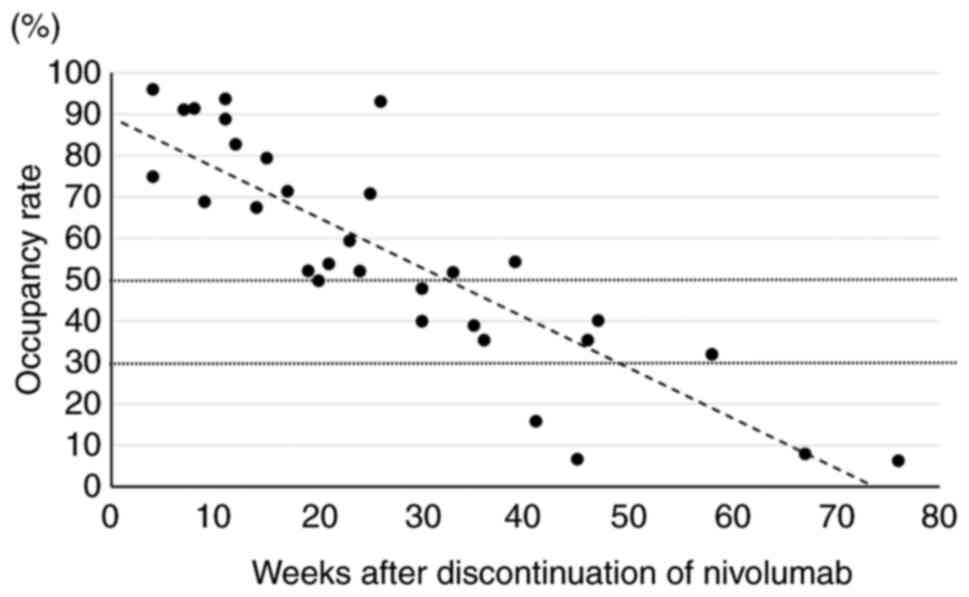
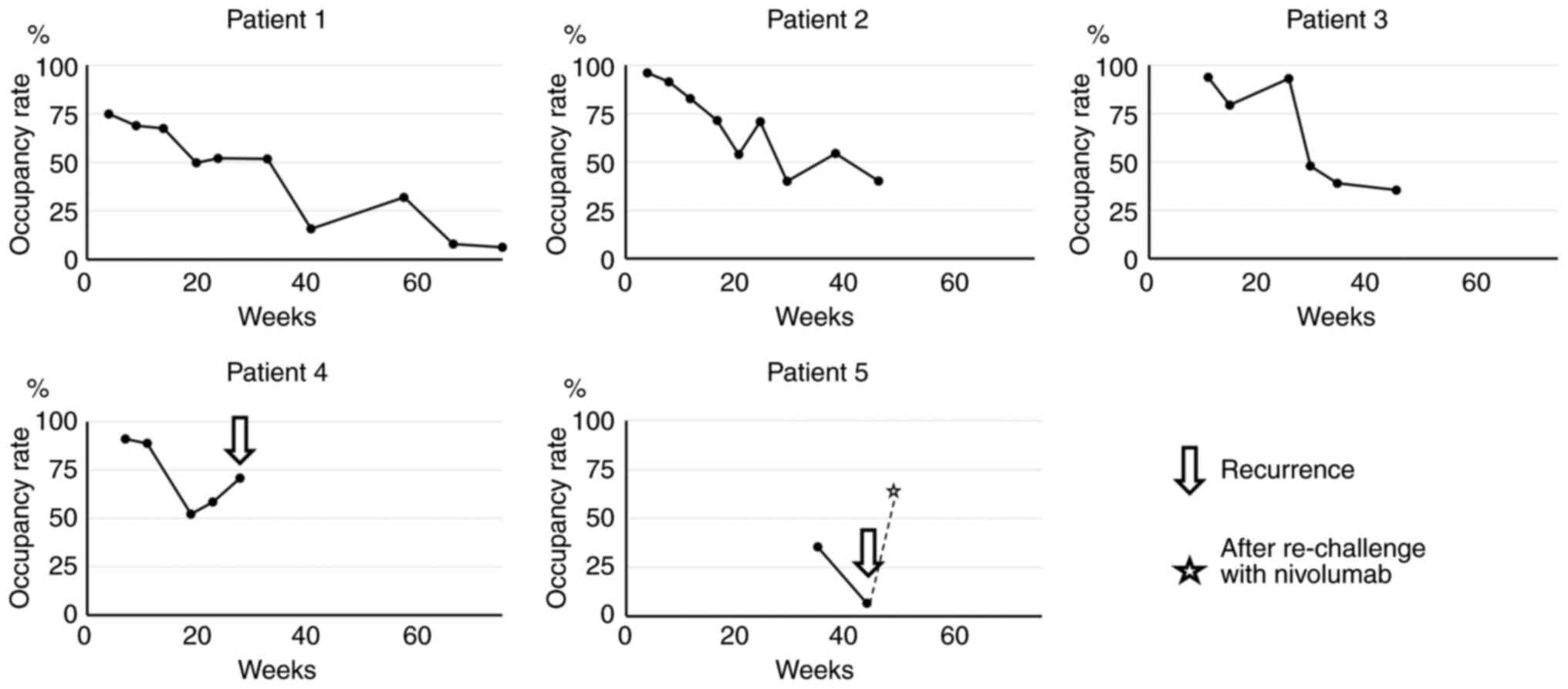
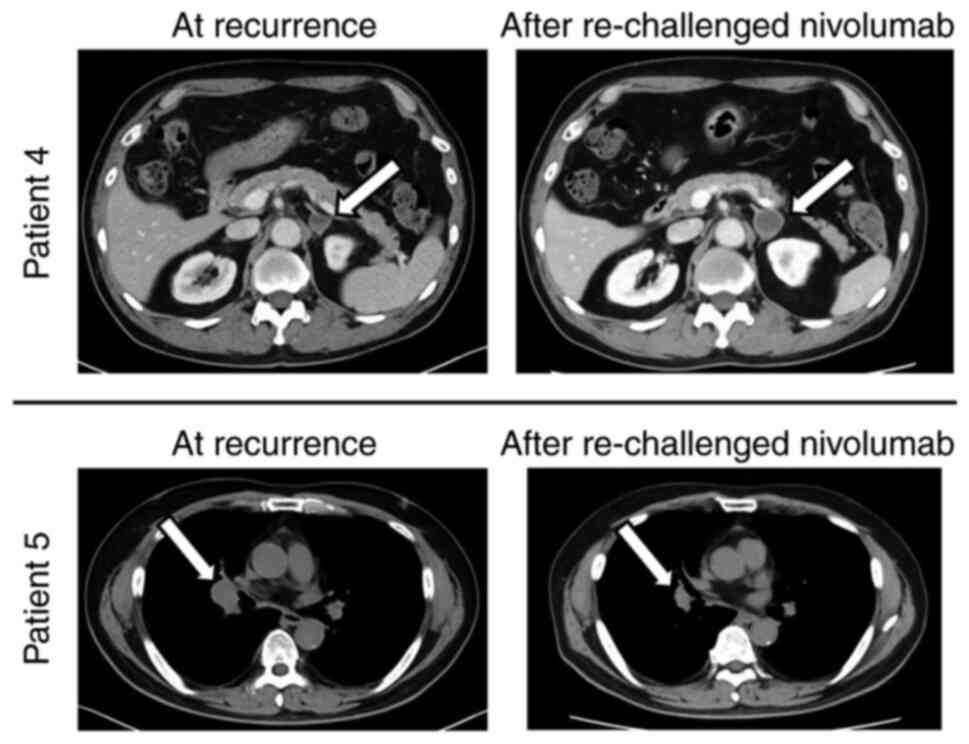
|
1
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Sosman JA,
Atkins MB, Leming PD, et al: Five-year survival and correlates
among patients with advanced melanoma, renal cell carcinoma, or
non-small cell lung cancer treated with nivolumab. JAMA Oncol.
5:1411–1420. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hodi FS, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J,
Dummer R, et al: Nivolumab plus ipilimumab or nivolumab alone
versus ipilimumab alone in advanced melanoma (CheckMate 067):
4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet
Oncol. 19:1480–1492. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Quinn C, Garrison LP, Pownell AK, Atkins
MB, de Pouvourville G, Harrington K, Ascierto PA, McEwan P, Wagner
S, Borrill J and Wu E: Current challenges for assessing the
long-term clinical benefit of cancer immunotherapy: A
multi-stakeholder perspective. J Immunother Cancer.
8(e000648)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Brahmer JR, Lacchetti C, Schneider BJ,
Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner
JM, Ginex P, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: American
Society of clinical oncology clinical practice guideline. J Clin
Oncol. 36:1714–1768. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Takagi T, Yoshida K, Kobayashi H, Kondo T,
Iizuka J, Okumi M, Ishida H, Fukuda H, Ishihara H and Tanabe K:
Durable response after discontinuation of nivolumab therapy in
patients with metastatic renal cell carcinoma. Jpn J Clin Oncol.
48:860–863. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yatsuda Y, Hirose S, Ito Y, Onoda T,
Sugiyama Y, Nagafuchi M, Suzuki H, Niisato Y, Tange Y, Ikeda T, et
al: A Durable response after the discontinuation of nivolumab in an
advanced gastric cancer patient. Intern Med. 60:1011–1017.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kimura H, Araya T, Yoneda T, Shirasaki H,
Kurokawa K, Sakai T, Koba H, Tambo Y, Nishikawa S, Sone T and
Kasahara K: Long-lasting responses after discontinuation of
nivolumab treatment for reasons other than tumor progression in
patients with previously treated, advanced non-small cell lung
cancer. Cancer Commun (Lond). 39(78)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brahmer JR, Drake CG, Wollner I, Powderly
JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller
TL, et al: Phase I study of single-agent anti-programmed death-1
(MDX-1106) in refractory solid tumors: Safety, clinical activity,
pharmacodynamics, and immunologic correlates. J Clin Oncol.
28:3167–3175. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Osa A, Uenami T, Koyama S, Fujimoto K,
Okuzaki D, Takimoto T, Hirata H, Yano Y, Yokota S, Kinehara Y, et
al: Clinical implications of monitoring nivolumab immunokinetics in
non-small cell lung cancer patients. JCI insight.
3(e59125)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Waterhouse DM, Garon EB, Chandler J,
McCleod M, Hussein M, Jotte R, Horn L, Daniel DB, Keogh G, Creelan
B, et al: Continuous versus 1-year fixed-duration nivolumab in
previously treated advanced non-small-cell lung cancer: CheckMate
153. J Clin Oncol. 38:3863–3873. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schachter J, Ribas A, Long GV, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al:
Pembrolizumab versus ipilimumab for advanced melanoma: Final
overall survival results of a multicentre, randomised, open-label
phase 3 study (KEYNOTE-006). Lancet. 390:1853–1862. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Danson S, Hook J, Marshall H, Smith A,
Bell S, Rodwell S and Corrie P: Are we over-treating with
checkpoint inhibitors? Br J Cancer. 121:629–630. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nomura S, Goto Y, Mizutani T, Kataoka T,
Kawai S, Okuma Y, Murakami H, Tanaka K and Ohe Y: A randomized
phase III study comparing continuation and discontinuation of PD-1
pathway inhibitors for patients with advanced non-small-cell lung
cancer (JCOG1701, SAVE study). Jpn J Clin Oncol. 50:821–825.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gauci ML, Lanoy E, Champiat S, Caramella
C, Ammari S, Aspeslagh S, Varga A, Baldini C, Bahleda R, Gazzah A,
et al: Long-term survival in patients responding to Anti-PD-1/PD-L1
therapy and disease outcome upon treatment discontinuation. Clin
Cancer Res. 25:946–956. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tachihara M, Negoro S, Inoue T, Tamiya M,
Akazawa Y, Uenami T, Urata Y, Hattori Y, Hata A, Katakami N and
Yokota S: Efficacy of anti-PD-1/PD-L1 antibodies after
discontinuation due to adverse events in non-small cell lung cancer
patients (HANSHIN 0316). BMC Cancer. 18(946)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shin DS, Zaretsky JM, Escuin-Ordinas H,
Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, Grasso CS, Hugo W,
Sandoval S, Torrejon DY, et al: Primary Resistance to PD-1 blockade
mediated by JAK1/2 mutations. Cancer Discov. 7:188–201.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen DS and Mellman I: Elements of cancer
immunity and the cancer-immune set point. Nature. 541:321–330.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Qiao M, Zhou F, Hou L, Li X, Zhao C, Jiang
T, Gao G, Su C, Wu C, Ren S and Zhou C: Efficacy of
immune-checkpoint inhibitors in advanced non-small cell lung cancer
patients with different metastases. Ann Transl Med.
9(34)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Herbst RS, Garon EB, Kim DW, Cho BC,
Gervais R, Perez-Gracia JL, Han JY, Majem M, Forster MD, Monnet I,
et al: Five year survival update from KEYNOTE-010: pembrolizumab
versus docetaxel for previously treated, programmed Death-Ligand
1-positive advanced NSCLC. J Thorac Oncol. 16:1718–1732.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Eggermont AM, Meshcheryakov A, Atkinson V,
Blank CU, Mandala M, Long GV, Barrow C, Di Giacomo AM, Fisher R,
Sandhu S, et al: Crossover and rechallenge with pembrolizumab in
recurrent patients from the EORTC 1325-MG/Keynote-054 phase III
trial, pembrolizumab versus placebo after complete resection of
high-risk stage III melanoma. Eur J Cancer. 158:156–168.
2021.PubMed/NCBI View Article : Google Scholar
|