Introduction
Immunotherapy in the form of immune checkpoint
inhibitors have become U.S. Food and Drug Administration (FDA)
approved in the treatment of metastatic colorectal cancer (mCRC)
that is microsatellite instability-high (MSI-H) or mismatch repair
deficient (dMMR) in both the first-line (1) and treatment-refractory settings
(2-5).
Despite promising overall response rates (ORRs) and durable
responses demonstrated with immunotherapy in mCRC, carcinoembryonic
antigen (CEA) remains the only conventional blood-based tumor
marker to assess systemic therapy responses. A more widely
applicable blood-based measure of tumor response to systemic
therapies inclusive of immunotherapy could prove useful given that
up to 34% of patients with CRC are CEA non-producers (6). Recently, circulating tumor DNA
(ctDNA) has been recognized as a reliable tool in oncology that
appears more sensitive to changes in tumor burden and monitoring of
tumor response to systemic therapies than conventional approaches
in CRC (7). In this case series,
we report the utility of serial plasma ctDNA analyses using a
modified Epi proColon® 2.0 CE (Epigenomics AG) assay for
ctDNA testing on the methylated SEPTIN9 gene (mSEPT9) as a
dynamic marker of response to immunotherapy in mCRC (8). The Epi proColon assay was modified
for cell free DNA extraction from just 1 ml plasma with
semi-quantification of mSEPT9 ctDNA levels that were calculated as
previously described (8). Serial
blood collections for mSEPT9 testing were performed under an
IRB-approved protocol Pro00054104.
Case report
Case 1
A 60-year-old woman with treatment-refractory
microsatellite stable (MSS) mCRC to the liver, lungs, and pelvis
was treated with third-line regorafenib (80 mg oral once a day for
21 days every 28-day cycles) and pembrolizumab (200 mg
intravenously every 3 weeks). Plasma ctDNA analysis of mSEPT9 at
treatment initiation showed positivity with a percentage of
methylation reference (PMR) of 510.50. After 4 cycles of
pembrolizumab and regorafenib, the mSEPT9 PMR value decreased to
389.12 correlating to a radiographic response on computed
tomography (CT) scan (Fig. 1).
Carcinoembryonic antigen (CEA) levels similarly decreased from
122.1 to 100.4 ng/ml along these same timepoints.
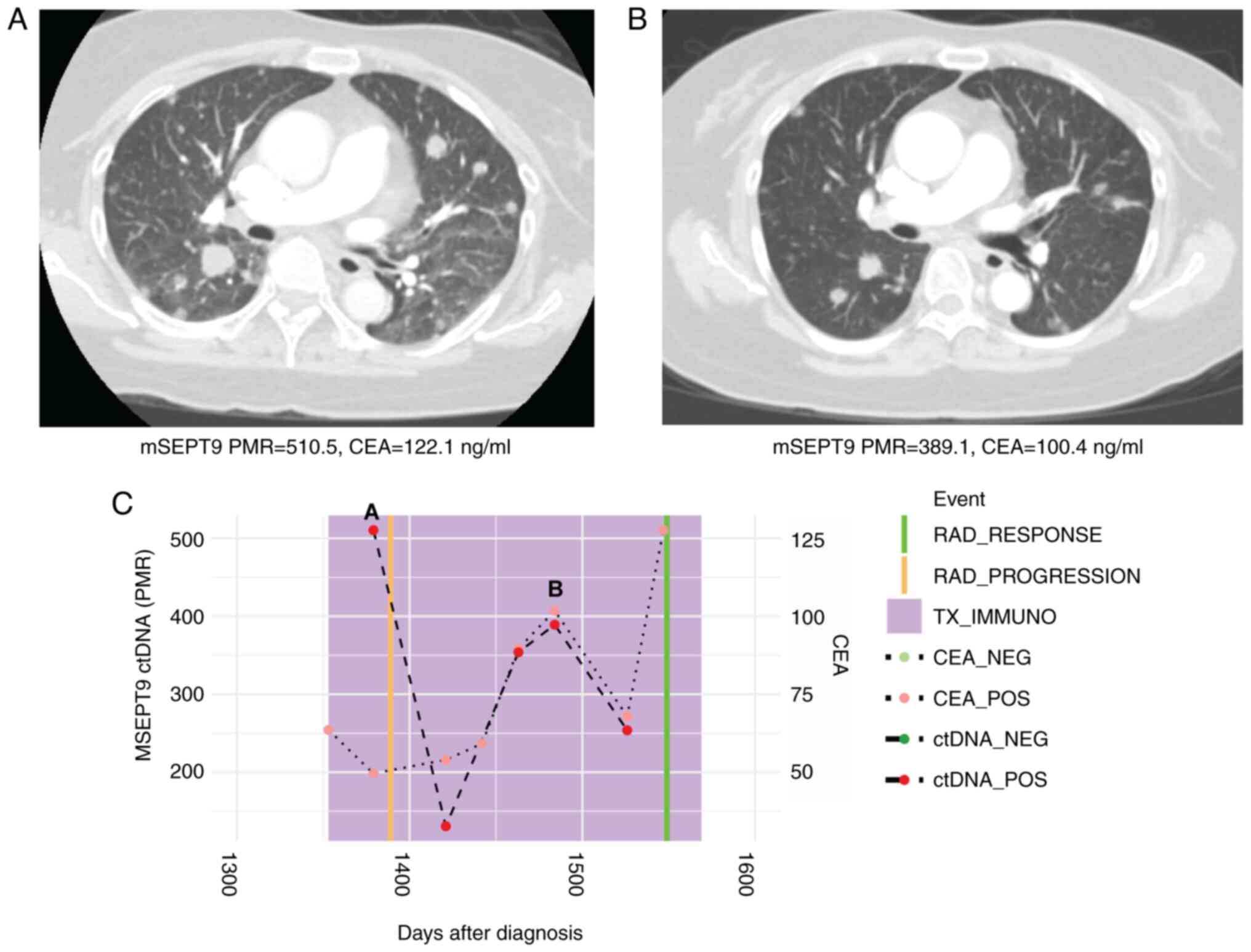 | Figure 1Case 1 with microsatellite stable
metastatic CRC shows decline in ctDNA levels with response to
immunotherapy. (A) CT scan at initiation of immunotherapy treatment
showed widespread pulmonary metastases. Beneath, mSEPT9 ctDNA and
CEA levels from blood drawn at this timepoint. (B) CT scan after
four cycles of third-line regorafenib and pembrolizumab showed
marked reduction in size of pulmonary metastases. Beneath, ctDNA
and CEA levels from blood drawn at this timepoint were decreased.
(C) Timeline of mSEPT9 and CEA levels over immunotherapy course.
The timepoints matching CT panels A and B above are shown on the
timeline graph. Reduced mSEPT9 ctDNA and CEA levels between
initiation, and post-cycle four of immunotherapy correlated with
response to treatment. mSEPT9, methylated SEPTIN9 gene; PMR,
percentage of methylation reference; CEA, carcinoembryonic antigen;
RAD, radiologic; TX_IMMUNO, treatment with immunotherapy; NEG,
negative; POS, positive; ctDNA, circulating tumor DNA; CT, computed
tomography. |
Case 2
A 60-year-old woman with MSI-H metastatic rectal
cancer to the lungs was initiated on first-line pembrolizumab (200
mg every 3 weeks). Plasma mSEPT9 ctDNA was positive at treatment
initiation with a PMR of 101.84. The PMR increased to 804.17 by
cycle 6 of pembrolizumab, which corresponded to an increase in size
of the primary rectal tumor on magnetic resonance imaging (MRI). On
clinical assessment, there were findings consistent with clinical
progression of disease as well. Levels of CEA increased from 60.9
to 85.0 ng/ml during these same timepoints corroborating disease
progression to pembrolizumab therapy (Fig. 2). Levels of ctDNA and CEA continued
to rise post-radiographic progression (Fig. 2C).
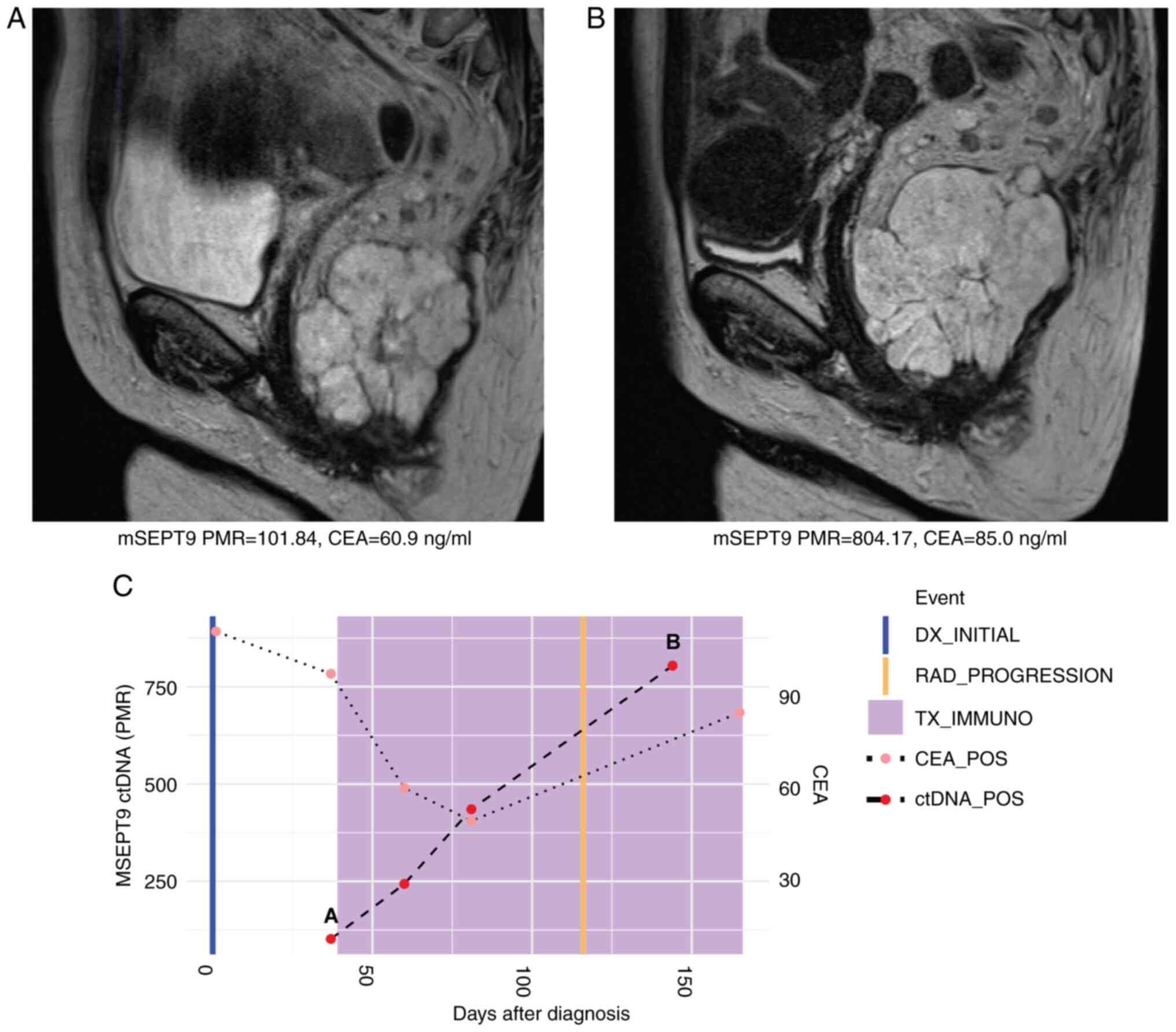 | Figure 2Case 2 with microsatellite
instability-high metastatic rectal cancer shows rise in ctDNA
levels consistent with radiographic and clinical progression to
immunotherapy. (A) MRI at initiation of immunotherapy treatment
showing primary rectal tumor. Beneath, mSEPT9 ctDNA and CEA levels
from blood drawn at this timepoint. (B) MRI at cycle six of
pembrolizumab showed growth in size of primary rectal tumor.
Beneath, ctDNA and CEA levels from blood draw at this timepoint
increased. (C) Timeline of mSEPT9 and CEA levels over immunotherapy
course. The timepoints matching MRI panels A and B above are shown
on the timeline graph. Rising mSEPT9 ctDNA and CEA levels between
initiation and cycle six of immunotherapy corresponded with
radiographic and clinical progression in the primary rectal tumor.
mSEPT9, methylated SEPTIN9 gene; PMR, percentage of
methylation reference; CEA, carcinoembryonic antigen; DX,
diagnosis; RAD, radiologic; TX_IMMUNO, treatment with
immunotherapy; POS, positive; ctDNA, circulating tumor DNA; MRI,
magnetic resonance imaging. |
Case 3
A 74-year-old man with MSI-H colon cancer metastatic
to the abdominal wall and peritoneum who progressed on adjuvant
5-fluorouracil and oxaliplatin (FOLFOX) was treated with
pembrolizumab (200 mg every 3 weeks). He was plasma mSEPT9 ctDNA
positive at cycle 1 of immunotherapy (PMR 139.24). However, by
cycle 4 of pembrolizumab, he became ctDNA negative and remained
negative by cycle 7 (PMR 0 for both timepoints), which corresponded
to a complete radiographic response at these same timepoints
(Fig. 3). Notably, serial CEAs
throughout his pembrolizumab treatment remained low at 2.2-2.3
ng/ml.
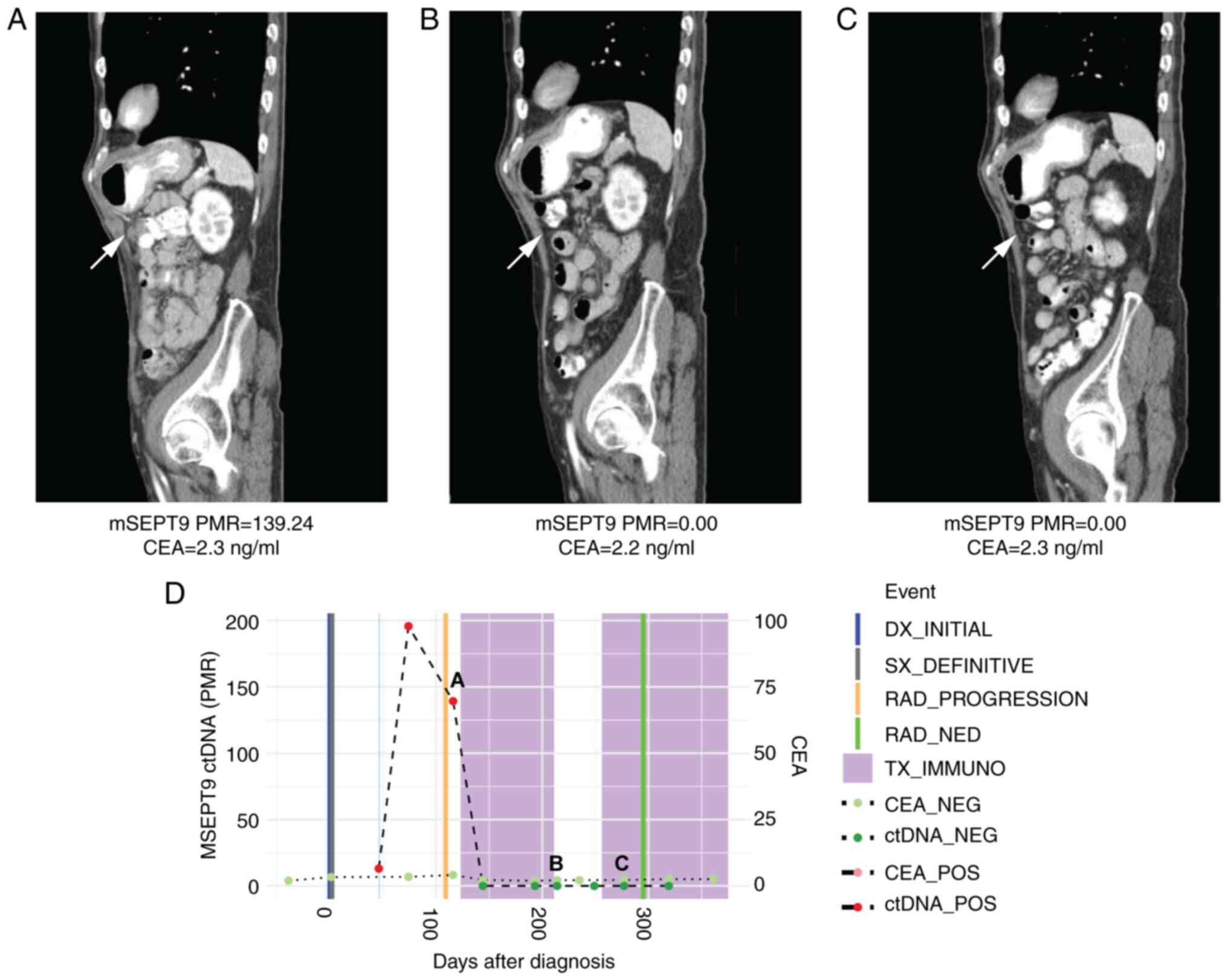 | Figure 3Case 3 with microsatellite
instability-high metastatic colorectal cancer shows decline in
ctDNA levels consistent with response to immunotherapy in a non-CEA
producer. (A) CT scan at initiation of immunotherapy treatment
showing peritoneal metastases. Beneath, mSEPT9 ctDNA and CEA levels
from blood drawn at this timepoint. (B) CT scan by cycle four of
pembrolizumab showed a complete radiographic response in peritoneal
metastases. Beneath, ctDNA levels normalized, while CEA levels
remained low. (C) CT scan by cycle seven of pembrolizumab showed a
sustained complete radiographic response in peritoneal metastases.
Beneath, ctDNA levels remained normalized, while CEA levels
remained low. (D) Timeline of mSEPT9 and CEA levels over
immunotherapy course. The timepoints matching CT panels A, B, and C
above are shown on the timeline graph. Compared with initiation,
mSEPT9 ctDNA levels normalized by cycle four and cycle seven of
immunotherapy, which correlated with response to treatment; CEA
levels remained low through all three timepoints. mSEPT9,
methylated SEPTIN9 gene; PMR, percentage of methylation
reference; CEA, carcinoembryonic antigen; DX, diagnosis; SX,
surgery; RAD, radiologic; NED, no evidence of disease; TX_IMMUNO,
treatment with immunotherapy; NEG, negative; POS, positive; ctDNA,
circulating tumor DNA; CT, computed tomography. |
Case 4
A 55-year-old woman with treatment-refractory,
unresectable colon cancer that was MSS was treated with third-line
regorafenib (80 mg oral once a day for 21 days every 28-day cycles)
and nivolumab (240 mg intravenously every 2 weeks). Following 4
cycles of regorafenib and nivolumab, her plasma mSEPT9 ctDNA values
(PMR) increased from 498.92 to 1432.83 with marked disease
progression on interval CT scans as evidenced by new hepatic
metastases (Fig. 4). She died 2
months later due to progressive disease. Her CEA levels were
uninformative and ranged from 5.0-7.4 ng/ml during her
immunotherapy course.
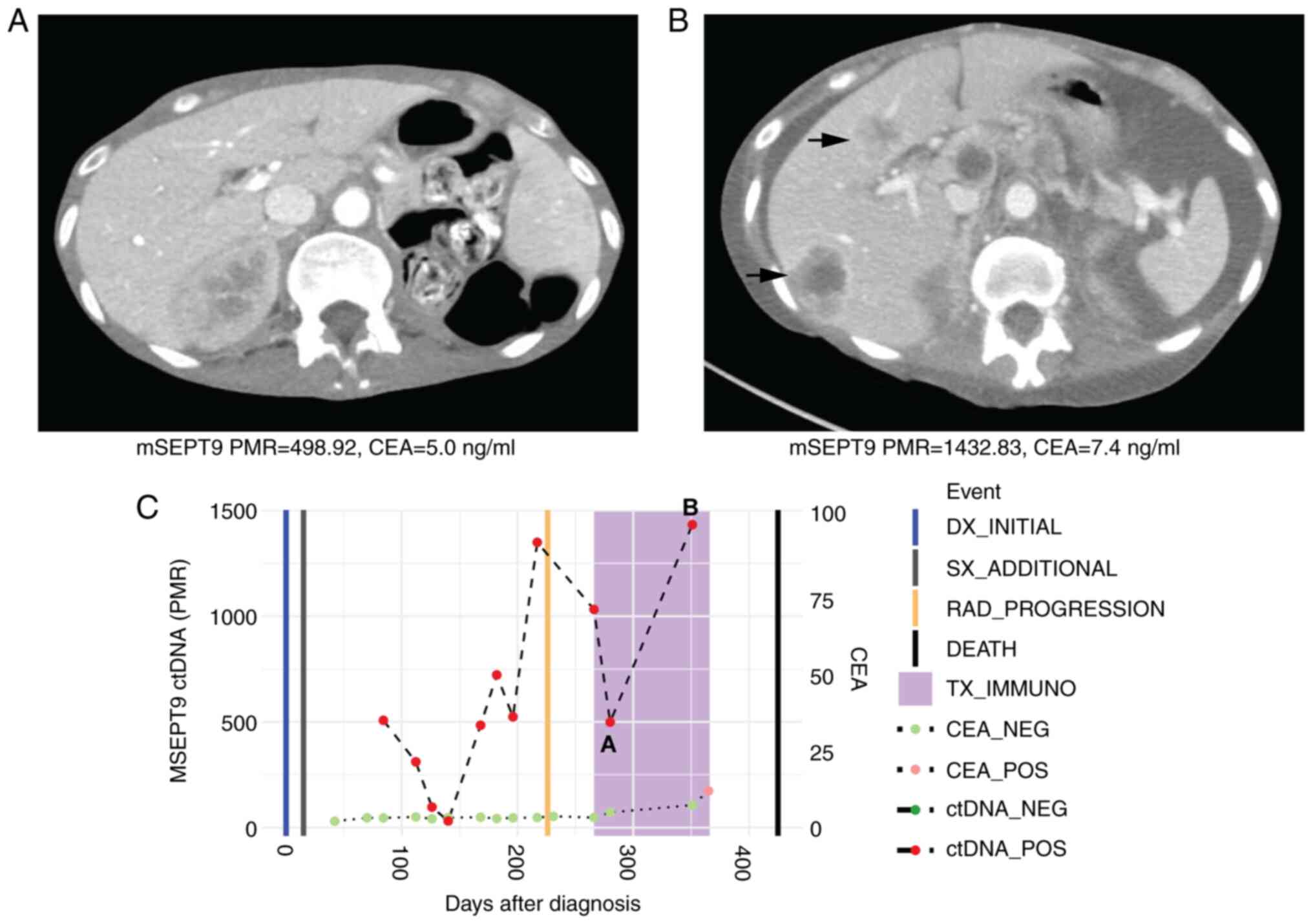 | Figure 4Case 4 with microsatellite stable
metastatic colorectal cancer shows rise in ctDNA levels consistent
with radiographic progression to immunotherapy. (A) CT scan at
initiation of immunotherapy treatment showed normal liver. Beneath,
mSEPT9 ctDNA and CEA levels from blood drawn at this timepoint. (B)
CT scan after four cycles of third-line regorafenib and nivolumab
showed radiographic progression with development of liver
metastases. Beneath, ctDNA and CEA levels from blood drawn at this
timepoint increased. (C) Timeline of mSEPT9 and CEA levels over
immunotherapy course. The timepoints matching CT panels A and B
above are shown on the timeline graph. Rising mSEPT9 ctDNA and CEA
levels between initiation and post-cycle four of immunotherapy
correlated with disease progression. mSEPT9, methylated
SEPTIN9 gene; PMR, percentage of methylation reference; CEA,
carcinoembryonic antigen; DX, diagnosis; SX, surgery; RAD,
radiologic; TX_IMMUNO, treatment with immunotherapy; NEG, negative;
POS, positive; ctDNA, circulating tumor DNA; CT, computed
tomography. |
Discussion
To the best of our knowledge, there have been only 3
studies to demonstrate that rises and declines in plasma ctDNA
levels predicted radiographic tumor progression and response,
respectively, in MSS or MSI-H mCRC treated with immunotherapy
(9-11).
In one study, changes in plasma ctDNA levels 4 weeks from
initiation of immunotherapy was predictive of radiographic
response, while undetectable ctDNA at week 8 from immunotherapy was
associated with prolonged progression-free survival and overall
survival in another study (9,11).
Our case series supports the use of plasma ctDNA as
a dynamic marker of response to immunotherapy in mCRC. In all
cases, a decrease or clearance of ctDNA corroborated radiographic
tumor response, while rises in plasma ctDNA levels corroborated
radiographic tumor progression to immunotherapy. In cases where
colorectal tumors produced CEA, ctDNA levels aligned with changes
in CEA, and furthermore, both were in concordance with radiographic
response assessments. Our findings are consistent with the
literature showing that plasma ctDNA correlates with conventional
measures of tumor response to systemic therapy (by CEA and imaging)
in mCRC (12). However, unlike
CEA, wherein measurable levels can be found in normal individuals
but a proportion of colorectal tumors can be non-secretors of CEA,
ctDNA as a measure of minimal residual disease (MRD) is a binary
metric whose presence indicates that a large burden of residual
metastatic cancer cells remain in one's body (13). It is therefore not surprising that
there is growing evidence to suggest that ctDNA demonstrates
superior sensitivity to CEA to detect recurrence in non-metastatic
CRC and radiographic progression events in mCRC (13-16).
In mCRC, changes in ctDNA levels from pretreatment to cycle 2 was a
better predictor of radiologic response to chemotherapy than CEA at
this early time point (17). In
resected, stage I-III CRC, ctDNA detection offers median lead times
of up to 11 months for radiographic detection of recurrence
(18). A recent large
observational cohort demonstrated that in those with postoperative
ctDNA positivity following curative-intent surgery for stage I-IV
CRC, clearance of MRD with adjuvant chemotherapy was also a
positive prognostic indicator (19).
Importantly, we report a novel finding that plasma
ctDNA levels predicted radiographic response (or lack of) to
immunotherapy in CEA non-producers. CEA represents the only
conventional and recognized blood-based test for monitoring
response to systemic therapy in mCRC. Notably, up to 34% of
patients with CRC are CEA non-producers, which represents a
clinically relevant proportion of patients without a marker to
monitor systemic treatment response short of interval surveillance
imaging (6). In Case 3, plasma
mSEPT9 ctDNA levels were detectable at baseline but subsequently
cleared over the course of treatment with pembrolizumab and
corresponded to a complete radiographic response by cycle 4 of
immunotherapy (Fig. 3). CEA was
uninformative in this case as the patient had low values of CEA
throughout the course of immunotherapy (non-producer). Conversely,
in Case 4, CEA levels were fairly low and would not have indicated
the degree of rapid disease progression that was detected by
interval imaging and a substantial rise in plasma ctDNA levels from
baseline to cycle 4 of immunotherapy. This patient died from
progressive disease shortly thereafter.
When purposed for detection of tumor mutations
through targeted next-generation sequencing (NGS), ctDNA can be
used to track dynamic changes in mutations in response to systemic
therapy to detect presence of resistance mutations (12). However, in our series we have used
a tumor-agnostic, methylated ctDNA marker (mSEPT9) and therefore
its assessment of systemic tumor burden is not influenced by tumor
mutational profile or prior therapies. When purposed for MRD
detection, the timing of therapy to the collection of blood for
ctDNA measurement can influence ctDNA levels. For example, surgery
can lead to elevation in ctDNA levels lasting as long as 4 weeks
from surgery, which is why many groups have recommended
postoperative measures of ctDNA to not occur until 4 weeks after
CRC surgery (12,20). Chemotherapy can also cause changes
in ctDNA as early as 48 h of chemotherapy infusion, although in
another series most changes in ctDNA occurred by cycle 2 of
chemotherapy (17,21). Early spikes in ctDNA levels within
the first few days of chemotherapy infusion may represent a rapid
release of ctDNA from lysing tumors (17). The collection of blood samples in
our 4 cases for ctDNA assessments occurred prior to the infusion of
immunotherapy and before the first dose of oral chemotherapy on
each day 1 of the respective cycle of therapy.
Although promising and durable responses have been
observed with immune checkpoint blockade in MSI-H mCRC, it should
be noted that the median time to onset of response is 2.2 months
(range 1.8 to 18.8 months) (1).
Therefore, during this initial critical period up until the first
interval assessment scan, having a convenient and minimally
invasive biomarker that can reliably monitor tumor response to
immunotherapy can be of immense benefit to the physician while
easing patient anxiety, particularly in the absence of high CEA
levels to begin with. This holds especially true in non-metastatic
settings where neoadjuvant immunotherapy has been used in MSI-H
locally advanced rectal cancer given increasing evidence that these
tumors are fairly resistant to standard chemotherapy and
chemoradiation (22). Neoadjuvant
immunotherapy has been explored in early-stage colon cancers as
well (23). Here, serial plasma
ctDNA assessments might provide an early indicator of benefit to
neoadjuvant immunotherapy and potentially allow a timely transition
to salvage chemoradiation or surgery, while preserving a curative
intent treatment pathway in the event of lack of tumor response to
immunotherapy.
Our group of mCRC cases demonstrates that
longitudinal plasma ctDNA assessments can predict true progressors
to immunotherapy, even in the context of a CEA non-producing tumor.
This is noteworthy, given that there are increasing efforts to
consistently detect the phenomenon of pseudoprogression to
immunotherapy (24). As evidence
accumulates in support of ctDNA being a more sensitive measure of
changes in tumor burden than conventional approaches in CRC, it
would be prudent to further investigate its role in the detection
of pseudoprogression and true progression in larger, prospective
cohorts of mCRC patients treated with immunotherapy (7). There are ongoing prospective studies
seeking to evaluate the impact of tumor response assessments using
plasma ctDNA assays in patients with mCRC and other advanced solid
tumors treated with immunotherapy that will hopefully provide more
insight in this context (NCT04761783).
Plasma ctDNA represents a minimally invasive tool
with promising potential as a measure of tumor burden and tumor
response to systemic therapies in CRC. In this case series, we
demonstrate that blood-based assessments of mSEPT9 ctDNA predicted
radiographic response to immunotherapy in patients with mCRC that
were MSS or MSI-H. Interestingly, plasma ctDNA was a predictor of
response to immunotherapy even in colorectal tumors that were CEA
non-producers. Our findings add to ongoing efforts to establish the
role of plasma ctDNA in monitoring response to immunotherapy in
CRC. Future studies are warranted to investigate the potential of
plasma ctDNA to detect hyperprogressors or differentiate true
progression from pseudoprogression in the context of
immunotherapy-based treatments in CRC.
Acknowledgements
Not applicable.
Funding
Funding: This project was supported by a Tower Cancer Research
Foundation Career Development Grant in Translational Cancer
Research.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JG, FA, DH, RA, LZ, AH, AO, KZ, MC, AG, and MH
assisted in analysis and interpretation of data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Serial blood collections for mSEPT9 testing and
written informed consent for publication of this case series were
obtained under IRB-approved protocol (approval no. Pro00054104) by
the Cedars-Sinai Medical Center Institutional Review Board. All
patients were treated at the Cedars-Sinai Medical Center Samuel
Oschin Comprehensive Cancer Institute.
Patient consent for publication
Patient written consent for publication was obtained
under an IRB-approved protocol (approval no. Pro00054104) overseen
by the Cedars-Sinai Medical Center Institutional Review Board.
Competing interests
JG and MC: Consultant or advisory role for Natera
and HalioDx; AH: Consultant or advisory role for Natera; KZ:
Speakers bureau for Natera. All other authors declare that they
have no competing interests.
References
|
1
|
André T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab in microsatellite-instability-high advanced
colorectal cancer. N Engl J Med. 383:2207–2218. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marcus L, Lemery SJ, Keegan P and Pazdur
R: FDA Approval summary: Pembrolizumab for the treatment of
microsatellite instability-high solid tumors. Clin Cancer Res.
25:3753–3758. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with nivolumab plus ipilimumab
in DNA mismatch repair-deficient/microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Andre T, Berton D, Curigliano G, Ellard S,
Pérez JMT, Arkenau HT, Abdeddaim C, Moreno V, Guo W, Im M and
Starling N: Safety and efficacy of anti-PD-1 antibody dostarlimab
in patients (pts) with mismatch repair-deficient (dMMR) solid
cancers: Results from GARNET study. J Clin Oncol. 39 (Suppl
3)(S9)2021.
|
|
6
|
Cho M, Akiba C, Lau C, Smith D, Telatar M,
Afkhami M, Sentovich S, Melstrom K and Fakih M: Impact of RAS and
BRAF mutations on carcinoembryonic antigen production and pattern
of colorectal metastases. World J Gastrointest Oncol. 8:128–135.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dasari A, Morris VK, Allegra CJ, Atreya C,
Benson AB III, Boland P, Chung K, Copur MS, Corcoran RB, Deming DA,
et al: ctDNA applications and integration in colorectal cancer: An
NCI colon and rectal-anal task forces whitepaper. Nat Rev Clin
Oncol. 17:757–770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hitchins MP, Vogelaar IP, Brennan K,
Haraldsdottir S, Zhou N, Martin B, Alvarez R, Yuan X, Kim S, Guindi
M, et al: Methylated SEPTIN9 plasma test for colorectal cancer
detection may be applicable to Lynch syndrome. BMJ Open
Gastroenterol. 6(e000299)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cabel L, Riva F, Servois V, Livartowski A,
Daniel C, Rampanou A, Lantz O, Romano E, Milder M, Buecher B, et
al: Circulating tumor DNA changes for early monitoring of anti-PD1
immunotherapy: A proof-of-concept study. Ann Oncol. 28:1996–2001.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Stein A, Simnica D, Schultheiß C, Scholz
R, Tintelnot J, Gökkurt E, von Wenserski L, Willscher E, Paschold
L, Sauer M, et al: PD-L1 targeting and subclonal immune escape
mediated by PD-L1 mutations in metastatic colorectal cancer. J
Immunother Cancer. 9(e002844)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang C, Chevalier D, Saluja J, Sandhu J,
Lau C and Fakih M: Regorafenib and nivolumab or pembrolizumab
combination and circulating tumor DNA response assessment in
refractory microsatellite stable colorectal cancer. Oncologist.
25:e1188–e1194. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gong J, Hendifar A, Gangi A, Zaghiyan K,
Atkins K, Nasseri Y, Murrell Z, Figueiredo JC, Salvy S, Haile R and
Hitchins M: Clinical applications of minimal residual disease
assessments by tumor-informed and tumor-uninformed circulating
tumor DNA in colorectal cancer. Cancers (Basel).
13(4547)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tie J, Cohen JD, Lo SN, Wang Y, Li L,
Christie M, Lee M, Wong R, Kosmider S, Skinner I, et al: Prognostic
significance of postsurgery circulating tumor DNA in nonmetastatic
colorectal cancer: Individual patient pooled analysis of three
cohort studies. Int J Cancer. 148:1014–1026. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Reinert T, Henriksen TV, Christensen E,
Sharma S, Salari R, Sethi H, Knudsen M, Nordentoft I, Wu HT, Tin
AS, et al: Analysis of plasma cell-free DNA by ultradeep sequencing
in patients with stages I to III colorectal cancer. JAMA Oncol.
5:1124–1131. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tie J, Cohen JD, Wang Y, Christie M,
Simons K, Lee M, Wong R, Kosmider S, Ananda S, McKendrick J, et al:
Circulating tumor DNA analyses as markers of recurrence risk and
benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol.
5:1710–1717. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tie J, Wang Y, Tomasetti C, Li L, Springer
S, Kinde I, Silliman N, Tacey M, Wong HL, Christie M, et al:
Circulating tumor DNA analysis detects minimal residual disease and
predicts recurrence in patients with stage II colon cancer. Sci
Transl Med. 8(346ra92)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tie J, Kinde I, Wang Y, Wong HL, Roebert
J, Christie M, Tacey M, Wong R, Singh M, Karapetis CS, et al:
Circulating tumor DNA as an early marker of therapeutic response in
patients with metastatic colorectal cancer. Ann Oncol.
26:1715–1722. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tarazona N, Gimeno-Valiente F, Gambardella
V, Zuñiga S, Rentero-Garrido P, Huerta M, Roselló S,
Martinez-Ciarpaglini C, Carbonell-Asins JA, Carrasco F, et al:
Targeted next-generation sequencing of circulating-tumor DNA for
tracking minimal residual disease in localized colon cancer. Ann
Oncol. 30:1804–1812. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kotaka M, Shirasu H, Watanabe J, Yamazaki
K, Hirata K, Akazawa N, Matsuhashi N, Yokota M, Ikeda M, Kato K, et
al: Association of circulating tumor DNA dynamics with clinical
outcomes in the adjuvant setting for patients with colorectal
cancer from an observational GALAXY study in CIRCULATE-Japan. J
Clin Oncol. 40 (4 Suppl)(S9)2022.
|
|
20
|
Henriksen TV, Reinert T, Christensen E,
Sethi H, Birkenkamp-Demtröder K, Gögenur M, Gögenur I, Zimmermann
BG, Dyrskjøt L and Andersen CL: IMPROVE Study Group. The effect of
surgical trauma on circulating free DNA levels in cancer
patients-implications for studies of circulating tumor DNA. Mol
Oncol. 14:1670–1679. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Moser T, Waldispuehl-Geigl J, Belic J,
Weber S, Zhou Q, Hasenleithner SO, Graf R, Terzic JA, Posch F, Sill
H, et al: On-treatment measurements of circulating tumor DNA during
FOLFOX therapy in patients with colorectal cancer. NPJ Precis
Oncol. 4(30)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Demisse R, Damle N, Kim E, Gong J, Fakih
M, Eng C, Oesterich L, McKenny M, Ji J, Liu J, et al: Neoadjuvant
immunotherapy-based systemic treatment in MMR-deficient or MSI-high
rectal cancer: Case series. J Natl Compr Canc Netw. 18:798–804.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chalabi M, Fanchi LF, Dijkstra KK, Van den
Berg JG, Aalbers AG, Sikorska K, Lopez-Yurda M, Grootscholten C,
Beets GL, Snaebjornsson P, et al: Neoadjuvant immunotherapy leads
to pathological responses in MMR-proficient and MMR-deficient
early-stage colon cancers. Nat Med. 26:566–576. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ma Y, Wang Q, Dong Q, Zhan L and Zhang J:
How to differentiate pseudoprogression from true progression in
cancer patients treated with immunotherapy. Am J Cancer Res.
9:1546–1553. 2019.PubMed/NCBI
|


















