of the patients according to cancer type
and stage. The most common disease stage was stage IV in 137
patients (65%), followed by stage III in 25 patients, stage I in 17
patients and stage II in 7 patients (unknown disease stage in 24
patients). As most patients were in stage IV and radiotherapy was a
local treatment, their post-treatment evaluation was assessed based
on local effects and not on overall survival (OS) or disease-free
survival. Of the 186 patients who could be followed up after the
treatment, according to RECIST version 1.1, 28 (15%) patients had a
CR, 59 (32%) had a PR, 73 (39%) had SD and 26 (14%) had PD. In
addition, the median survival time of all 28 CR patients was 30
months (range, 2-96 months), and the 2-, 3-, and 5-year OS rates
were 83.1, 76.2 and 66.7%, respectively. Table II shows the adverse events
associated with KORTUC, which were assessed according to CTCAE
version 4, and included the following: Radiation dermatitis, grade
III in 6 patients and grade II in 76 patients; leukopenia, grade
III in 2 patients (receiving concurrent chemotherapy);
postoperative recurrence of perforation at the intestinal
anastomotic site, grade IV in 1 patient; oral mucositis, grade III
in 2 patients; radiation esophagitis, grade II in 22 patients;
radiation enteritis, grade II in 3 patients; and pain during
injection of the radiosensitizer, grade I in 20 patients.
 | Table IResults of Kochi oxydol radiation
therapy for unresectable carcinomas performed in Nagasaki
Prefecture Shimabara Hospital. |
Table I
Results of Kochi oxydol radiation
therapy for unresectable carcinomas performed in Nagasaki
Prefecture Shimabara Hospital.
| |
Clinical
responsea of patients
at different diagnostic stages, n | |
|---|
| | Stage I (n=17) | Stage II (n=7) | Stage III (n=25) | Stage IV (n=137) | Responders, n |
|---|
| Cancer type | Total treated, n | UD, n | CR | PR | SD | PD | CR | PR | SD | PD | CR | PR | SD | PD | CR | PR | SD | PD | CR+PR |
|---|
| Breast | 49 | 8 | 8 | 1 | | | | | | | | | | 1 | 5 | 11 | 11 | 4 | 25 |
| Lung | 46 | 2 | | 1 | 1 | 1 | | | 1 | | | 1 | 3 | | 5 | 12 | 17 | 2 | 19 |
| Colon/rectum | 36 | | | | 1 | | | | | 1 | 1 | 2 | 1 | 2 | 2 | 6 | 17 | 3 | 11 |
| Esophagus | 29 | 3 | | 2 | | | 1 | 1 | | | 2 | 5 | 1 | 1 | | 5 | 5 | 3 | 16 |
| Bone and soft
tissue | 11 | 4 | | 1 | | | 1 | | | | | | | | | 2 | 2 | 1 | 4 |
| Urinary system | 8 | 2 | | 1 | | | | | | | 1 | | 2 | | | 2 | | | 4 |
| Pancreas | 7 | | | | | | | | | | | | | 1 | | | 4 | 2 | 0 |
| Stomach | 5 | 1 | | | | | | | | | | | | | | | 1 | 3 | 0 |
| Head and neck | 3 | 1 | | | | | | | | | | | | | 1 | | | 1 | 1 |
| Lymphoma | 6 | 1 | | | | | | 1 | | | | | | | | 3 | 1 | | 4 |
| Biliary system | 4 | 1 | | | | | | | | | | | | | | 1 | 2 | | 1 |
| Pleura | 1 | | | | | | | | | | | | | | | | 1 | | 0 |
| Ovary | 1 | | | | | | | | | | | | | | | | 1 | | 1 |
| Liver | 2 | | | | | | | 1 | | | | | | | | | 1 | | 0 |
| Unknown
primary | 2 | 1 | | | | | | | | | 1 | | | | | | | | 1 |
| Total | 210 | 24 | 8 | 6 | 2 | 1 | 2 | 3 | 1 | 1 | 5 | 8 | 7 | 5 | 13 | 42 | 63 | 19 | 87 |
 | Table IIAdverse events following Kochi oxydol
radiation therapy for unresectable carcinomas according to Common
Terminology Criteria for Adverse Events version 4 (n=210). |
Table II
Adverse events following Kochi oxydol
radiation therapy for unresectable carcinomas according to Common
Terminology Criteria for Adverse Events version 4 (n=210).
| Adverse Events | Severity
(Grade) | Number of
cases |
|---|
| Radiation
dermatitis | II | 76 |
| | III | 6 |
| Radiation
esophagitis | II | 22 |
| Pain during
injection of the radiosensitizer | I | 20 |
| Radiation
enteritis | II | 3 |
| Leukopenia
(receiving concurrent chemotherapy) | III | 2 |
| Oral mucositis | III | 2 |
| Postoperative
recurrence of perforation at the intestinal anastomotic site | IV | 1 |
Representative case reports
The following are case reports of 4 patients who had
failed conventional therapy but maintained a CR following
KORTUC.
Case 1: Advanced and inoperable breast
cancer
A woman in her 50s noticed a lump in the right
breast, which was left untreated. The patient presented to a local
doctor 1 year later with gradually worsening shortness of breath
and lower extremity edema accompanied by anemia, and was diagnosed
with stage IV breast cancer [invasive ductal carcinoma,
papillotubular to scirrhous carcinoma; lymph node, liver and lung
metastases; immunohistochemical examination (IH) result: ER(3+),
PgR(+) and HER2(3+)]. The patient responded inadequately to seven
courses of 840 mg pertuzumab/420 mg trastuzumab/100 mg docetaxel,
seven courses of 200 mg trastuzumab emtansine, 330 mg
trastuzumab/1,800 mg gemcitabine and fulvestrant for ~3 years. The
primary lesion gradually grew into a giant lesion, 15 cm along its
major axis, and was elevated enough to break through the skin. The
tumor was hemorrhagic, had white exudate on its surface and had an
unusually offensive odor. The breast cancer was resistant to
treatment, and an experienced radiation oncologist in the same
hospital was consulted about administering radiation therapy to the
patient. It was considered that radiotherapeutic efficacy against
the tumor progression could not be expected either. The patient was
then referred to Nagasaki Prefecture Shimabara Hospital for KORTUC
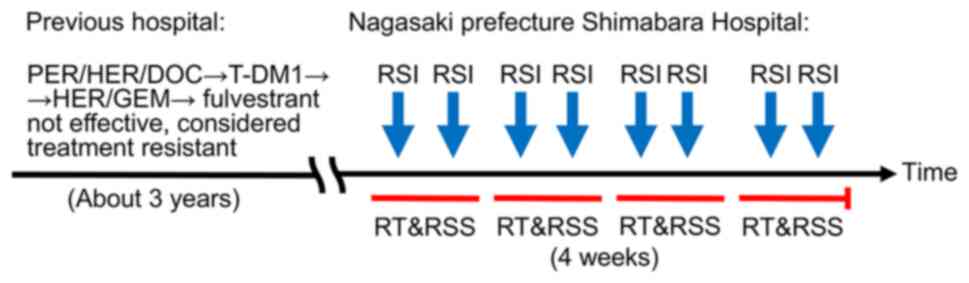
in August 2019 at the age of 56. Standard therapy had previously
been used, but no treatment resistance factors had been identified.
Therefore, it was considered that previous treatments had not been
used effectively.
The patient now received intensity modulated
radiation therapy (IMRT) with 44 Gy in 16 fractions for 3 weeks to
the breast and the regional lymph nodes, followed by boosts of 9 Gy
in three fractions to the primary lesion and involved ipsilateral
lymph nodes. In addition, twice weekly, the patient received
radiosensitizer injected into the breast cancer and multiple
regional metastatic lymph nodes within the irradiation field.
Furthermore, a hydrogen peroxide solution was sprayed on the
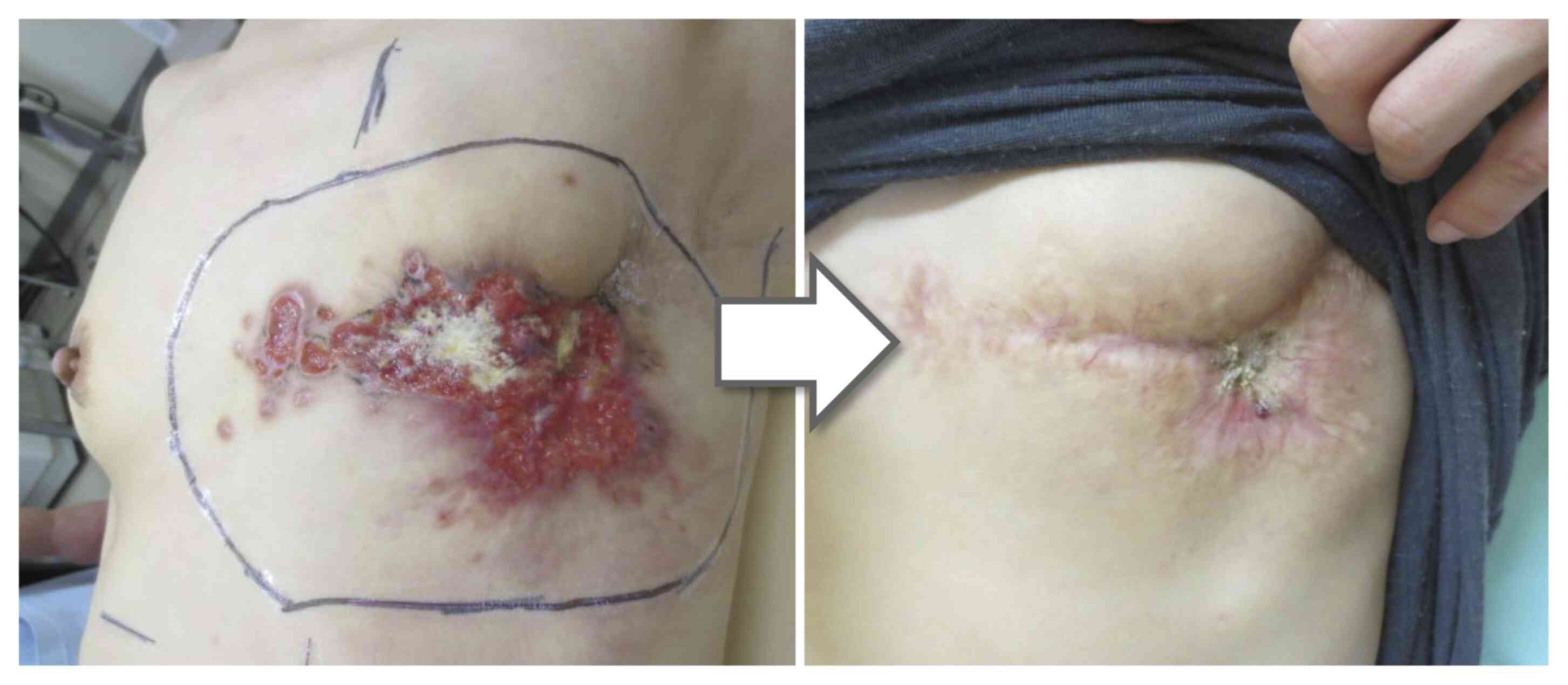
surface of the tumor (KORTUC I & II) (Fig. 3).
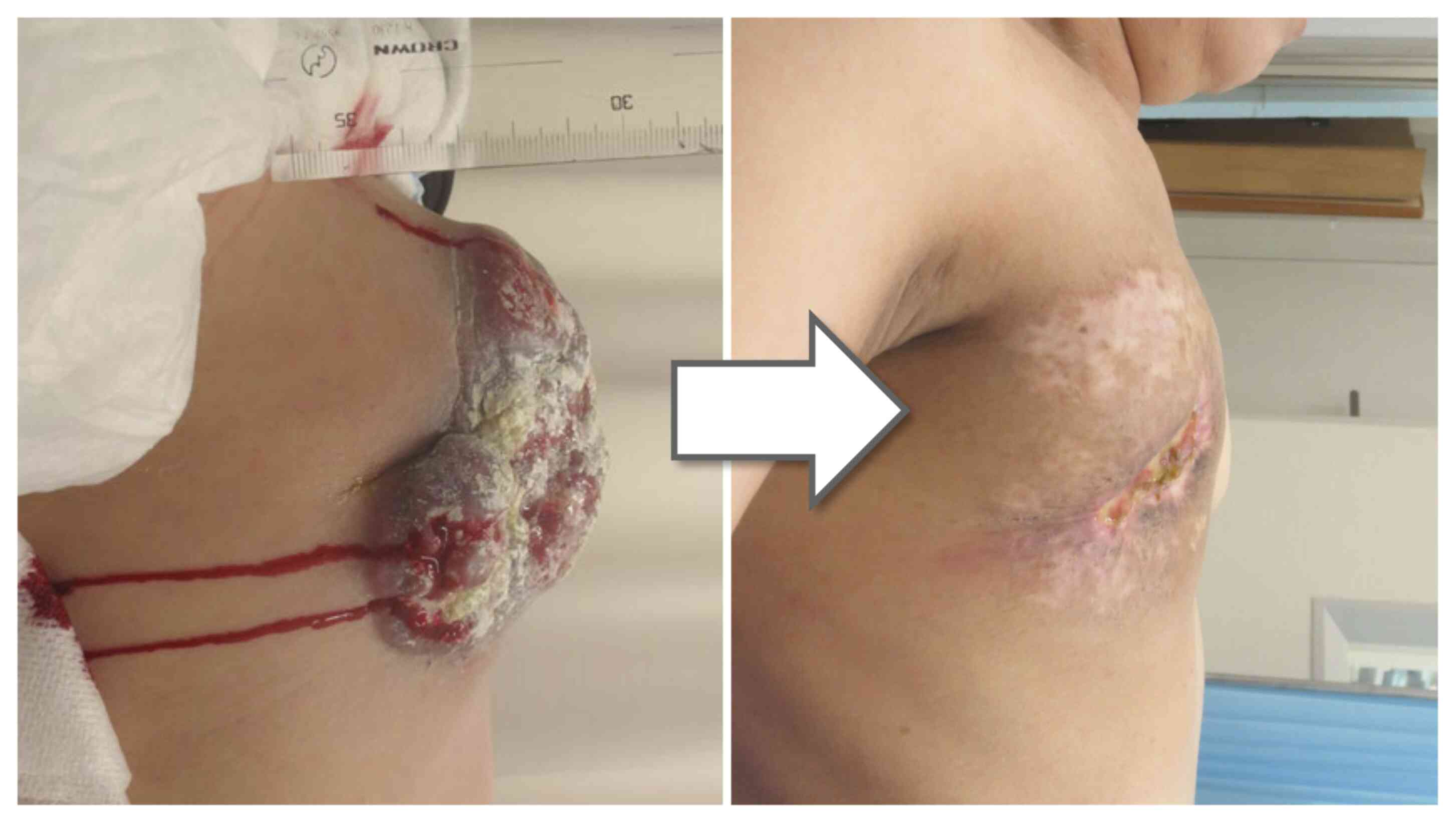
No serious adverse events were observed. At 4 months
after KORTUC, the large mass and swollen lymph nodes within the
radiation field regressed. A concavity was present on the right
chest wall where the lesion had been present, but the size of the
concavity reduced over time (Fig.
4). After 14 months, the patient succumbed to cancer, but no
regrowth of the right chest wall lesion was observed (however, it
should be noted that this case is not included in the
aforementioned data due to the collection period).
Case 2: A refractory patient with
recurrence a decade after postoperative irradiation
A woman in her 40s noticed a lump in the left breast
and underwent breast conserving surgery and axillary lymph node
dissection for left breast cancer [invasive ductal carcinoma; n(+);
IH: ER(+), PgR(+), HER2(2+); FISH unknown], and radiotherapy with
50 Gy in 25 fractions, at the Nagasaki Prefecture Shimabara
Hospital. This was followed by adjuvant chemotherapy and endocrine
therapy with tegafur/uracil and Nolvadex for the remaining breast
tissue post-surgery. The patient voluntarily stopped visiting the
Outpatient Department after 5 years. At 10 years after the first
visit, irregular erosions and an ulcer, 10 cm long along its major
axis and surrounded by multiple red nodules, developed in the
remaining breast (Fig. 5).
Metastasis was confirmed by skin biopsy [IH: ER(+), PgR(-),
HER2(3+); Ki-67 34%]. Femara was administered and then substituted
with 1 mg anastrozole per day, and 16 courses of trastuzumab
therapy were administered, once every 3 weeks (first course of 342
mg and then 252 mg). Despite these treatments, the disease
worsened, and KORTUC with irradiation of 45 Gy in 25 fractions was
performed only at the site of recurrence in February 2015 at the
age of 53.
Acute-phase grade III dermatitis was observed. The
local lesion disappeared, with no serious adverse events after 6
years. During that time, bone metastasis and lung metastasis were
observed, and adjuvant chemotherapy in the form of nine courses of
840 mg pertuzumab/252 mg trastuzumab and 152 mg trastuzumab
emtansine (once every 3 weeks), and 4 mg zoledronic acid hydrate
(once every 4 weeks) was administered. A solitary small cerebellar
metastasis was treated with stereotactic brain irradiation. The
re-irradiated left chest wall lesion showed partial ulcer formation
4.5 years after KORTUC, and was accompanied by secondary infection.
Cytology was class III, but has since improved.
It has been reported that ~10% of patients have
local recurrence after standard treatment (13), suggesting that re-irradiated KORTUC
may become an important salvage therapy for patients with recurrent
disease.
Case 3: Advanced and inoperable rectal
cancer
A 57-year-old female who visited the Emergency
Outpatient Department of Nagasaki Prefecture Shimabara Hospital for
left abdominal pain, appetite loss and nausea was diagnosed with
rectal cancer at stage IVA, T4bN2aM1a, as a result of an inpatient
examination in August 2015. The Eastern Cooperative Oncology Group
performance status (PS) (14) was
3. The patient was confined to bed, with decreased appetite, severe
anemia (hemoglobin: 6.5 g/dl; normal range, 11.6-14.8 g/dl), and
weight loss of 10 kg over half a year. The patient had a type 2
tumor (moderately differentiated adenocarcinoma), which bleed
regularly and was covered with white exudate, invading the total
circumference of the lower rectum and extending to the perineum
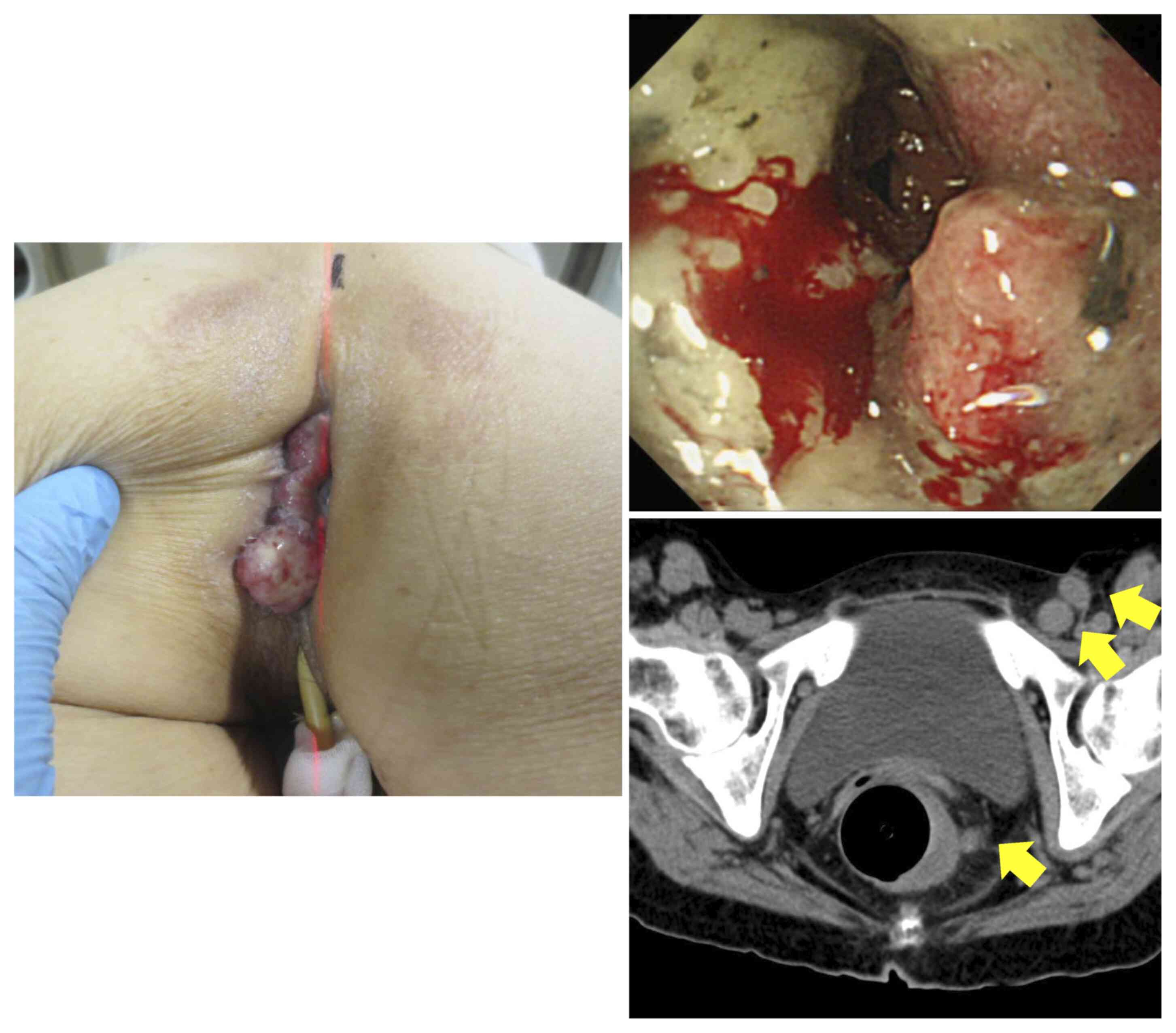
beyond the anal canal (Fig. 6). A
CT scan revealed a partially unclear border with the uterus,
suggestive of infiltration, and multiple perirectal, left internal
iliac, left obturator, left external iliac and left inguinal lymph
nodes of various sizes up to 2 cm, strongly suggestive of lymph
node involvement (Fig. 6). Based
on these findings, the patient was informed that they had an
inoperable cancer and a life expectancy of 2 weeks to 1 month, and
it was recommended that best supportive care should be administered
for symptomatic relief, instead of aggressive curative treatment.
The patient was also reluctant to undergo curative therapy.
However, the patient's family could not understand the sudden
declaration of terminal cancer and consulted a gastroenterologist.
This doctor also recommended palliative care, as chemotherapy could
further shorten the lifespan of the patient. The family was still
not satisfied with this recommendation and was eventually referred
to the Department of Radiology and Radiotherapy, Nagasaki
Prefecture Shimabara Hospital. Based on a discussion with the
patient and their family, the patient was scheduled to receive
KORTUC in combination with chemotherapy instead of standard
treatment.
The patient underwent IMRT with 50.4 Gy in 30
fractions for 6 weeks to the primary lesion and regional lymph
nodes, in the prone position using a belly board device. This was
combined with twice-weekly injections and spray of the
radiosensitizer on/into the perineal lesion, and use of both
methods on/into the rectal lesion with endoscopic guidance. Two
courses of modified FOLFOX6 at reduced doses (90% doses; 241 mg
leucovorin and 103 mg oxaliplatin over 2 h of continuous
intravenous infusion, respectively; 482 mg fluorouracil
intravenously by slow bolus over 5 min, and 2,894 mg fluorouracil
over 46 h of continuous intravenous infusion) were administered
concomitantly with radiation therapy. In addition, immediately
before treatment initiation, a central venous port was placed, and
intravenous hyperalimentation was initiated to improve the poor
general condition of the patient, to reduce adverse events and to
increase the therapeutic effect. A colostomy was placed proximally
to the descending colon to prepare for the development of ileus
secondary to transient swelling caused by irradiation of the lower
rectum.
At 9 months post-KORTUC, the rectal lesion had
disappeared completely and an endoscopic rectal biopsy revealed no
malignancy. The tumor extending to the perineum had disappeared.
The perineal dermatitis was mild, possibly due to the low dose
administered, and no serious adverse events were observed. The
multiple lymph node metastases located on the left side of the
pelvis had also regressed and could not be identified (Fig. 7). These included metastatic lymph
nodes within the radiation field that had not been injected with
the radiosensitizer.
This phenomenon has also been observed in other
patients undergoing KORTUC. A possible explanation is that, due to
its low molecular weight, the radiosensitizer injected into the
primary lesion flows via the lymphatic drainage into the regional
metastatic lymph nodes, similar to the way a dye or radioisotope is
injected for sentinel lymph node identification in breast cancer,
thereby sensitizing even lymph nodes that have not been injected.
We named this phenomenon the ‘sentinel effect’ of KORTUC, as it is
like the method of sentinel lymph node identification in breast
cancer (15).
Elevated pre-treatment levels of carcinoembryonic
antigen (264.5 ng/ml; normal range, 0-5 ng/ml) and carbohydrate
antigen 19-9 (117 U/ml; normal range, 0-37 U/ml) returned to normal
rapidly after KORTUC. Subsequently, the patient received adjuvant
chemotherapy, 11 courses of mFOLFOX6 once every 3 weeks, and
changing to 2nd line therapy (FOLFIRI, 90% dose; 300 mg
levofolinate calcium and 230 mg irinotecan hydrochloride hydrate
over 2 h of continuous intravenous infusion, respectively; 600 mg
fluorouracil intravenously by slow bolus over 5 min, and 3,600 mg
fluorouracil over 46 h of continuous intravenous infusion),
periodically for 4.5 years until the appearance of adverse events
in the form of skin ulcers. The patient achieved and has continued
to achieve a CR, with normal weight and general overall condition
for 6 years now without adjuvant cancer therapy. Currently, the
patient is followed up once a month by the referral
gastroenterologist.
Case 4: A huge lymph node
metastasis
A 69-year-old male presented with advanced non-small
cell lung cancer (NSCLC; RLL, adenocarcinoma, cT1bN3M1c, stage IVB;
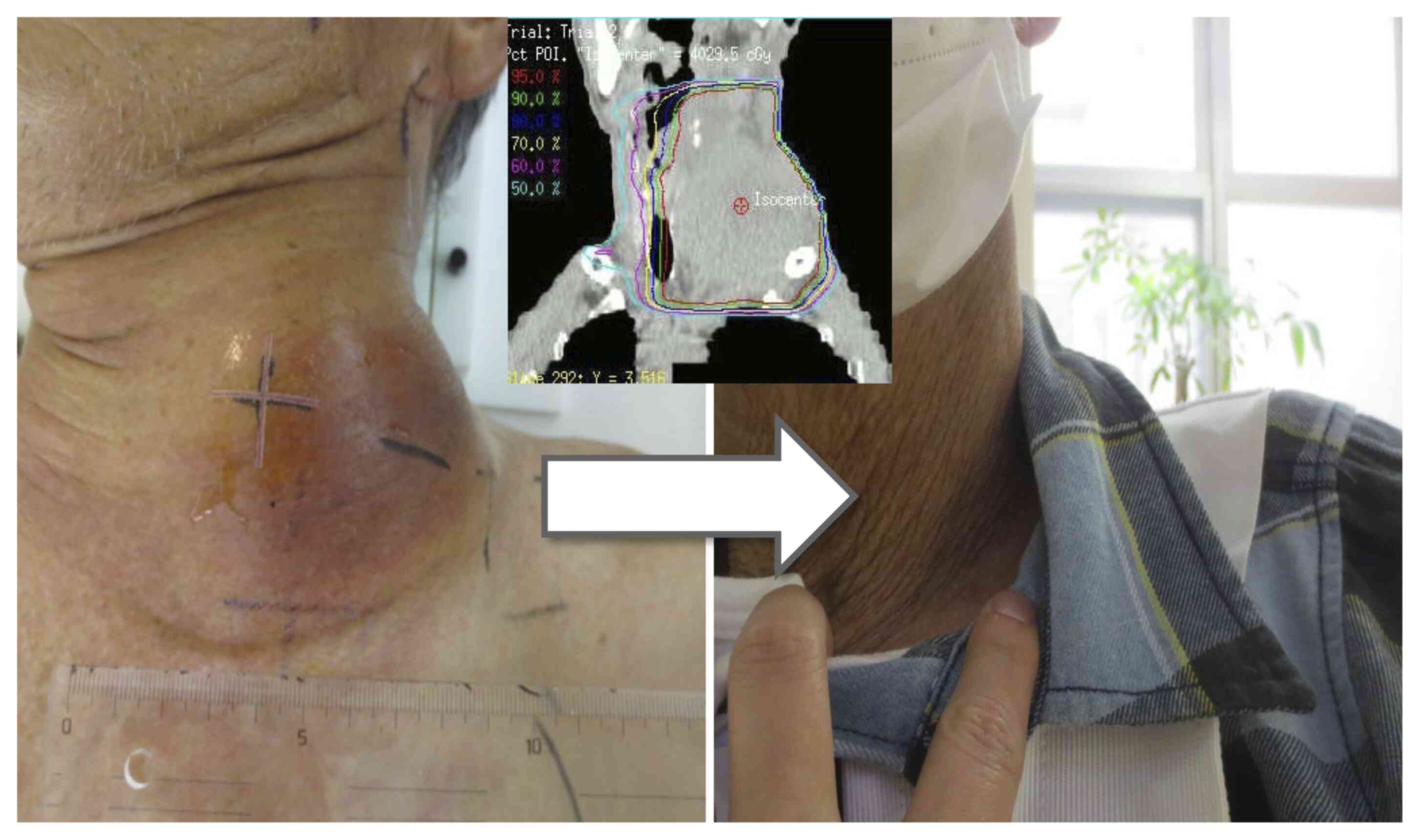
PS: 2) in September 2017. A left neck lesion appeared 1.5 months
previously and increased in size rapidly to 12 cm along its major
axis (Fig. 8). Due to the lesion,
the patient complained of neck pain and numbness of the left upper
extremity. The patient could not rotate their neck or elevate the
ipsilateral upper arm, and developed Horner's syndrome. At first,
the primary lesion was unknown; however, as a result of subsequent
pathological findings, which suggested adenocarcinoma of the lung,
and finding of a small nodule in the right lower lung on whole-body
CT, this was considered to be the primary lesion. The tumor was
consistent with lymph node metastasis and involved surrounding
blood vessels, so was considered difficult to surgically resect.
Radiation therapy could also have caused the rupture and collapse
of blood vessels, and sudden changes. However, if left untreated,
the tumor was expected to grow more rapidly and invade the
surrounding tissues and blood vessels more widely, breaking through
the skin, causing secondary infections and fatal bleeding. Impaired
quality of life could then result through severe pain and motor
dysfunction progression, followed by sudden death. Therefore, the
patient was referred from the Department of Respiratory Medicine,
Nagasaki Prefecture Shimabara Hospital, for palliative
radiotherapy. It was decided that the patient should be treated
using IMRT, with 40 Gy in 16 fractions for 3 weeks to the entire
left neck lymph node lesion, followed by boosts of 9 Gy in three
fractions to the shrinking lesion, in combination with twice-weekly
injection of the radiosensitizer under ultrasound guidance, and 60
mg oral S-1 twice a day for 3 weeks, withdrawn for 2 weeks.
At 8 months after KORTUC initiation, the lesion
disappeared, and concomitant conditions such as neck pain, motor
dysfunction and Horner's syndrome subsided. Subsequently, the
patient received adjuvant chemotherapy at the aforementioned dose
periodically for 2 years, and no tumor regrowth was present before
the patient succumbed to aspiration pneumonia.
Original abilities of radiation
therapy
The adaptive size of lesions in stereotactic
irradiation, which aims to have a therapeutic effect on cancer
using large doses, is generally ≤5 cm, and it is difficult to
expect a curative effect on lesions of larger sizes. The combined
use of the new sensitizer has shown a marked improvement in lesions
with a size of ≥10 cm without increasing the total dose of
radiation.
Discussion
Tailor-made treatments that have selective effects
on certain types of cancer have become popular, such as KYMRIAH
(tisagenlecleucel) (16). KORTUC,
which broadly eliminates the source of cancer, appears to swim
against such current trends, as it sensitizes radiotherapy, so can
be considered effective for basically any type of cancer. However,
KORTUC does not require many biopsied tissue samples to determine
potential selective treatment effects and has favorable cost
performance, as it does not require the reagents and tests that
would be needed for treatments with limited indications. These
advantages reduce the financial burden on patients and healthcare
providers, state healthcare systems, and ultimately, taxpayers.
As the pursuit of tailor-made treatments, in other
words, the diversity concerning malignant transformation, is
thought to be the same as the pursuit of the infinite, potential
adaptability of organisms, the development of tailor-made
treatments should overcome the complexity of malignant
transformation. It might be beneficial to reconsider the use of a
single approach to treat the underlying cause of all cancer
types.
KORTUC mainly exerts its effects through
reoxygenation. After reoxygenation has been achieved, almost all
molecular-targeted drugs would theoretically show the same local
effects as those observed when the drug was administered to the
tumor. Although it is known that an improved low-oxygen environment
or reoxygenation enhances treatment effects, attempts to achieve
reoxygenation have failed for a long period. In an attempt to apply
this approach to clinical settings, however, attention was paid to
the tumor growth, invasion and metastasis, as well as to the growth
factors involved in the acquisition of metastatic potential, such
as vascular endothelial growth factor (VEGF), epidermal growth
factor (EGF) and other molecules, which underlie the influence of
hypoxia (17). Based on the
assumption that inhibiting the action of these factors may at least
put a brake on cancer progression, molecular-targeted therapy has
been developed. VEGF, EGF and other molecules, which are targets
for molecular-targeted therapy, are produced in response to
stimulation by hypoxia-inducible factor (HIF)-1α. However, HIF-1α
is rapidly ubiquitinated and deactivated if the underlying
low-oxygen environment is improved. KORTUC can increase oxygen
tension in hypoxic intratumor environments and thus KORTUC therapy
could theoretically result in a halt to further production of
target factors such as VEGF and EGF for molecular-targeted drugs.
This means that KORTUC could eradicate the target molecules of
molecular-targeted therapy. KORTUC not only provides local
treatment, but also reduces the risk of tumor growth and
metastasis. In the future, molecular-targeted therapy may only need
to be used in patients who already have distant metastasis (M1) or
are at a high risk for distant metastasis.
The threat that reoxygenation poses to cancer cells
also involves the cancer cell cycle and cancer stem cells. Cancer
stem cells are also called dormant cells, a number of which show
treatment resistance in the G0 phase (quiescent stage).
Once the cancer stem cells switch from the quiescent stage to the
proliferation stage, radiotherapy and chemotherapy could exert the
same effects as those observed in normal cancer cells (18-21).
Since oxygen concentration appears to be involved in the transition
from the quiescent stage to the proliferation stage, reoxygenation
would enable this transition. This means that KORTUC can activate
cancer stem cells and reduce the risk of recurrence after
treatment.
In healthy conditions, reactive oxygen species (ROS)
harm the body, and the presence of antioxidant enzymes that
neutralize ROS is therefore essential. However, once a carcinogenic
state occurs, the role is reversed and ROS are needed to eliminate
the cancer, but the ROS become ineffective if neutralized by
antioxidant enzymes. To survive and protect itself from ROS attack,
cancer utilizes this neutralization reaction and uses transcription
factors, thereby excessively producing antioxidant enzymes
(22,23). In the process of achieving
reoxygenation, KORTUC neutralizes the excessive antioxidant enzymes
that interfere with treatment; therefore, it may enhance the
effects of radiotherapy and chemotherapy (24). However, unlike radiotherapy, which
has high penetrability, chemotherapy requires an angioarchitecture
that can deliver anticancer drugs to cancer. The resistance factors
of both radiation therapy and anticancer drugs may overlap, and it
cannot be denied that sensitizers affect both. Furthermore, it is
undeniable that sensitizers were intentionally used in anticipation
of the sensitizing effects of both radiation therapy and
chemotherapy, especially in refractory patients. A number of good
outcomes have been recorded that were not achieved with
conventional chemoradiotherapy. A number of patients with terminal
diagnoses have been cured using KORTUC, and this is a fact that
cannot be overlooked, as it suggests that KORTUC may exceed the
limits of current treatments. Based on the present results, it is
strongly hoped that clinical trials will be conducted and data will
be gained at other facilities worldwide, and that a therapeutic
effect will be achieved in patients who are suffering from
intractable cancer. Hypoxia and excessive antioxidant enzymes are
among the main factors conferring resistance to both radiotherapy
and chemotherapy. By neutralizing excessive antioxidant enzymes in
cancer cells with hydrogen peroxide, which is a readily available
ROS, antioxidant enzymes disappear, and oxygen occurs as the end
product. By combining the end product with the remaining excessive
ROS, irradiation of this state acts as a devastating attack against
cancer. This is the core principle of KORTUC action (25-27).
A common question asked is whether the damage to the
surrounding normal tissues is increased by KORTUC. When the oxygen
partial pressure is >30 mmHg, the influence of irradiation
reaches a plateau (28). ROS
produces water and oxygen as end products. Therefore, excessive ROS
(unreacted dilute hydrogen peroxide) is the only factor that may
increase adverse events, and no obvious increase in the incidence
of adverse events was recorded in the present study following the
addition of KORTUC to the general treatment.
In conclusion, the KORTUC radiosensitization method
showed good efficacy and tolerable safety for various types of
radioresistant tumors, and it has the potential for immediate
worldwide use.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SO, AK, YO and TK conceived and designed the study.
All authors performed the treatments. SO, YIs, YIn, SM, MN, RN, KM,
SK and AK analyzed and interpreted the data. SO, AK, YIs, YIn, SM,
SK and MN wrote, reviewed, and revised the manuscript. SO and AK
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study involving human data was approved
by the Ethics Committee of Nagasaki Prefecture Shimabara Hospital
(approval no. 21SH103 from January 14, 2010; and additional no.
26SH185 from September 4, 2014). All patients provided written
informed consent prior to their inclusion in the study. The study
was conducted in accordance with the Declaration of Helsinki of the
World Medical Association.
Patient consent for publication
All patients included in the present study provided
consent for participation and publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ogawa Y, Yamashita T, Masunaga S, Kariya
S, Tokuhiro S, Aoyama N, Tsuzuki A, Yaogawa S, Kubota K, Nishioka
A, et al: A new enzyme-targeting and radio-sensitization treatment;
basics and clinical practice of KORTUC Shinoharashinsha Pub. Inc.,
Tokyo, 2015 (In Japanese).
|
|
2
|
Ogawa Y, Takahashi T, Kobayashi T, Kariya
S, Nishioka A, Hamasato S, Moriki T, Seguchi H, Yoshida S and
Sonobe H: Immunocytochemical characteristics of human osteosarcoma
cell line HS-Os-1: Possible implication in apoptotic resistance
against irradiation. Int J Mol Med. 14:397–403. 2004.PubMed/NCBI
|
|
3
|
Kariya S, Sawada K, Kobayashi T, Karashima
T, Shuin T, Nishioka A and Ogawa Y: Combination treatment of
hydrogen peroxide and X-rays induces apoptosis in human prostate
cancer PC-3 cells. Int J Radiat Oncol Biol Phys. 75:449–454.
2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ogawa Y, Takahashi T, Kobayashi T, Toda M,
Nishioka A, Kariya S, Seguchi H, Yamamoto H and Yoshida S:
Comparison of radiation-induced reactive oxygen species formation
in adult articular chondrocytes and that in human peripheral T
cells: Possible implication in radiosensitivity. Int J Mol Med.
11:455–459. 2003.PubMed/NCBI
|
|
5
|
Ogawa Y: Paradigm shift in radiation
biology/radiation oncology-exploitation of the
‘H2O2 Effect’ for radiotherapy using low-LET
(Linear Energy Transfer) Radiation such as X-rays and high-energy
electrons. Cancers (Basel). 8(28)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ogawa Y, Kubota K, Aoyama N, Yamanishi T,
Kariya S, Hamada N, Nogami M, Nishioka A, Onogawa M and Miyamura M:
Non-surgical breast-conserving treatment (KORTUC-BCT) using a new
radio-sensitization method (KORTUC II) for patients with stage I or
II breast cancer. Cancers (Basel). 7:2277–2289. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nimalasena S, Gothard L, Anbalagan S,
Allen S, Sinnett V, Mohammed K, Kothari G, Musallam A, Lucy C, Yu
S, et al: Intratumoral hydrogen peroxide with radiation therapy in
locally advanced breast cancer: Results from a phase 1 clinical
trial. Int J Radiat Oncol Biol Phys. 108:1019–1029. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tokuhiro S, Ogawa Y, Tsuzuki K, Akima R,
Ue H, Kariya S and Nishioka A: Development of a new
enzyme-targeting radiosensitizer (KORTUC) containing hydrogen
peroxide for intratumoral injection for patients with low linear
energy transfer (LET) radioresistant neoplasms. Oncol Lett.
1:1025–1028. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Christensen ES: Iatrogenic dissemination
of tumor cells-dissemination of tumor cells along the needle track
after percutaneous, transthoracic lung biopsy. Dan Med Bull.
25:82–87. 1978.PubMed/NCBI
|
|
10
|
Sinner WN and Zajicek J: Implantation
metastasis after percutaneous transthoracic needle aspiration
biopsy. Acta Radiol Diagn (Stockh). 17:473–480. 1976.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
National Cancer Institute (NCI): Common
Terminology Criteria for Adverse Events (CTCAE) v4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/.
Accessed November 25, 2021.
|
|
13
|
Gebski V, Lagleva M, Keech A, Simes J and
Langlands AO: Survival effects of postmastectomy adjuvant radiation
therapy using biologically equivalent doses: A clinical
perspective. J Natl Cancer Inst. 98:26–38. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
ECOG-ACRIN cancer Research Group: ECOG
Performance Status. https://ecog-acrin.org/resources/ecog-performance-status.
Accessed November 25, 2021.
|
|
15
|
Obata S, Ogihara Y, Ohta Y, Kan T, Kanegae
S, Inoue Y, Kuroiwa A, Inoue K, Yamanishi M, Hayashi T, et al:
Possibility of a ‘sentinel effect’ in chemoradiotherapy and a new
radiosensitizer injection (KORTUC). Jpn J Clin Radiol. 63:317–327.
2018.(In Japanese).
|
|
16
|
U.S. Food and Drug Administration: KYMRIAH
(tisagenlecleucel) https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel.
Accessed November 25, 2021.
|
|
17
|
Vaupel P: The role of hypoxia-induced
factors in tumor progression. Oncologist. 9 (Suppl 5):S10–S17.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Li L and Bhatia R: Stem cell quiescence.
Clin Cancer Res. 17:4936–4941. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Takeishi S, Matsumoto A, Onoyama I, Naka
K, Hirao A and Nakayama KI: Ablation of Fbxw7 eliminates
leukemia-initiating cells by preventing quiescence. Cancer Cell.
23:347–361. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Iriuchishima H, Takubo K, Matsuoka S,
Onoyama I, Nakayama KI, Nojima Y and Suda T: Ex vivo maintenance of
hematopoietic stem cells by quiescence induction through Fbxw7 and
alpha; overexpression. Blood. 117:2373–2377. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Taniyama Y and Griendling KK: Reactive
oxygen species in the vasculature: Molecular and cellular
mechanisms. Hypertension. 42:1075–1081. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pizzino G, Irrera N, Cucinotta M, Pallio
G, Mannino F, Arcoraci V, Squadrito F, Altavilla D and Bitto A:
Oxidative stress: Harms and benefits for human health. Oxid Med
Cell Longev. 2017(8416763)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kobayashi M and Harada H: Hypoxic stress
and HIF. Seikagaku. 85:187–195. 2013.PubMed/NCBI(In Japanese).
|
|
25
|
Ogawa Y, Ue H, Tsuzuki K, Tadokoro M,
Miyatake K, Sasaki T, Yokota N, Hamada N, Kariya S, Hitomi J, et
al: New radio-sensitization treatment (KORTUC I) using hydrogen
peroxide solution-soaked gauze bolus for unresectable and
superficially exposed neoplasms. Oncol Rep. 19:1389–1394.
2008.PubMed/NCBI
|
|
26
|
Miyatake K, Kubota K, Ogawa Y, Hamada N,
Murata Y and Nishioka A: Non-surgical care for locally advanced
breast cancer: Radiologically assessed therapeutic outcome of a new
enzyme-targeting radio-sensitization treatment, Kochi
Oxydol-radiation therapy for unresectable Carcinomas, Type II
(KORTUC II) with systemic chemotherapy. Oncol Rep. 24:1161–1168.
2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ogawa Y, Kubota K, Ue H, Tadokoro M,
Matsui R, Yamanishi T, Hamada N, Kariya S, Nishioka A, Nakajima H,
et al: Safety and effectiveness of a new enzyme-targeting
radio-sensitization treatment (KORTUC II) for intratumoral
injection for low-LET radio-resistant tumors. Int J Oncol.
39:553–560. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chapman JD, Dugle DL, Reuvers AP, Meeker
BE and Borsa J: Studies on the radio-sensitizing effect of oxygen
in Chinese hamster cells. Int J Radiat Biol Relat Stud Phys Chem
Med. 26:383–389. 1974.PubMed/NCBI View Article : Google Scholar
|