Introduction
A collision tumor is a coexistence of two
diagnostically distinct tumors in a common anatomic space (1). Small cell carcinoma (SmCC) is a
high-grade tumor derived from neuroendocrine cells. Extrapulmonary
SmCC is rare, accounting for 2.5-5% of all cases of SmCC. The
genitourinary and gastrointestinal systems are the most common
sites (2). In the maxillary
sinuses, the most common malignancy is squamous cell carcinoma
(SCC), followed by adenocarcinoma (3,4).
SmCC is a highly aggressive tumor with high recurrence rates and
propensity for distant metastasis, hence its poor prognosis
(4).
In the head and neck region, the collision of
neuroendocrine tumors is uncommon. Only a small number of cases in
the oral region and sinonasal area have been reported (3,5,6).
This occurrence has been reported more frequently in the larynx
(7). Composite tumor in the
sinonasal area mostly comprises adenocarcinoma and neuroendocrine
carcinoma (5). Collision tumors
made up of SmCC and SCC are rare. The present study reported on a
case of SmCC of the maxillary sinus; at the same site, SCC was
detected during follow-up. The radiologic findings, including
dynamic contrast-enhanced (DCE)-magnetic resonance imaging (MRI),
are discussed.
Case report
An 82-year-old female was referred to Okayama
University Hospital (Okayama, Japan) with pain in the upper right
gingiva in the proximity of the partial denture with associated
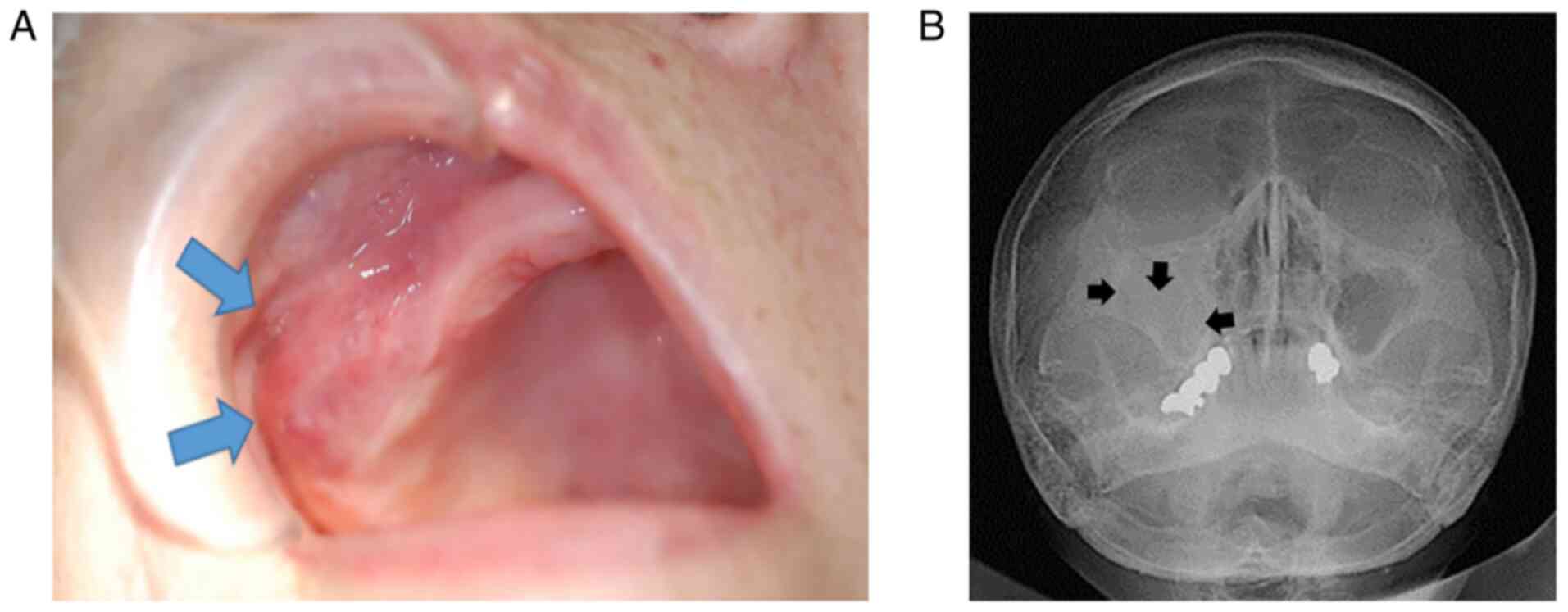
cheek swelling. Intra-oral examination revealed a 26-mm
compressible swelling of the right cheek and a non-tender mass in
the upper right edentulous gingiva extending from the canine region
to the molar region. In addition, the Water's projection revealed a
radiopaque mass in the right maxillary sinus (Fig. 1A and B). The patient's medical history was
significant for hypertension and myocardial infarction.
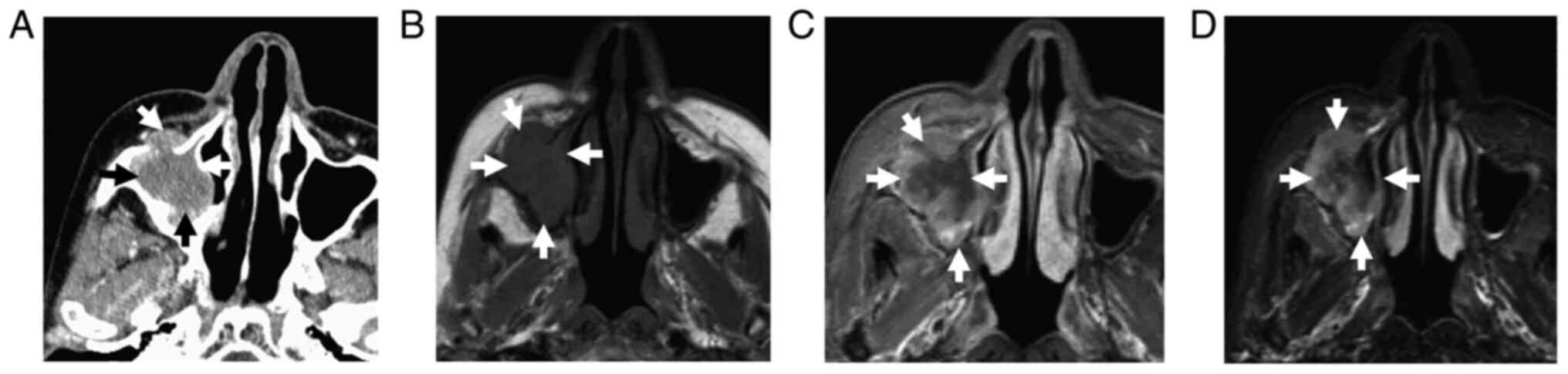
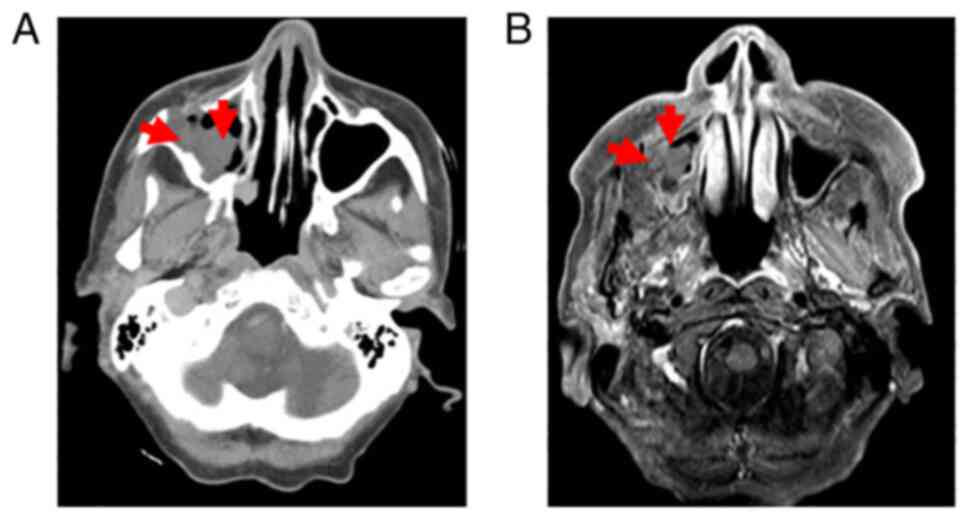
Axial computed tomography (CT) images displayed a
mass in the maxillary sinus with a bony defect of the anterior wall
(Fig. 2A). No destruction of the
posterior wall, ethmoid sinuses or pterygoid plate was present. On
MRI, the mass exhibited heterogeneous enhancement on post-contrast
images. MRI examination was performed using a 1.5 T device
(Magnetom Vision®; Siemens AG) with a head and neck
coil. T1-weighted images (T1WI) were acquired with a spin-echo
sequence using repetition time (TR)/echo time (TE) parameters of
450/10 msec in addition to short T1 inversion recovery (STIR)
images using turbo-spin echo sequence TR/TE/inversion time
parameters of 4,500/60/140 msec. In addition, DCE-MRI images were
acquired with 3D fast imaging with a steady-state precession
sequence using the following parameters: TR, 5 msec; TE, 2 msec;
flip angle, 25˚; 16 partitions in a 48 slab; section thickness, 3
mm; 250x188-mm rectangular fields of view; and a 256x192 matrix
resulting in a 0.98x0.98-mm pixel size. The first image series was
obtained in 14 consecutive scans. Gadolinium-diethylentriamine
pentaacetic acid (Magnevist Syringe; Nihon Schering) was
administered intravenously for 6 sec at a rate of ~0.2 ml/kg via
manual injection between the first and second scans in the first
series. Second- and third-series scans were performed at 440 and
880 sec after the injection. All scans were acquired over 14 sec
with a 1-sec interval between each scan.
The mass demonstrated heterogeneous low signal
intensity (SI) on T1WI (Fig. 2B),
high SI on STIR images (Fig. 2C)
and heterogeneous solid enhancement on contrast-enhanced (CE) T1WI
(Fig. 2D). Dynamic CE images were
analyzed with regions of interest using a workstation (Synapse
Vincent®; Fujifilm Medical) to obtain contras index
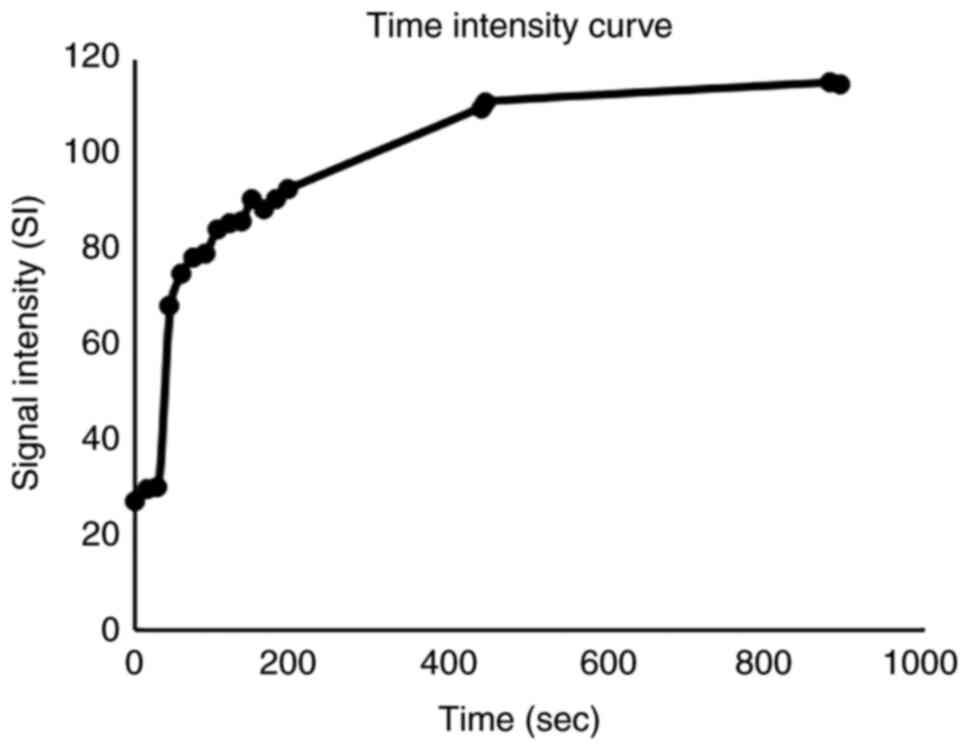
curves (CI curves). The CI curves exhibited a rapid increase over
~100 sec and then a further increase without washout of the
contrast medium (Fig. 3). The
initial radiologic diagnosis was a lymphoproliferative lesion. The
DCE-MRI suggested non-SCC with salivary gland tumor and
adenocarcinoma differential. On 18F fluorodeoxyglucose
positron-emission tomography (FDG-PET) indicated no other abnormal
sites, suggesting that the maxillary sinus mass was a primary
lesion with a maximum standardized uptake value (SUVmax) of
24.81.
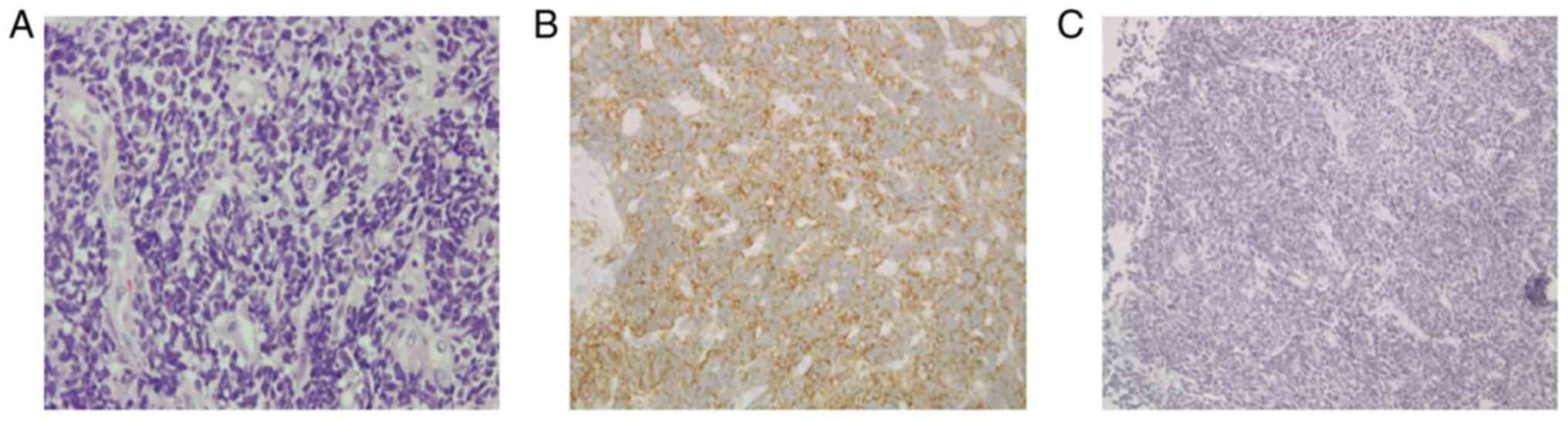
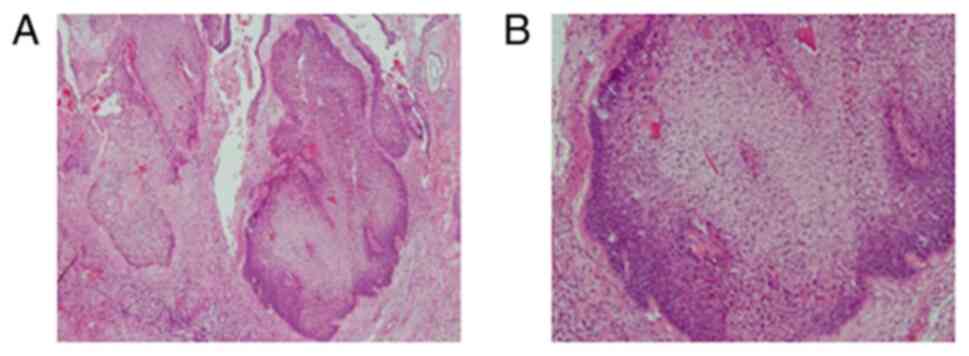
Histopathologic examination of a biopsy specimen
revealed a large amount of chromatin and non-uniform cells on
H&E staining. The maxillary sinus tissue was fixed with
formaline and embedded in paraffin (10%) at 60˚C and cut into
2.5-µm sections. The sections were incubated with antibodies
against CD56 (1:125 dilution; cat. no. 123C3; Roche Diagnostics),
Chromogranin A (1:1,000 dilution; cat. no. LH2H10; Roche
Diagnostics), Synaptophysin (1:200 dilution; cat. no. SP11; Roche
Diagnostics) and Ki-67 (1:500 dilution; cat. no. 30-9; Roche
Diagnostics) at 37˚C for 15 min. Immunostaining indicated that 2 of
3 neuroendocrine tumor markers, CD56 and chromogranin A were
expressed, while synaptophysin was negative. However, Ki-67 was
positive (Fig. 4A-C). Based on
these findings, the case was diagnosed with SmCC.
At seven weeks after the initial visit, the patient
underwent concurrent chemoradiotherapy (CCRT). Carboplatin and
etoposide were administered intravenously at a dose of 225 and 110
mg/day, respectively. The CCRT procedure was performed for three
days with three-week intervals for each cycle with four cycles. The
patient also underwent radiotherapy during the chemotherapy course.
The total radiotherapy dose was 60 Gy with 30 fractions of 2 Gy
given five days per week. The patient had no adverse events
associated with CCRT. CT (Fig. 5A)
and MRI (Fig. 5B) evaluation
indicated a residual tumor on the right of the maxillary sinus. A
Denker procedure was performed to excise the residual tumor.
Histologic examination of the surgical specimen of residual tumor
using H&E staining (cat. no. H9627; MilliporeSigma) revealed
positivity for cytokeratin 7 (CK7; cat. no. M7018; Dako) and CK20
(cat. no. PA0022; Leica Biosystems GmbH), consistent with the
diagnosis of SCC (Fig. 6). The
imunostaining result of neuroendocrine was negative and therefore,
SmCC was no longer apparent. The patient underwent chemotherapy
using cisplatin. However, treatment was stopped after the first
course due to adverse effects of the therapy. Subsequently, the
patient regularly underwent follow-ups until six months after the
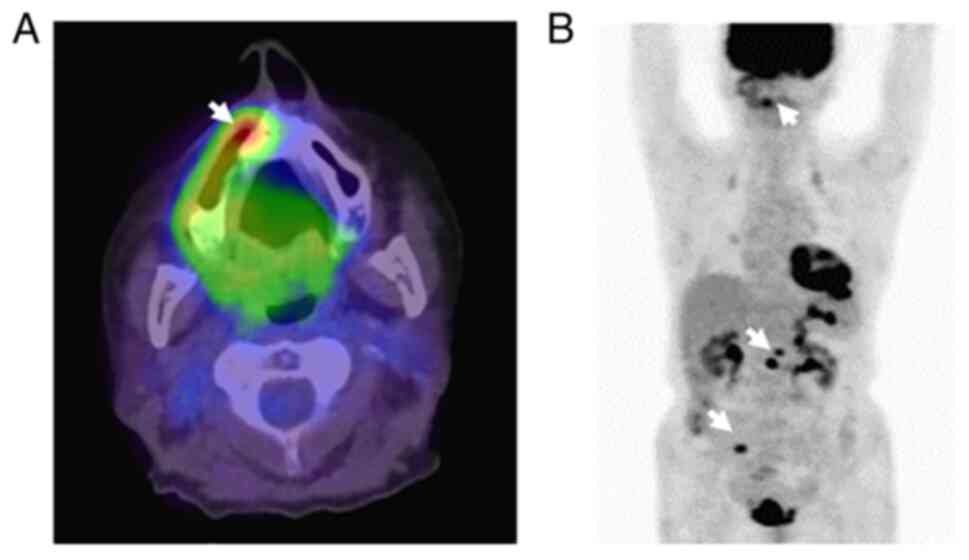
second surgery. 18F FDG-PET revealed tumor recurrence in
the maxillary sinus (SUVmax=6.0) (Fig.
7A) and multiple metastases to organs in the abdomen (Fig. 7B). The patient was transferred to a
palliative care facility and died two months after the detection of
metastases.
Discussion
Collision tumor refers to two malignant tumors
coexisting and the components originate from the same area or organ
but with different morphologies according to histologic
examination. There are various theories related to collision
tumors; however, due to low frequency and individuality,
controversy still exists regarding the pathogenesis and definition
(8). Reports on the synchronous
and metachronous coexistence of SmCC and SCC in the same anatomic
space in the head and neck are rare. One study proposed two
possible explanations for this phenomenon. The first is that the
tumors arise from a typical pluripotential stem cell with
subsequent divergent differentiation. The second theory is that two
separate tumors coincide through two independent molecular
processes (3).
SmCC of the head and neck is an aggressive tumor
type with a propensity to spread locoregionally with simultaneous
distant metastases (3,4). Head and neck SmCC is more common in
the larynx and rare in the paranasal sinuses (2). The diagnostic markers for
neuroendocrine tumors include CD56, chromogranin A and
synaptophysin (6,9). Kontogianni et al (10) reported that SmCC was positive for
CD56 and negative for chromogranin and synaptophysin.
In the case of the present study, Immunostaining
indicateded that CD56 and chromogranin A were positive, while
synaptophysin was negative and Ki-67 was positive on initial
examination, suggesting the diagnosis of SmCC. Other results on
post-CCRT treatment revealed epithelial tumor markers of a high
level of squamous cell antigen (11.3 ng/ml) and a positive result
of keratin AE1/AE3 was evident, which was suggestive of SCC, an
epithelial tumor. The residual tumor was precisely in the same area
of the primary tumor and was a different type of tumor, and it was
hypothesized that the occurrence of SCC after CCRT of SmCC may have
been due to the initial tumor containing epithelial tumor
cells.
On conventional radiographs and CT, the present case
exhibited destruction of the anterior wall and tumor progression
through an anterior wall. There was no destruction of the posterior
wall, ethmoid sinuses or pterygoid plate. Most cases of SmCC in the
head and neck extend into adjacent spaces without extensive bone
destruction (3). A previous study
reported on SmCC of the nasal cavity with extensive and aggressive
destruction of bone at the skull base and invasion of the right
orbit (8).
The MR findings of SmCC in the paranasal sinuses
include moderate SI on T1WI, slightly high SI on T2WI high SI on
STIR (11) and mild-to-moderate
homogenous enhancement after administration of contrast agent
(12). In the present case, the
mass demonstrated heterogeneous low-to-moderate SI on T1WI and high
SI on STIR images, with contrast-enhanced T1WI revealing intense
heterogeneous enhancement. A previous study by our group reported
on a case of malignant lymphoma with low SI on T1WI and high SI and
slightly high enhancement on T2WI and CE-T1WI, respectively
(13). CI curve analysis indicated
a rapid increase until ~100 sec and further expansion without
washout. This finding is different from other malignancies, such as
SCC and malignant lymphoma, which rapidly increase and gradually
decrease with varying peak times (14).
The limitation of the present study wass that on
initial presentation, the entire primary lesion was not examined
and the border of each lesion was not defined, but only a biopsy
was performed. The histopathological examination only revealed
SmCC, although the subsequent result indicated epithelial tumor
cells. Histopathologic examination of the complete specimen of is
critical to the detection of collision tumor.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received for this study.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
IS: Conceptualization, design, drafting of the
manuscript; YY: Acquisition of data and critical revision; MH, SO,
YT and BOB: Conceptualization and critical revision; JA:
Acquisition and analysis of the data, critical revision and final
approval of the manuscript. All authors read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The authors have obtained the appropriate consent
from the patient's relatives to publish the case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nabili V, Natarajan S, Hirschovitz S,
Bhuta S and Abemayor E: Collision tumor of thyroid: Metastatic lung
adenocarcinoma plus papillary thyroid carcinoma. Am J Otolaryngol.
28:218–220. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Van der Heijden HF and Heijdra YF:
Extrapulmonary small cell carcinoma. South Med J. 98:345–349.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Barham HP, Said S and Ramakrishnan VR:
Colliding tumor of the paranasal sinus. Allergy Rhinol
(Providence). 4:e13–e16. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Day TA, Beas RA, Schlosser RJ, Woodworth
BA, Barredo J, Sharma AK and Gillespie MB: Management of paranasal
sinus malignancy. Curr Treat Options Oncol. 6:3–18. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang SF, Chuang WY, Cheng SD, Hsin LJ,
Lee LY and Kao HK: A colliding maxillary sinus cancer of
adenosquamous carcinoma and small cell neuroendocrine carcinoma-a
case report with EGFR copy number analysis. World J Surg Oncol.
8(92)2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mochizuki Y, Omura K, Sakamoto K,
Nakanishi S, Satoh K, Marukawa E and Yamaguchi A: A case of primary
combined neuroendocrine carcinoma with squamous cell carcinoma in
the upper gingiva. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 109:e34–e39. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Davies-Husband CR, Montgomery P,
Premachandra D and Hellquist H: Primary, combined, atypical
carcinoid and squamous cell carcinoma of the larynx: A new variety
of composite tumour. J Laryngol Otol. 124:226–229. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yu Q, Chen YL, Zhou SH, Chen Z, Bao YY,
Yang HJ, Yao HT and Ruan LX: Collision carcinoma of squamous cell
carcinoma and small cell neuroendocrine carcinoma of the larynx: A
case review and review of the literature. World J Clin Cases.
7:242–252. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hosokawa S, Okamura J, Takizawa Y and
Mineta H: Long-term survival of a patient with primary small cell
neuroendocrine carcinoma of the maxillary sinus: A case report. J
Oral Maxillofac Surg. 71:e248–e252. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kontogianni K, Nicholson AG, Butcher D and
Sheppard MN: CD56: A useful tool for the diagnosis of small cell
lung carcinomas on biopsies with extensive crush artefact. J Clin
Pathol. 58:978–980. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Joyce EA, Kavanagh J, Sheehy N, Beddy P
and O'Keeffe SA: Imaging features of extrapulmonary small cell
carcinoma. Clin Radiol. 68:953–961. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu Q, Zhu W, Wu J and Zhang H: The CT and
MRI observations of small cell neuroendocrine carcinoma in
paranasal sinuses. World J Surg Oncol. 13(54)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Matsuzaki H, Hara M, Yanagi Y, Asaumi J,
Katase N, Unetsubo T, Hisatomi M, Konouchi H, Takenobu T and
Nagatsuka H: Magnetic resonance imaging (MRI) and dynamic MRI
evaluation of extranodal non-Hodgkin lymphoma in oral and
maxillofacial regions. Oral Surg Oral Med Oral Pathol Oral Radiol.
113:126–133. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Asaumi J, Yanagi Y, Konouchi H, Hisatomi
M, Matsuzaki H and Kishi K: Application of dynamic
contrast-enhanced MRI to differentiate malignant lymphoma from
squamous cell carcinoma in the head and neck. Oral Oncol.
40:579–584. 2004.PubMed/NCBI View Article : Google Scholar
|