Introduction
Non-Hodgkin lymphoma mimicking a Klatskin tumor is a
very rare clinical entity. In the staging of non-Hodgkin lymphoma,
the liver is implicated in 40% of cases; however, primary liver
non-Hodgkin lymphoma represents <1% of all non-Hodgkin lymphoma
cases worldwide (1). Nguyen
(2) first described a case of
primary non-Hodgkin lymphoma of the extra-hepatic bile duct in
1982. The clinical manifestation is very similar to a Klatskin
tumor and imaging studies cannot easily differentiate these
lesions. Because of its rarity as a cause of biliary obstruction,
non-Hodgkin lymphoma is rarely considered in differential
diagnosis; consequently, major surgeries are undertaken in most
patients with a presumptive diagnosis of cholangiocarcinoma
(3,4).
Since Nguyen described the first case of primary
non-Hodgkin lymphoma of the extra-hepatic bile duct, only 42
similar cases have been reported in the English literature
(5). The present case report
described another rare case of non-Hodgkin lymphoma of the common
hepatic duct with obstructive jaundice, for which differential
diagnosis was complex.
Case report
A 61-year-old woman was admitted to the Third
Department of Surgery, National and Kapodistrian University of
Athens, Attikon University Hospital (Athens, Greece) with a 10-day
history of jaundice associated with generalized pruritus, stool
discoloration and dark urine, combined with a history of nausea and
vomiting. A 5 kg weight loss was reported over the previous 3
months. Her past medical history was significant only for arterial
hypertension and she reported no past radiation exposure. Physical
examination revealed a non-distended, non-tender, supple abdomen
and the patient remained afebrile after the onset of her symptoms.
Laboratory tests indicated elevated total and direct bilirubin
levels of 9.95 mg/dl (normal value, <1.2 mg/dl) and 9.53 mg/dl
(normal value, <0.2 mg/dl), respectively; AST of 80 U/l (normal
value, <32 U/l); ALT of 81 U/l (normal value, <33 U/l);
alkaline phosphatase of 298 U/l (normal values, 35-104 U/l);
γ-glutamyl transferase of 232 U/l (normal values, 5-36 U/l);
lactate hydrogenase of 180 U/l (normal values, 135-225 U/l); and
complete blood count within normal values. Tumor markers
demonstrated a CA 19-9 of 24.9 U/ml (normal value, <27 U/ml),
with normal α-fetoprotein levels of 1.6 ng/ml (normal value, <7
ng/ml) and carcinoembryonic antigen levels of 2.1 ng/ml (normal
value, <3.8 ng/ml). The serology results for hepatitis B and C
were negative. An abdominal ultrasound (US) performed before
admission indicated a 9.8 mm dilatation of the intra-hepatic ducts
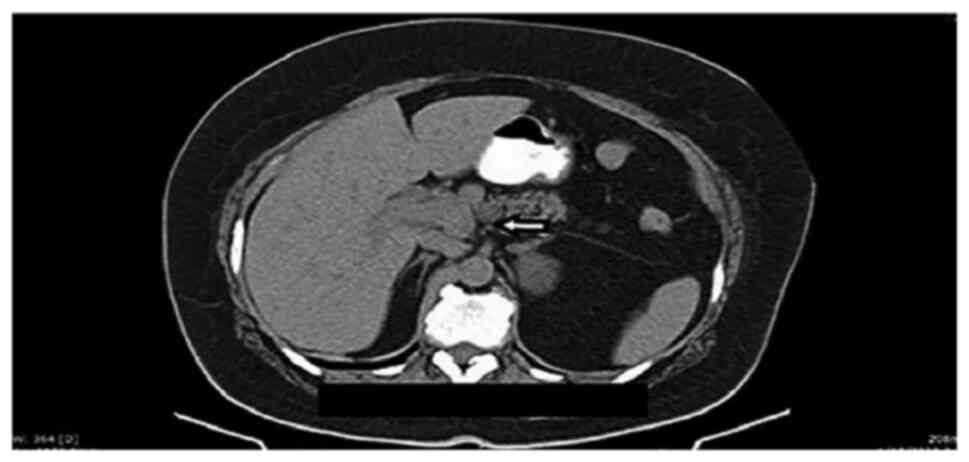
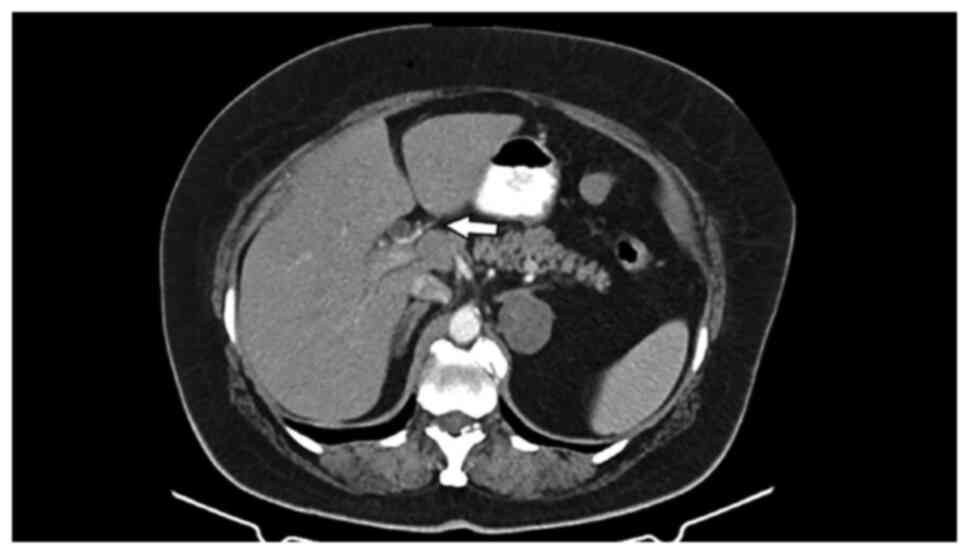
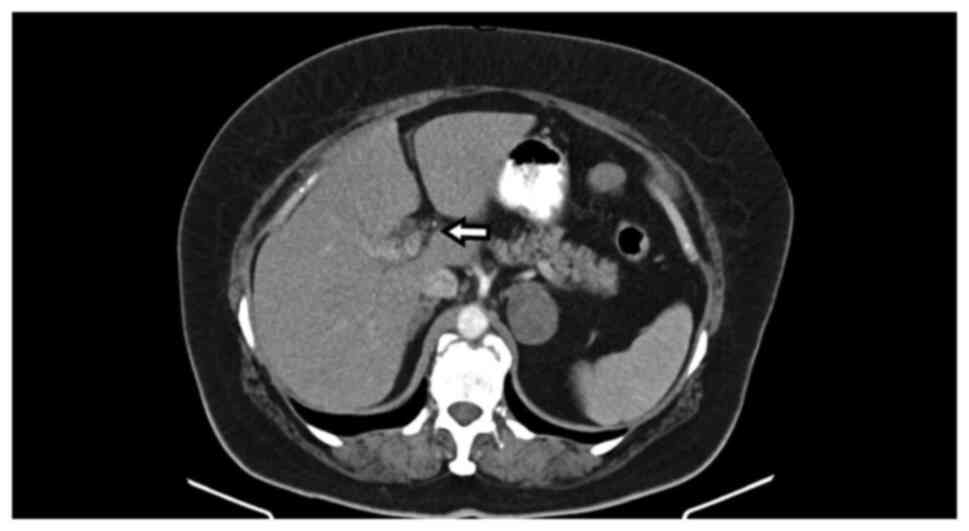
and the common hepatic duct. Abdominal computerized tomography
(CT), also performed before admission, indicated dilatation of the
common hepatic duct, and of the intra- and extra-hepatic ducts, and
a mass situated in the liver hilum (Fig. 1, Fig.
2 and Fig. 3), as well as a
4x3 cm round mass in the left adrenal gland, with features
indicative of probable adenoma.
On admission, magnetic resonance
cholangiopancreatography revealed an obstructing mass measuring
3.5x3 cm in the liver hilum, probably emerging along the
extra-hepatic course of the common hepatic duct, with possible
extension within the gallbladder and causing dilatation of the
intra-hepatic ducts through common hepatic duct compression. The
peripheral part of the common hepatic duct, the pancreatic duct and
the pancreas appeared to be normal. A small (11 mm) lesion in
segment VII of the liver was also identified, which was considered
a possible metastatic lesion. The left adrenal mass was confirmed
to measure 4x3 cm and to be an adenoma.
The case was then discussed during the tumor board
meeting and a biopsy from the hilar mass was requested before
further treatment for extra-hepatic cholangiocarcinoma. Endoscopic
US was performed to meet this goal and showed an endoluminal
obstructing mass in the common hepatic duct, causing central duct
dilatation and enlarged lymph nodes of the liver hilum, thus
raising suspicion for lymphoproliferative disorder. Fine needle
aspiration from the mass was subsequently performed, and
cytological analysis revealed a high-grade lymphoproliferative
disease, without evidence of adenocarcinoma cells. However, the
specimen was not adequate to enable a definite diagnosis. To
further identify the disease and investigate the possibility of a
lymphoma mimicking a Klatskin tumor, a bone marrow biopsy was
performed, which showed reactive changes from the B and T cell
series with the presence of two lymphoid aggregates consisting of
both small CD3+ T cells and CD20+ B cells
(B=T).
Thereafter, diagnostic laparoscopy was undertaken to
extract an adequate tissue sample for pathology. The mass in the
liver hilum was identified during the operation, and a lymph node
from the hepatoduodenal ligament was retrieved. The specimen was
fixed in 10% neutral buffered formalin for 24 h at room temperature
(RT), processed by routine methods and embedded in paraffin. For
routine histology, sections were stained at room temperature for 30
min with hematoxylin (cat. no. CS700; Dako; Agilent Technologies,
Inc.) and eosin (cat. no. CS701; Dako; Agilent Technologies, Inc.)
in Dako Coverstainer (Dako; Agilent Technologies, Inc.) and studied
under a light microscope (Nikon Eclipse E200; Nikon Corporation).
Immunostaining was performed on 3-µm deparaffinized sections.
Primary antibodies against CD3 (rabbit polyclonal, 1:300 dilution;
Zytomed Systems GmbH), CD20 (mouse monoclonal, clone L26 1:100
dilution; Dako; Agilent Technologies, Inc.), Pax-5 (mouse
monoclonal, clone DAK-PAX5, ready to use; Dako; Agilent
Technologies, Inc.), CD5 (rabbit monoclonal, clone SP19/4C7, 1:200
dilution; Dako; Agilent Technologies, Inc.), CD10 (mouse
monoclonal, clone 56C6, ready to use; Dako; Agilent Technologies,
Inc.), Bcl-6 (mouse monoclonal, clone PG-B6P, 1:20 dilution; Dako;
Agilent Technologies, Inc.), Bcl-2 (mouse monoclonal, clone 124,
1:40 dilution; Dako; Agilent Technologies, Inc.), MUM-1 (mouse
monoclonal, clone MUM-1P, 1:40 dilution; Dako; Agilent
Technologies, Inc.), MYC (mouse monoclonal, clone EP121, 1:50
dilution; Bio SB, Inc.), CD30 (mouse monoclonal, clone BER-H2, 1:50
dilution; Dako; Agilent Technologies, Inc.), Ki67 (mouse
monoclonal, clone MIB-1, 1:200 dilution; Dako; Agilent
Technologies, Inc.) were used (6).
For antigen retrieval, slides were immersed in a high pH Dako
EnVision Flex Target Retrieval Solution (50X, cat. no. DM828; Dako;
Agilent Technologies, Inc.) at 97˚C for 20 min and endogenous
peroxidase activity was blocked using the Dako EnVision FLEX
Peroxidase Blocking Reagent before antibody incubation (cat. no.
SM801; Dako; Agilent Technologies, Inc.) (6). Immunohistochemistry was performed
using the Dako Autostainer Link 48 device, with
EnVision™ detection and visualization kit (cat. no.
K5007) and Dako EnVision DAB + Chromogen Flex substrate buffer
(dilution 1 drop/ml) for colour development (all from Dako; Agilent
Technologies, Inc.). All sections were lightly counterstained with
Harris Hematoxylin (Biognost) for 45 sec at RT, prior to mounting
(6).
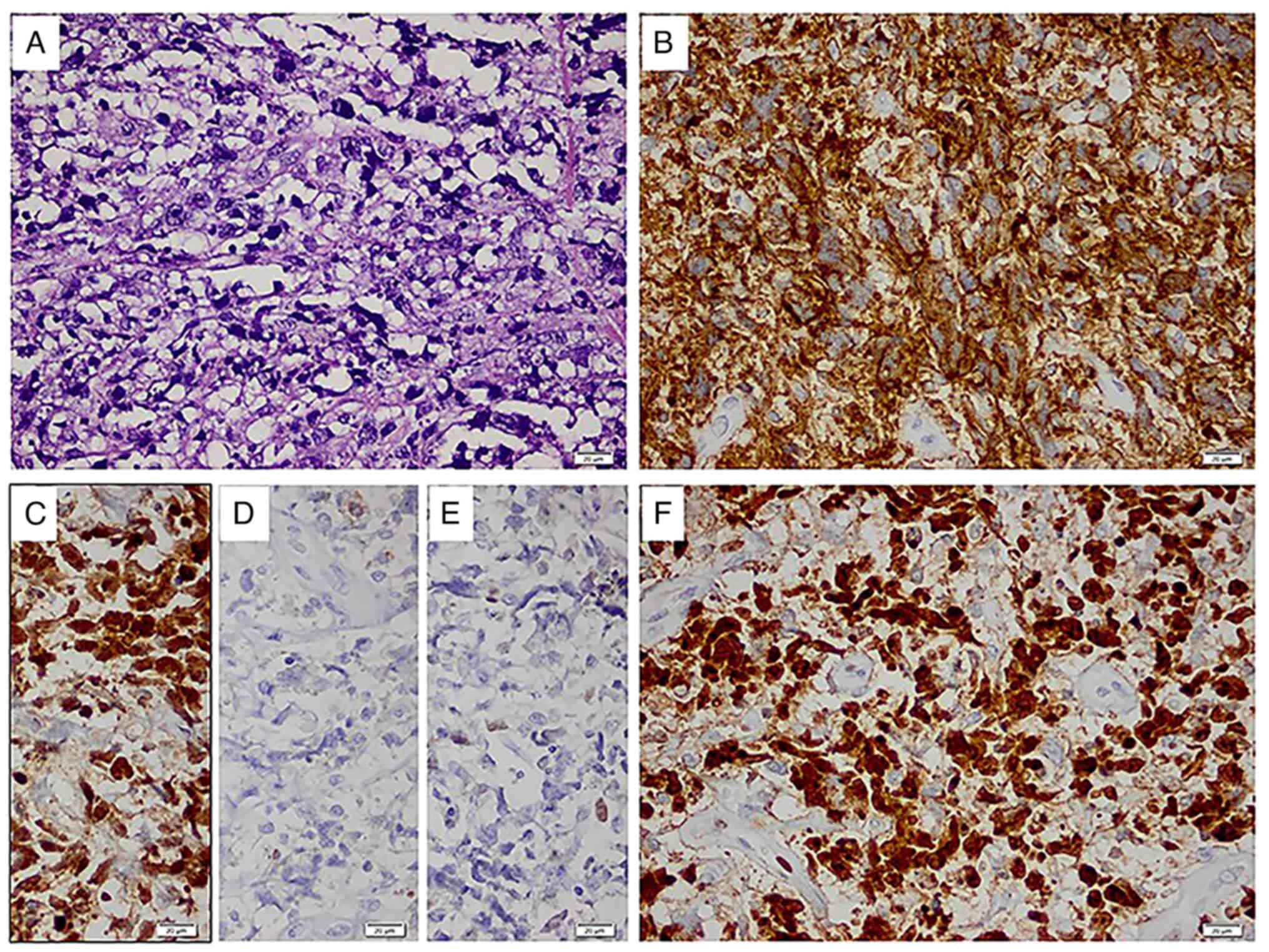
Histology showed a diffuse infiltrate, that
disrupted normal lymph node architecture, consisting of large
lymphoma cells with centroblastic morphology and the following
immunophenotype: CD20+, PAX5+ (data not
shown), Bcl-6+, CD10-, MUM-1- and
CD30-, with a high Ki67 proliferation index (~90%)
(Fig. 4). Accordingly, the
neoplasm was classified as diffuse large B-cell lymphoma, not
otherwise specified, germinal center B-cell-like according to Hans
algorithm (7). The patient
provided written informed consent for the publication of the data
and accompanying images.
The revised International Prognostic Index (R-IPI)
score (8) for this patient was 2,
indicating a good prognosis and an estimated overall survival of
79%. Therefore, after the diagnosis of primary non-Hodgkin lymphoma
of the common hepatic duct was reached, chemotherapy with
cyclophosphamide, doxorubicin, vincristine and prednisolone in
conjunction with the anti-CD20 monoclonal antibody rituximab
(R-CHOP regimen) was scheduled. The patient was in good health 8
months after the diagnosis, without recurrence of the cholestatic
phenomena.
Discussion
Non-Hodgkin lymphoma is found in only 1-2% of all
malignant biliary obstruction cases in adults worldwide (9). Because of its rarity, it is not
usually considered in the differential diagnosis for patients
presenting with jaundice (10).
Its presentation with obstructive jaundice is mainly due to
compression of the extra-hepatic ducts by periportal, perihepatic
or peripancreatic lymphadenopathy (11,12).
Primary non-Hodgkin lymphoma of the extra-hepatic bile duct is very
rare. According to a review of the English literature, since the
first case was reported by Nguyen (2) in 1982, a total of 43 total cases have
been described, including the present report. In 2007, Odemiş et
al (13) reported an incidence
of primary biliary non-Hodgkin lymphoma in patients with malignant
cholangiocarcinoma of 0.6%, accounting for 0.016% of all cases of
non-Hodgkin lymphoma globally.
Hepatitis B virus, hepatitis C virus, HIV, Epstein
Barr virus, elevated lactate hydrogenase levels or low immunity
have been associated with the development of primary biliary
non-Hodgkin lymphoma (1,14,15).
The clinical manifestations are jaundice, fever, abdominal pain,
weight loss and the presence of an abdominal mass. Diagnosing a
primary lymphoma of the extra-hepatic duct is difficult through CT,
MRI or magnetic resonance cholangiopancreatography because, in most
cases, the associated clinical and radiological features closely
resemble those of cholangiocarcinoma. Therefore, tissue biopsy is
required; however, preoperative histological diagnosis remains a
major challenge, particularly in lesions of the hepatic hilum.
In adults, Klatskin tumor, malignant lymph nodes
(metastatic or primary) and hepatoma are the most likely causes of
hilar biliary obstruction. Minimally invasive techniques for
verifying the diagnosis include US- or CT-guided biopsy of the
tumor mass, and endoscopic brushing in endoscopic retrograde
cholangiopancreatography, percutaneous transluminal cholangiography
or cholangioscopy in patients with cholangiocarcinoma. The success
rate for these techniques varies widely, from 20 to 80% (16). Success with endosonographically
guided fine-needle aspiration cytology has previously been reported
(16). In the present case report,
attempts to perform this technique were unsuccessful, because the
tissue obtained was insufficient for a diagnosis. Hence, in some
cases, surgery is inevitable to obtain an adequate sample and
achieve an accurate diagnosis (17,18).
Because of the rarity of primary biliary non-Hodgkin
lymphoma, the treatment options are unclear. However, as soon as a
diagnosis is verified, chemotherapy is considered the gold standard
treatment. Immunochemotherapy with doxorubicin, rituximab plus
cyclophosphamide, vincristine and prednisone (R-CHOP) is generally
used (3,19). The original IPI was developed and
validated before the addition of rituximab to curative
anthracycline-based chemotherapy (20). Clinical trials (15-18)
have confirmed that rituximab can improve the survival of patients
with diffuse large B-cell lymphoma.
The R-IPI score was developed to predict the
outcomes of individuals receiving rituximab with chemotherapy. The
score can differentiate patients into three groups (very good, good
or poor); at present, all of these groups have a survival rate of
>50% (21). Although it remains
unclear as to whether chemotherapy should be administered prior to
biliary drainage in patients with non-Hodgkin lymphoma presenting
with jaundice, chemotherapy alone usually alleviates obstructive
jaundice without the need for biliary drainage (22). Dudgeon and Brower (22) described five cases of patients with
non-Hodgkin lymphoma causing obstructive jaundice that were treated
with combined chemotherapy without prior surgical or endoscopic
biliary decompression or radiation therapy. The therapy rapidly
alleviated the jaundice in all patients and had acceptable toxic
side effects; notably, all five patients achieved remission
(22). In some patients,
radiotherapy is helpful and can also be used to treat patients with
residual disease after the primary chemotherapy or to relieve pain.
Nevertheless, whether radiation therapy is an essential treatment
remains to be further explored, because of the scarcity of cases of
the disease (10). Moreover,
studies on the prognostic implications of primary biliary
non-Hodgkin lymphoma are lacking.
In conclusion, the present case report described a
rare case of primary non-Hodgkin lymphoma of the common hepatic
duct. Although it is difficult to diagnose preoperatively, primary
biliary non-Hodgkin lymphoma should be considered in the
differential diagnosis for obstructive jaundice. The clinical and
radiological findings resemble those of cholangiocarcinoma;
therefore, preoperative diagnosis is quite challenging, owing to
nonspecific clinical manifestations, laboratory profiles and
imaging findings. Because misdiagnosis could lead to inappropriate
treatment, tissue biopsy is mandatory. Although surgery is
essential for accurate histological diagnosis and local tumor
control, multidisciplinary therapy, including systemic
chemotherapy, may be necessary to improve the prognosis associated
with this disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NP performed data collection, substantially
contributed to the conception and the design of the study, and was
a major contributor in writing the manuscript. PGF performed the
pathological analysis and reviewed the paper. AP performed
experiments and data collection, contributed to manuscript drafting
and critically revised the intellectual content. GB performed data
collection and was a major contributor in writing the manuscript.
DP substantially contributed to the acquisition, analysis and
interpretation of the data. VP performed the hematological analysis
and reviewed the paper. KN and EP substantially contributed to the
conception and the design of the study, and confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of the data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Emile JF, Azoulay D, Gornet JM, Lopes G,
Delvart V, Samuel D, Reynès M, Bismuth H and Goldwasser F: Primary
non-Hodgkin's lymphomas of the liver with nodular and diffuse
infiltration patterns have different prognoses. Ann Oncol.
12:1005–1010. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nguyen GK: Primary extranodal
non-Hodgkin's lymphoma of the extrahepatic bile ducts. Report of a
case. Cancer. 50:2218–2222. 1982.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Joo YE, Park CH, Lee WS, Kim HS, Choi SK,
Cho CK, Rew JS, Kim SJ and Maetani I: Primary non-Hodgkin's
lymphoma of the common bile duct presenting as obstructive
jaundice. J Gastroenterol. 39:692–696. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yoon MA, Lee JM, Kim SH, Lee JY, Han JK,
Choi BI, Kim SW and Jang JJ: Primary biliary lymphoma mimicking
cholangiocarcinoma: A characteristic feature of discrepant CT and
direct cholangiography findings. J Korean Med Sci. 24:956–959.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu J, Zhou Y, Li Q, Zhang J and Mao Y:
Primary biliary non-Hodgkin's lymphoma: A case report. Medicine
(Baltimore). 100(e26110)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gallamini A and Juweid M (eds): Lymphoma.
Exon Publications, Brisbane, AU, 2021.
|
|
7
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sehn LH, Berry B, Chhanabhai M, Fitzgerald
C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J,
et al: The revised international prognostic index (R-IPI) is a
better predictor of outcome than the standard IPI for patients with
diffuse large B-cell lymphoma treated with R-CHOP. Blood.
109:1857–1861. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lokich JJ, Kane RA, Harrison DA and
McDermott WV: Biliary tract obstruction secondary to cancer:
Management guidelines and selected literature review. J Clin Oncol.
5:969–981. 1987.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ravindra KV, Stringer MD, Prasad KR,
Kinsey SE and Lodge JP: Non-Hodgkin lymphoma presenting with
obstructive jaundice. Br J Surg. 90:845–849. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
André SB, Farias AQ, Bittencourt PL,
Guarita DR, Machado MC, Viana R and Laudanna AA: Primary extranodal
non-Hodgkin lymphoma of the extrahepatic bile duct mimmicking
Klatskin tumor. Rev Hosp Clin Fac Med Sao Paolo. 51:192–194.
1996.PubMed/NCBI
|
|
12
|
Das K, Fisher A, Wilson DJ, dela Torre AN,
Seguel J and Koneru B: Primary non-Hodgkin's lymphoma of the bile
ducts mimicking cholangiocarcinoma. Surgery. 134:496–500.
2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Odemiş B, Parlak E, Başar O, Yüksel O and
Sahin B: Biliary tract obstruction secondary to malignant lymphoma:
Experience at a referral center. Dig Dis Sci. 52:2323–2332.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Noronha V, Shafi NQ, Obando JA and Kummar
S: Primary non-Hodgkin's lymphoma of the liver. Crit Rev Oncol
Hematol. 53:199–207. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dlouhy I, Filella X, Rovira J, Magnano L,
Rivas-Delgado A, Baumann T, Martínez-Trillos A, Balagué O, Martínez
A, González-Farre B, et al: High serum levels of soluble
interleukin-2 receptor (sIL2-R), interleukin-6 (IL-6) and tumor
necrosis factor alpha (TNF) are associated with adverse clinical
features and predict poor outcome in diffuse large B-cell lymphoma.
Leuk Res. 59:20–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fritscher-Ravens A, Broering DC, Sriram
PV, Topalidis T, Jaeckle S, Thonke F and Soehendra N: EUS-guided
fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: a
case series. Gastrointest Endosc. 52:534–540. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kosuge T, Makuuchi M, Ozaki H, Kinoshita
T, Takenaka T and Mukai K: Primary lymphoma of the common bile
duct. Hepatogastroenterology. 38:235–238. 1991.PubMed/NCBI
|
|
18
|
Maymind M, Mergelas JE, Seibert DG,
Hostetter RB and Chang WW: Primary non-Hodgkin's lymphoma of the
common bile duct. Am J Gastroenterol. 92:1543–1546. 1997.PubMed/NCBI
|
|
19
|
Luigiano C, Ferrara F, Fabbri C, Ghersi S,
Bassi M, Polifemo AM, Billi P, Fornelli A, Cinquantini F and
D'Imperio N: Primary lymphoma of the common bile duct presenting
with acute pancreatitis and cholangitis. Endoscopy. 42 (Suppl
2):E265–E266. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
International Non-Hodgkin's Lymphoma
Prognostic Factors Project. A predictive model for aggressive
non-Hodgkin's lymphoma. N Engl J Med. 329:987–994. 1993.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Coiffier B, Lepage E, Briere J, Herbrecht
R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G,
Gaulard P, et al: CHOP chemotherapy plus rituximab compared with
CHOP alone in elderly patients with diffuse large-B-cell lymphoma.
N Engl J Med. 346:235–242. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Dudgeon DJ and Brower M: Primary
chemotherapy for obstructive jaundice caused by intermediate-grade
non-Hodgkin lymphoma. Cancer. 71:2813–2816. 1993.PubMed/NCBI View Article : Google Scholar
|