Introduction
Metastatic malignant melanoma is a common cause of
cancer-related morbidity and mortality (1) and has been historically associated
with poor prognosis (2). The
introduction of novel therapies such as immunotherapy and BRAF/MEK
inhibition for patients harboring a BRAFV600-mutation have changed
drastically the course of the disease and are now considered a
standard of care for this population (3).
Approximately 50% of cutaneous melanomas exhibit
activating mutations in the B-raf proto-oncogene (BRAF) gene, more
commonly in the position V600E. BRAF is part of the
mitogen-activated protein kinases (MAPK) pathway and plays a key
role in regulation of cellular growth and survival. Activating
mutations in the BRAF gene result in continuous downstream
activation of the MAPK cascade, including mitogen-activated
extracellular signal-regulated kinase (MEK), thus leading to
uncontrolled cell proliferation and malignant transformation
(4). BRAF inhibition presents
significant antitumor activity in BRAF-mutated melanoma, although
early development of resistance through alternative MAPK-activating
mechanisms is a common clinical problem. Combination of BRAF and
MEK inhibition is known to have proven synergic effect and delay
the development of acquired resistance (4).
Sarcoidosis is an inflammatory disease of unknown
origin characterized by the development of granulomas in various
organs, with a possible epidemiological and causal correlation to
multiple hematologic and solid malignancies (5). For example, incidence of sarcoidosis
in melanoma patients has been estimated at 0.58%, a rate
significantly higher in comparison to that of the general
population (6). In the last years
since the establishment of modern anticancer agents, sarcoidosis
and sarcoid-like reactions (SLR) occur more often as a result of
treatment, with immunotherapy causing the majority of those cases
(7,8). Few cases of drug-induced sarcoidosis
and SLR have been described in connection to BRAF inhibitors, alone
or combined with MEK inhibitors, and present, in most cases,
exclusively as skin reactions (9).
In this report, we discuss the case of a patient
developing mediastinal and right hilar lymphadenopathy as the only
manifestation of SLR while on dabrafenib and trametinib for
metastatic melanoma, and summarize the cases of such granulomatous
reactions published in the literature.
Case report
A 55-year-old female, with no significant medical
history, no family history of cancer and no current medication,
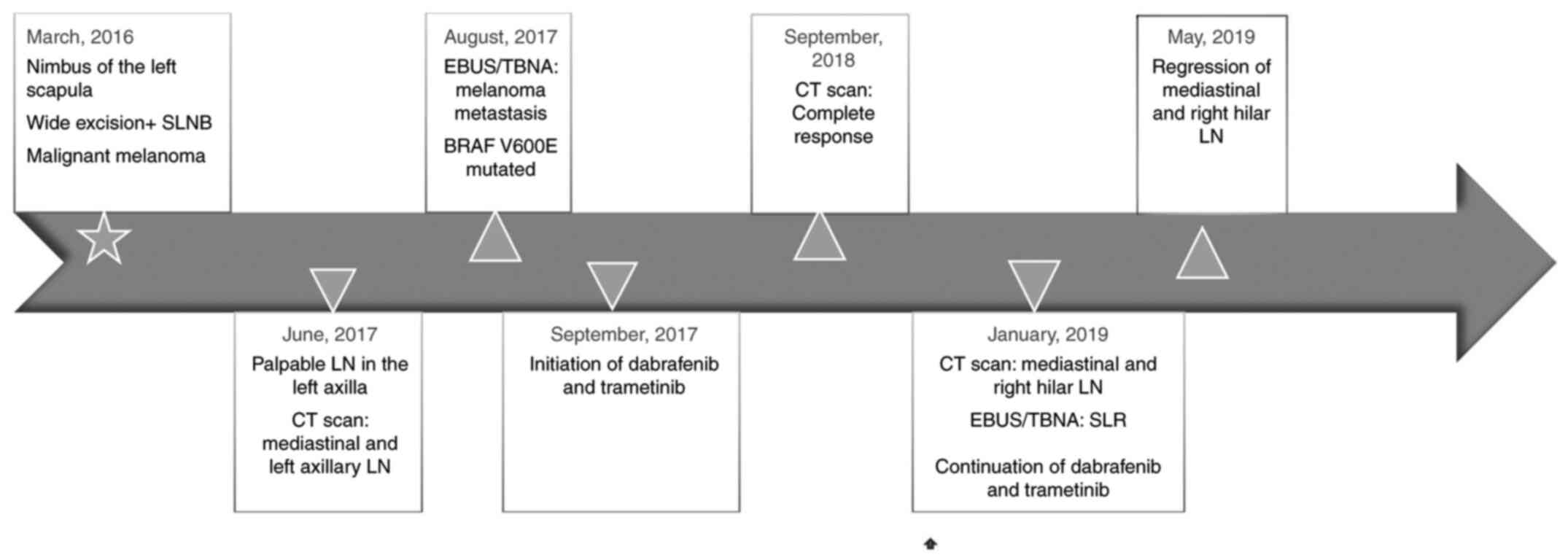
presented in 2016 with a newly detected asymmetric and ulcerated
mole in the area of the left scapula. The patient did not report
any symptoms, physical examination and laboratory tests revealed no
other significant findings.
Wide excision and sentinel lymph node biopsy (SLNB)
were performed, leading to the diagnosis of AJCC (7th edition)
stage IIb cutaneous melanoma [pT2b (1.5 mm), N0 (SLNB), Clark level
3]. The patient was then scheduled for follow-up. Fifteen months
later, physical examination revealed a palpable lymph node of the
left axilla. Computed Tomography (CT) scan confirmed
lymphadenopathy of the left axilla and mediastinum measuring 1.8 cm
in diameter, as well as a 1.7-cm nodule of the left lower lobe. The
patient underwent endobronchial ultrasound bronchoscopy (EBUS) with
transbronchial needle aspiration (TBNA) and histologic examination
of material from the 2L station verified the metastatic nature of
the lymphadenopathy along with the presence of
BRAFV600E-mutation.
First-line treatment with BRAF/MEK inhibitors
dabrafenib 150 mg per os twice daily and trametinib 2 mg per os
once daily was initiated on September 2017. Treatment was well
tolerated without any adverse events during the first months, and
CT scan at 12 months showed complete remission of both the
lymphadenopathy and lung nodule. Sixteen months after therapy
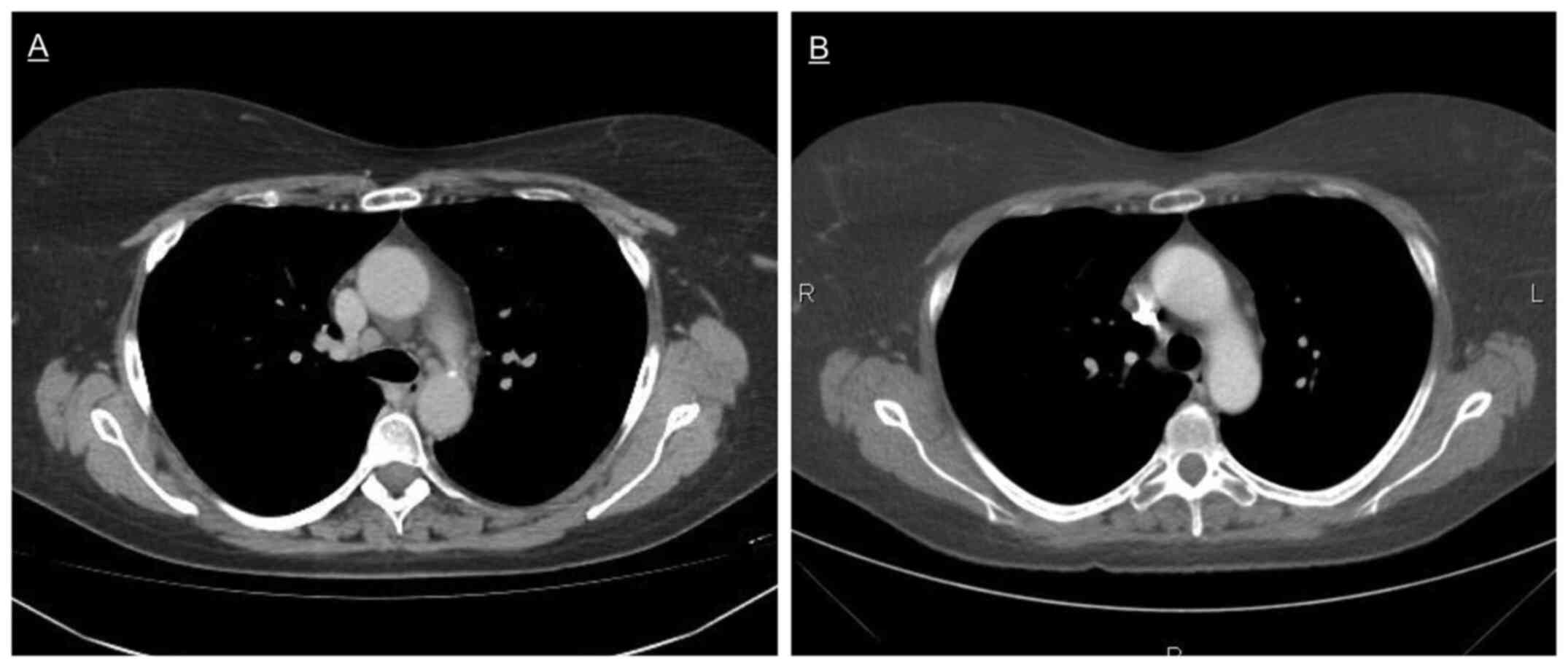
initiation, CT scan raised the suspicion of disease progression
with new marginal mediastinal and right hilar lymphadenopathy of
1.1 cm in short axis (Fig. 1A).
The patient had no symptoms, physical and laboratory tests were
once again normal. Histopathology obtained through EBUS-TBNA from
the stations 4R and 11R showed sarcoid-like non-necrotizing
granulomas composed of epithelioid cells, macrophages and
T-lymphocytes. Since the patient was asymptomatic, no additional
treatment was started and antineoplastic therapy with
dabrafenib/trametinib was continued. The lymphadenopathy regressed
spontaneously in the next five months, while the patient remained
in complete remission (Fig. 1B). A
timeline of all relevant data is presented in Fig. 2.
Discussion
BRAF inhibition has been associated with the
development of granulomatous reactions such as sarcoidosis and SLR
through immunomodulatory mechanisms that affect the
immunosuppressive tumor microenvironment (10). More specifically, patients treated
with inhibitors of the MAPK-pathway tend to present increased TNF-α
and IFN-γ serum levels (11),
which are associated with formation of granulomas. Furthermore, it
has been hypothesized that granulomatous reactions could be a
paradoxical autoimmune response to BRAF inhibition through
CD8+ T-cell infiltration and PD-L1 expression (10). Onset of granulomatosis soon after
the initiation of treatment with BRAF/MEK inhibitors further
supports an etiological relation (9).
In any case, such adverse events remain uncommon. We
identified 17 documented cases of histologically confirmed
granulomatosis under combined treatment with dabrafenib and
trametinib in the literature (10,12-20),
which are summarized in Table I.
Men and women seem to be almost equally affected (8/17 vs. 9/17),
while patient age varies between 19 and 82 years. All but one
patient (16/17) suffered from stage IV melanoma, while in one case
granulomatosis was diagnosed during adjuvant treatment for stage
III disease (20). Time of
treatment until the onset of reactions also varies significantly
from 1-20 months. Skin lesions were almost universally present
(15/17) and the only manifestation in the majority of patients
(12/17), while five patients had systemic disease including
mediastinal lymph node, kidney, heart, salivary gland, liver and
eye involvement. Granulomatous reactions had a generally mild
course, with most patients (12/17) reaching a remission:
discontinuation of dabrafenib and trametinib was necessary in three
cases, topical corticosteroids in eleven and systemic
corticosteroid treatment in two. One patient died due to
granulomatous myocarditis before receiving any specific treatment
(15). Melanoma response was more
diverse: nine patients responded to BRAF/MEK inhibition and another
five progressed.
 | Table ICases of sarcoidosis/sarcoid-like
reactions under dabrafenib and trametinib. |
Table I
Cases of sarcoidosis/sarcoid-like
reactions under dabrafenib and trametinib.
| First author,
year | Sex | Age, years | Stage | Months until
onset | Site | Treatment
discontinuation | CS | Outcome of
SAR/SLR | Melanoma
response | (Refs.) |
|---|
| Green, 2013 | F | 62 | IV M1d | 9 | Skin | No | No | Remission | NR | (12) |
| | M | 72 | IV M1b | 8 | Skin | No | Topical | Remission | NR | |
| Park, 2014 | F | 82 | IV M1a | 2 | Skin | No | Topical | Remission | PR | (13) |
| Jansen, 2015 | M | 63 | IV M1a | 18 | Skin, kidney | Yes | Systemic | Remission | CR | (14) |
| Winkler, 2018 | M | 46 | IV M1b | 2 | Myocardium | No | No | Death | CR | (15) |
| Rueda-Rueda,
2018 | F | 39 | IV | 2 | Uvea | Yes | Systemic +
Topical | Remission | PD | (16) |
| Dimitriou, 2018 | M | 19 | IV M1c | 1 | Skin | No | Topical | Remission | CR | (10) |
| Korman, 2018 | F | 49 | IV M1b | 12 | Skin | No | No | Progress | CR | (17) |
| Giet, 2019 | M | 30 | IV M1c | 4 | Skin | NR | Topical | Stable | NR | (18) |
| Huynh, 2020 | F | 61 | IV M1c 1 | 8 | Skin | No | No | Remission | PD | (19) |
| | F | 77 | IV M1a 0 | 20 | Skin | No | Topical | Remission | CR | |
| | M | 45 | IV M1d 0 | 2 | Skin, salivary
glands, mediastinal lymph nodes | No, dose
reduction | No | Remission | PD | |
| | F | 49 | IV M1c 0 | 15 | Skin | No, dose
reduction | Topical | Relapse | CR | |
| | F | 66 | IV M1a 0 | 2 | Skin | No | Topical | Remission | PD | |
| | M | 57 | IV M1b 0 | 6.5 | Skin | No | Topical | Remission | CR | |
| | F | 82 | IV M1b 1 | 11 | Skin | No, dose
reduction | Topical | Stable | PD | |
| Boutros, 2020 | M | 38 | III | 3 | Skin, liver, uvea,
mediastinal lymph nodes | Yes | Topical | Remission | Relapsefree | (20) |
In accordance with, to date, published data, SLR in
our case had benign clinical behavior and resolved without special
treatment. However, skin lesions were absent and mediastinal or
hilar lymphadenopathy was the only manifestation. To our knowledge,
this is the first documented case of isolated lymphadenopathy due
to SLR in a patient receiving the combination of dabrafenib and
trametinib. Other BRAF-inhibitors such as vemurafenib, with or
without cobimetinib, have also been associated with granulomatous
reactions (13,21-23),
but isolated mediastinal or hilar lymphadenopathy has not been
described with these agents either. This observation raises the
question if SLR without skin involvement is an extremely rare
entity under BRAF/MEK inhibition or if it could sometimes be
mistaken for progressive disease in clinical practice. In any case,
it is reasonable to acquire tissue biopsy in case of newly detected
lymphadenopathy in order to establish a definite diagnosis
(9).
Due to the small number of documented cases and the
lack of prospective studies, many questions remain unanswered. It
is not known which patients develop drug-induced granulomatous
reactions, what is the optimal therapy, if any, and in which cases
discontinuation of BRAF/MEK inhibition is necessary (9). It has been suggested that
treatment-related sarcoidosis is associated with better efficacy of
BRAF inhibitors (24), but data is
very limited.
In summary, granulomatous reactions in patients
receiving dabrafenib and trametinib for advanced melanoma are rare,
usually mild adverse events. Our case describes the uncommon entity
of SLR with isolated mediastinal and hilar lymphadenopathy and
underlines the importance of differential diagnosis from tumor
progress. Further research is needed in order to define the optimal
management of these patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
VMB and MM treated the patient, designed the study
and wrote the manuscript. KL treated the patient, analyzed the
findings and critically revised the manuscript. EP, EL and DIL
analyzed and interpreted the findings, and reviewed and edited the
manuscript. GA made substantial contributions to conception and
design, and reviewed the manuscript. VMB and MM confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rubin KM and Lawrence DP: Your patient
with melanoma: Staging, prognosis, and treatment. Oncology
(Williston Park). 23 (Suppl 8):S13–S21. 2009.PubMed/NCBI
|
|
3
|
Michielin O, van Akkooi ACJ, Ascierto PA,
Dummer R and Keilholz U: ESMO Guidelines Committee. Electronic
address: simpleclinicalguidelines@esmo.org.
Cutaneous melanoma: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up†. Ann Oncol. 30:1884–1901.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mandalà M and Voit C: Targeting BRAF in
melanoma: Biological and clinical challenges. Crit Rev Oncol
Hematol. 87:239–255. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
El Jammal T, Pavic M, Gerfaud-Valentin M,
Jamilloux Y and Sève P: Sarcoidosis and cancer: A complex
relationship. Front Med (Lausanne). 7(594118)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Seve P, Schott AM, Pavic M, Broussolle C,
Gilis L and Thomas L: Sarcoidosis and melanoma: A referral center
study of 1,199 cases. Dermatology. 219:25–31. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cohen Aubart F, Lhote R, Amoura A, Valeyre
D, Haroche J, Amoura Z and Lebrun-Vignes B: Drug-induced
sarcoidosis: An overview of the WHO pharmacovigilance database. J
Intern Med. 288:356–362. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gkiozos I, Kopitopoulou A, Kalkanis A,
Vamvakaris IN, Judson MA and Syrigos KN: Sarcoidosis-like reactions
induced by checkpoint inhibitors. J Thorac Oncol. 13:1076–1082.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rubio-Rivas M, Moreira C and Marcoval J:
Sarcoidosis related to checkpoint and BRAF/MEK inhibitors in
melanoma. Autoimmun Rev. 19(102587)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dimitriou F, Frauchiger AL,
Urosevic-Maiwald M, Naegeli MC, Goldinger SM, Barysch M, Franzen D,
Kamarachev J, Braun R, Dummer R and Mangana J: Sarcoid-like
reactions in patients receiving modern melanoma treatment. Melanoma
Res. 28:230–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wilmott JS, Haydu LE, Menzies AM, Lum T,
Hyman J, Thompson JF, Hersey P, Kefford RF, Scolyer RA and Long GV:
Dynamics of chemokine, cytokine, and growth factor serum levels in
BRAF-mutant melanoma patients during BRAF inhibitor treatment. J
Immunol. 192:2505–2513. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Green JS, Norris DA and Wisell J: Novel
cutaneous effects of combination chemotherapy with BRAF and MEK
inhibitors: A report of two cases. Br J Dermatol. 169:172–176.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park JJ, Hawryluk EB, Tahan SR, Flaherty K
and Kim CC: Cutaneous granulomatous eruption and successful
response to potent topical steroids in patients undergoing targeted
BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol.
150:307–311. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jansen YJ, Janssens P, Hoorens A, Schreuer
MS, Seremet T, Wilgenhof S and Neyns B: Granulomatous nephritis and
dermatitis in a patient with BRAF V600E mutant metastatic melanoma
treated with dabrafenib and trametinib. Melanoma Res. 25:550–554.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Winkler JK, Buder-Bakhaya K, Ellert E,
Herpel E, Martens UM, Enk A and Hassel JC: Acute heart failure as a
result of granulomatous myocarditis: Case report on a patient with
metastatic melanoma treated with dabrafenib and trametinib. J Eur
Acad Dermatol Venereol. 32:e31–e32. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rueda-Rueda T, Sánchez-Vicente JL,
Moruno-Rodríguez A, Molina-Socola FE, Martínez-Borrego AC and
López-Herrero F: Uveitis and serous retinal detachment secondary to
systemic dabrafenib and trametinib. Arch Soc Esp Oftalmol (Engl
Ed). 93:458–462. 2018.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
17
|
Korman AM, Nisar MS and Somach SC:
Subclinical granulomas in benign skin lesions heralding the onset
of BRAF and MEK inhibitor-associated granulomatous dermatitis in a
patient with metastatic melanoma. JAAD Case Rep. 4:722–724.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Giet G, Lebas E, Rorive A, Arrese JE and
Nikkels AF: Granulomatous reactions from tattoos following BRAF
inhibitor therapy. Case Rep Dermatol. 11:101–107. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huynh S, Lheure C, Franck N, Goldman-Lévy
G, Aractingi S, Dupin N, Kramkimel N and Guégan S: Induced
sarcoid-like reactions in patients with metastatic melanoma treated
with dabrafenib and trametinib: A monocentric retrospective study.
Melanoma Res. 30:317–320. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Boutros A, Schiavi C, Cecchi F, Spagnolo
F, Guadagno A, Tanda ET, Giusti F, Murdaca G and Queirolo P: Case
report: Immune-related toxicity during adjuvant treatment with BRAF
plus MEK inhibitors in a melanoma patient. Front Immunol.
11(579523)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Adam A, Thomas L, Bories N, Zaharia D,
Balme B, Freymond N and Dalle S: Sarcoidosis associated with
vemurafenib. Br J Dermatol. 169:206–208. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hui Ong EL, Sinha R, Jmor S and Fearfield
L: BRAF inhibitor-associated granulomatous dermatitis: A report of
3 cases. Am J Dermatopathol. 41:214–217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Assan F, Schlemmer F, Assie JB, Mahevas M,
Sustronck P, Ortonne N, Velter C, Fardet L and Zehou O: Atypical
systemic sarcoid-like granulomatosis in two patients treated with
BRAF and MEK inhibitors. Eur J Dermatol. 29:556–557.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lheure C, Kramkimel N, Franck N,
Laurent-Roussel S, Carlotti A, Queant A, Goldwasser F, Avril MF and
Dupin N: Sarcoidosis in patients treated with vemurafenib for
metastatic melanoma: A paradoxical autoimmune activation.
Dermatology. 231:378–384. 2015.PubMed/NCBI View Article : Google Scholar
|