Introduction
Borderline ovarian tumors (BOT) represent 10-12% of
ovarian cancers with a higher prevalence in young patients
(1). An incidence of 4.8 new cases
per 100.000 women is reported in Europe (2) with a 13.9% relapse rate in
comprehensive surgical treatment cases (3). International guidelines recommend
unilateral salpingo-oophorectomy and multiple peritoneal biopsies
in patients with childbearing potential who wish to maintain their
fertility (4). Minimally invasive
or ultra-minimally invasive approaches are feasible in this patient
subset (5-8).
Although reproductive outcomes are satisfactory after conservative
treatment, several authors reported a higher relapse rate in
patients undergoing fertility-sparing surgery compared to radical
treatment (9,10). Furthermore, Uzan et al
reported a higher rate of progression to cancer for mucinous and
micropapillary BOTs undergoing conservative surgery (11). Therefore, completion surgery after
childbearing is often suggested, although not for all BOT
histological types (4). In this
scenario, early recognition of relapsing cases is a cornerstone for
correct management when conservative treatment is pursued (12). Previous studies showed several
molecular and pathological features associated with relapse in BOT
patients (13-15).
Among these, the micropapillary architecture and microinvasive
tumors are the main culprits of recurrence in serous BOTs (16). Abnormal tumor markers,
advanced-stage at diagnosis, and cystectomy are factors associated
with BOT relapse in radical treatment cases (17). Despite this, valid scientific
evidence on BOT recurrence predictors in selected patients
undergoing conservative surgery is still lacking.
The aim of this study is to identify predictive
factors of BOT recurrence in patients with childbearing potential
undergoing conservative treatment with unilateral
salpingo-oophorectomy.
Materials and methods
From January 2010 to December 2020 all patients with
childbearing potential undergoing conservative treatment for
early-stage BOT at the University Hospital of Parma, at the “Sacro
Cuore-Don Calabria” Hospital in Negrar, at the department of
gynecologic oncology of the University of Palermo, at the Hospital
USL-IRCCS of Reggio Emilia, and the University Hospital of Verona
were included in the analysis. Inclusion criteria were age >18
years, patients affected by apparent early International Federation
of Gynecology and Obstetrics (FIGO) stage BOT of any histological
type, women undergoing conservative treatment with both
laparoscopic and laparotomic approaches. Patients of childbearing
potential were defined as all patients of any childbearing age
wishing to become pregnant. Patients undergoing ‘ultraconservative’
surgery (cystectomy), patients undergoing bilateral
salpingo-oophorectomy, and cases with missing clinical-pathological
data were excluded from the analysis. Conservative treatment was
defined as conservation of at least a portion of one ovary and the
uterus. Clinical and demographic characteristics, ultrasound
aspects of the ovarian lesion, intraoperative data, and
postoperative instrumental investigations were analyzed of all
patients meeting inclusion criteria. Preoperative assessment
included transvaginal gynecological ultrasound, computed tomography
(CT), and neoplastic markers. Expert sonographers with at least 10
years of experience performed the ultrasounds and classified the
ovarian lesion according to International Ovarian Tumor Analysis
(IOTA) criteria (18). According
to international guidelines, conservative treatment meant
unilateral salpingo-oophorectomy with peritoneal biopsies and
peritoneal washing (4). The open
vs. minimally invasive surgical approach was chosen relating the
tumor size and the risk of intraoperative cyst rupture (19). In the case of laparoscopic surgery,
an endobag was used to safely remove the ovarian lesion avoiding
tumor spillage. Gynecological examination with transvaginal
ultrasound and neoplastic markers were performed during the
follow-up period. CT scan was required annually or in case of
neoplastic markers alteration. Ca125 testing was carried out four
times a year for the first two years and two times a year
thereafter. All patients before surgery gave their written consent
to the surgical procedure and the use of their anonymous data for
scientific purposes. The study was approved by the ethics committee
of the University Hospital of Parma.
Statistical analysis
Quantitative variables are expressed in median with
range. The associations between categorical variables were analyzed
using Chi-square or the Fisher exact test when required. The
identification of the independent variables associated with BOT
recurrence (dependent variable) was performed by linear regression.
Odds Ratio (OR) and 95% confidence interval (CI) were reported for
statistically significant variables. A logistic regression model
was used to identify the variables with a P-value <0.05 in
univariate analysis correlated to the BOT recurrence. A P-value
<0.05 was considered statistically significant. Analyzes were
performed using SPSS 25 (IBM Corp.).
Results
Two hundred thirty BOT patients undergoing surgical
treatment during the study period were analyzed. Of these, 82
patients met the inclusion criteria. Relapse was experienced in 11
cases (13.4%), one (1.2%) peritoneal surface, and 10 (12.2%)
recurrences on the contralateral ovary. Median age was 33.5 years,
range 31 (18-49), median Body Mass Index (BMI) was 23.0
kg/m2, range 33 (17-50), and median follow-up was 42.5
months, range 118 (6-120). Of the 57 women (69.5%) >30 years 9
(15.8%) had relapse (P=0.283), 5 relapses (12.8%) occurred in
patients >35 years (P=0.570), and one case (5.6%) of relapse
presented in >40 years women (P=0.268). Furthermore, among 12
(14.6%) obese patients (BMI >30), 3 (25.0%) had recurrence on
the contralateral ovary (P=0.202). Nine nulliparous patients
(15.3%) out of 59 cases (72.0%) diagnosed relapse (P=0.350).
Furthermore, neither comorbidities (previous cancer P=0.866,
hypertension P=0.898, cigarette smoking P=0.446), nor previous
surgical interventions (ovarian cyst enucleation P=0.216,
appendectomy P=0.236, caesarean section P=0.599), nor
intraoperative tumor spillage (P=0.586), nor increased neoplastic
markers (Ca125 P=0.510, Ca 19-9 P=0.608, Ca 15-3 P=0.233, CEA
P=0.444) showed a statistically significant correlation with BOT
recurrence. Detailed clinical anamnestic correlations of BOT
patients with disease relapses are shown in Table I.
 | Table IPatients' characteristics and
correlation with relapse. |
Table I
Patients' characteristics and
correlation with relapse.
| Characteristic | Cases (n=82) (%) | Relapse (n=11)
(%) | P-value |
|---|
| Age | | | |
|
>30
years | 57 (69.5) | 9 (15.8) | 0.283 |
|
>35
years | 39 (47.6) | 5 (12.8) | 0.570 |
|
>40
years | 18 (22.0) | 1 (5.6) | 0.268 |
| BMI >30
(kg/m2) | 12 (14.6) | 3 (25.0) | 0.202 |
| Previously given
birth | | | |
|
Nulliparous | 59 (72.0) | 9 (15.3) | 0.350 |
|
Previous
vaginal birth | 13 (15.9) | 1 (7.7) | 0.446 |
|
Previous
caesarean section | 10 (12.2) | 1 (10.0) | 0.599 |
| Comorbidities | | | 0.400 |
|
Previous
cancer | 1 (1.2) | 0 | 0.866 |
|
Smoke | 13 (15.9) | 1 (7.7) | 0.446 |
|
Hypertension | 3 (3.7) | 0 | 0.898 |
| Previous surgery | | | 0.592 |
|
Ovarian cyst
enucleation | 10 (12.2) | 0 | 0.216 |
|
Appendectomy | 7 (8.5) | 2 (28.6) | 0.236 |
| Tumor markers | | | 0.615 |
|
Ca125 | 34 (41.5) | 5 (14.7) | 0.510 |
|
Ca 19-9 | 11 (13.4) | 1 (9.1) | 0.608 |
|
Ca 15-3 | 2 (2.4) | 1 (50.0) | 0.233 |
|
CEA | 4 (4.9) | 1 (25.0) | 0.444 |
| Surgical
approaches | | | 0.451 |
|
Laparoscopy | 64 (78.0) | 8 (12.5) | |
|
Laparotomy | 18 (22.0) | 3 (16.7) | |
| Histology | | | |
|
Serous | 57 (59.5) | 9 (15.8) | 0.283 |
|
Mucinous | 23 (28.0) | 2 (8.7) | 0.350 |
|
Others | 2 (2.4) | 0 | 0.748 |
| Tumor Spillage | 10 (12.2) | 1 (10.0) | 0.586 |
| Peritoneal
Washing | 2 (2.4) | 1 (50.0) | 0.331 |
| FIGO Stage | | | |
|
IA | 61 (74.4) | 9 (14.8) | 0.425 |
|
IC | 17 (20.7) | 1 (5.9) | 0.281 |
|
IIIC | 4 (4.9) | 1 (25.0) | 0.444 |
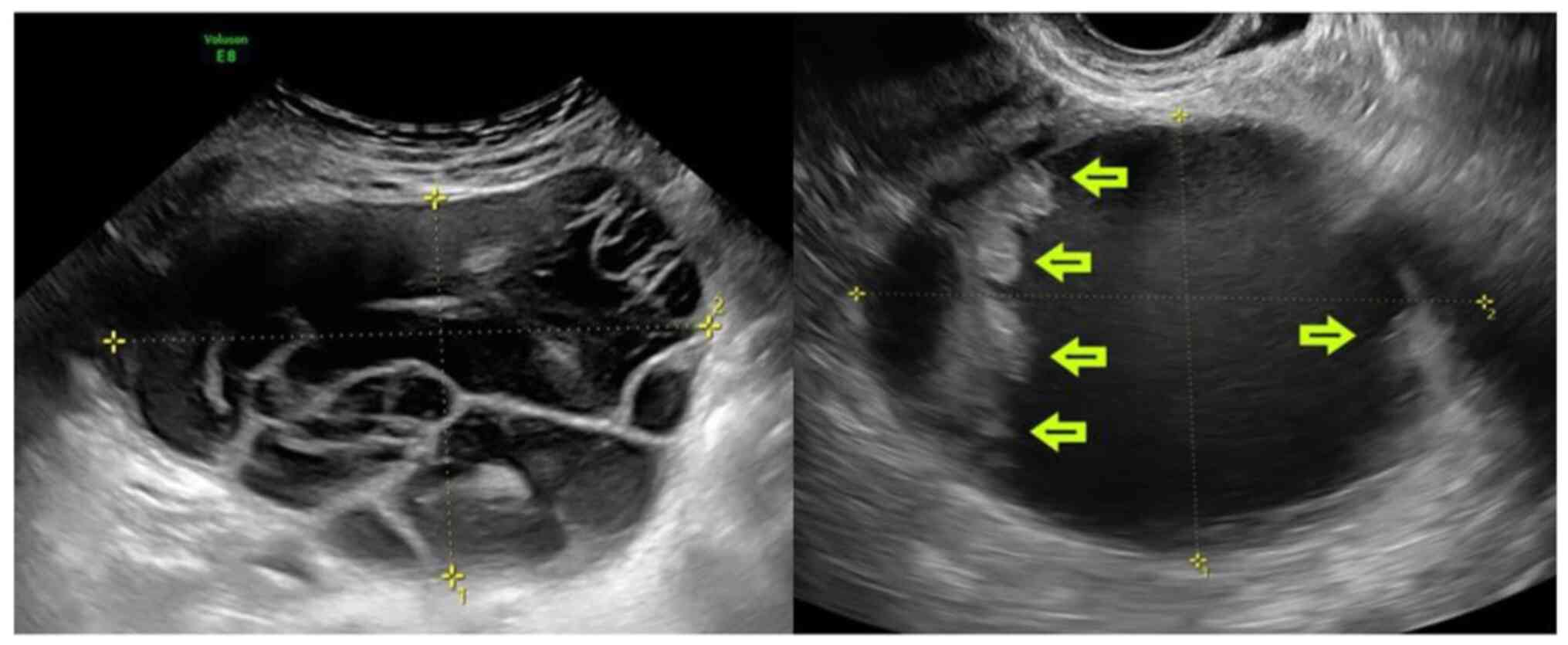
Analyzing the ultrasound characteristics of ovarian
lesions, the mean tumor diameter was 93.6 mm (standard deviation
68.53). Ovarian tumor size >50 mm (P=0.032, OR 7.317, 95% CI
0.89-60.29), Multilocular cysts >10 loculi (P=0.016, OR 7.543,
95% CI 1.64-34.78), cysts with >4 papillae (P=0.025, OR 6.190,
95% CI 1.40-27.36) were statistically correlated with recurrent BOT
(Fig. 1). In contrast, the solid
component diameter > 10 mm (P=0.526), the regular vs. irregular
cyst surface (P=0.505), and the color score (P=0.581) showed no
correlation with recurrence. The sonographic characteristics of
ovarian lesions are summarized in Table II. Besides, surgical approach
(laparoscopy vs. laparotomic approach P=0.451), histological
subtype (serous P=0.283, mucinous P=0.350, others P=0.748), and
FIGO stage (IA P=0.425, IC P=0.281, IIIC P=0.444) did not influence
recurrence. Finally, logistic regression analysis showed lesions
with maximum diameter >50 mm (P=0.014), multilocular cysts
>10 loculi (P=0.012), cysts with >4 papillae (P=0.003)
independent predictive factors of BOT recurrence (P<0.001,
correlation coefficient R=0.481).
 | Table IIOvarian sonographic characteristics
and correlation with recurrence. |
Table II
Ovarian sonographic characteristics
and correlation with recurrence.
| Characteristic | Cases (n=82)
(%) | Relapse (n=11)
(%) | P-value |
|---|
| Tumor size >50
mm | 51 (62.2) | 10 (19.6) | 0.032 |
| Solid
component | | | |
|
≥10 mm | 33 (40.2) | 4 (12.1) | 0.526 |
|
≥15 mm | 27 (32.9) | 4 (14.8) | 0.521 |
|
≥20 mm | 20 (24.4) | 4 (20.0) | 0.260 |
|
≥25 mm | 17 (20.7) | 2 (11.8) | 0.592 |
|
≥30 mm | 15 (18.3) | 2 (13.3) | 0.678 |
| Cyst surface | | | 0.505 |
|
Regular | 70 (85.4) | 9 (12.9) | |
|
Irregular | 12 (14.6) | 2 (16.7) | |
| Color Score | | | |
|
C1 | 46 (56.1) | 6 (13.0) | 0.581 |
|
CS 2 | 24 (29.3) | 2 (8.3) | 0.305 |
|
CS 3 | 10 (12.2) | 2 (20.0) | 0.401 |
|
CS 4 | 2 (2.4) | 1 (50.0) | 0.252 |
| Loculi
(number) | | | |
| Unilocular | 51 (62.2) | 4 (7.8) | 0.058 |
|
>10 | 9 (11.0) | 4 (44.4) | 0.016 |
|
<10 | 22 (26.8) | 3 (13.6) | 0.613 |
| Papillae
(number) | | | |
|
Any | 36 (43.9) | 2 (5.6) | 0.061 |
|
<4 | 36 (43.9) | 5 (13.9) | 0.581 |
|
>4 | 10 (12.2) | 4 (40.0) | 0.025 |
Discussion
The study identified tumor size >50 mm,
multilocular cyst >10 loculi, and ovarian cysts with >4
papillae as independent predictors of BOT recurrence in patients
with childbearing potential undergoing conservative treatment.
In the literature, conflicting results were reported
on the prognostic impact of tumor size in BOT patients (20). Recently, Niu et al (3), in a retrospective analysis including
both conservative and radical treatment, recognized the ovarian
tumor size at diagnosis as associated with increased Ca125 level
and recurrence events in BOT cases. Furthermore, in a large
prospective study by Kobayashi et al (21) involving 6398 Japanese women with an
initial diagnosis of endometrioma, the tumor diameter was also an
independent risk factor for developing ovarian cancer. As
previously hypothesized, possible correlating causes of ovarian
tumor size and recurrence would be intraoperative tumor spillage in
case of large lesions difficult to mobilize or attached to
surrounding structures (22). On
the other hand, the large size of the ovarian mass was
statistically correlated with the poor diagnostic accuracy of the
frozen section due to the pathologist's errors in selecting a
sufficient number of anatomical slices for analysis (23). These insufficient pathological
samplings could lead to an under-FIGO stage or a missed ovarian
cancer diagnosis which could justify the higher relapse rate also
in BOT cases. However, few cases of tumor spillage were reported in
our series. This result could be justified by the intraoperative
use of the endobag in case of minimally invasive treatment. In
addition, all surgeries were performed by surgeons experienced in
oncological gynecology. Furthermore, an average tumor diameter of
less than 10 cm was reported. Therefore, pathologist errors may
have been particularly reduced in our study. Indeed, no cases of
ovarian cancer misdiagnosis were shown on the frozen section
analysis compared to the definitive histological examination in our
series.
As known, the multilocular cyst and the presence of
papillae are known risk factors for malignancies (24). In an interesting study by Franchi
et al (25), multilocular
or multilocular solid cysts were the most frequent ultrasound
manifestations in cases of recurrent BOT. Furthermore, the same
authors stated that recurrent ovarian lesions mimicked the same
ultrasound multilocular appearance of the primary lesion,
especially in mucinous BOTs. Similar results were reported by
Zanetta et al (26) showing
the presence of cysts with papillae as the most frequent recurring
BOT pattern. In line with these authors, our study identified
multilocularity and the presence of papillae on the primary lesion
as independent predictors of recurrence BOT.
Finally, in contrast with other authors, neither
abnormal neoplastic markers level nor FIGO stage predicted BOT
recurrence (27). However,
selecting only patients with childbearing potential undergoing
conservative treatment, we presented extremely homogeneous data
with 95.1% of FIGO stage I cases and only 45.1% of patients with
abnormal neoplastic markers. These aspects could justify our
results.
The present study has the limitations of its
retrospective nature. Furthermore, due to the high patient
selection, a low number of relapse events were reported. On the
other hand, the article could have great clinical relevance, as a
closer follow-up of BOT patients with tumor size >50 mm,
multilocularity, and the presence of papillae could predict relapse
early. Besides, by excluding patients who underwent cystectomy and
not reporting cases of intraoperative tumor spillage we reduced the
presence of bias influencing recurrence.
In conclusion, the present study showed tumor size
>50 mm, multilocularity >10 loculi, and the presence of >4
papillae at the primary ovarian lesions as independent predictors
of BOT recurrence in patients with potential childbearing
undergoing conservative treatment. Closer follow-up for these
patients could be required. Prospective studies would be needed to
confirm our findings.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
VAC, SC, MC, GS, VDM, SU, VC, MF and RB conceived
and designed the study. ES, LM, AC, GB acquired the data. VAC, RB
and VC analyzed and interpreted data. All the authors have read and
approved the final manuscript. VAC and RB confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The Ethics Committee of the University Hospital of
Parma approved this retrospective study on 12 August 2021.
Patient consent for publication
All patients gave their written informed consent for
their anonymized data to be used for scientific purposes.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Vito Andrea Capozzi, ORCID
0000-0003-4720-5663.
References
|
1
|
Skírnisdóttir I, Garmo H, Wilander E and
Holmberg L: Borderline ovarian tumors in Sweden 1960-2005: Trends
in incidence and age at diagnosis compared to ovarian cancer. Int J
Cancer. 123:1897–1901. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Abascal-Saiz A, Sotillo-Mallo L, de
Santiago J and Zapardiel I: Management of borderline ovarian
tumours: A comprehensive review of the literature.
Ecancermedicalscience. 17(403)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Niu L, Tian H, Xu Y, Cao J, Zhang X, Zhang
J, Hou J, Lv W, Wang J, Xin L, et al: Recurrence characteristics
and clinicopathological results of borderline ovarian tumors. BMC
Womens Health. 21(134)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
National Comprehensive Cancer Network.
Ovarian cancer. (Version 1.2022) http//www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
Accessed January 29, 2022.
|
|
5
|
Cianci S, Perrone E, Rossitto C, Fanfani
F, Tropea A, Biondi A, Scambia G and Gueli Alletti S:
Percutaneous-assisted vs mini-laparoscopic hysterectomy: Comparison
of ultra-minimally invasive approaches. Updates Surg. 73:2347–2354.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Song T, Kim MK, Jung YW, Yun BS, Seong SJ,
Choi CH, Kim TJ, Lee JW, Bae DS and Kim BG: Minimally invasive
compared with open surgery in patients with borderline ovarian
tumors. Gynecol Oncol. 145:508–512. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Perrone E, Rossitto C, Fanfani F, Cianci
S, Fagotti A, Uccella S, Vizzielli G, Vascone C, Restaino S, Fedele
C, et al: Percutaneous-assisted versus laparoscopic hysterectomy: A
prospective comparison. Gynecol Obstet Invest. 85:318–326.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cianci S, Rosati A, Rumolo V, Gueli
Alletti S, Gallotta V, Turco LC, Corrado G, Vizzielli G, Fagotti A,
Fanfani F, et al: Robotic Single-Port platform in general,
urologic, and gynecologic surgeries: A systematic review of the
literature and Meta-analysis. World J Surg. 43:2401–2419.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kumari S, Kumar S, Bhatla N, Mathur S,
Thulkar S and Kumar L: Oncologic and reproductive outcomes of
borderline ovarian tumors in Indian population. Gynecol Oncol Rep.
36(100756)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Johansen G, Dahm-Kähler P, Staf C, Flöter
Rådestad A and Rodriguez-Wallberg KA: Reproductive and obstetrical
outcomes with the overall survival of fertile-age women treated
with fertility-sparing surgery for borderline ovarian tumors in
Sweden: A prospective nationwide population-based study. Fertil
Steril. 115:157–163. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Uzan C, Nikpayam M, Ribassin-Majed L, Gouy
S, Bendifallah S, Cortez A, Rey A, Duvillard P, Darai E and Morice
P: Influence of histological subtypes on the risk of an invasive
recurrence in a large series of stage I borderline ovarian tumor
including 191 conservative treatments. Ann Oncol. 25:1312–1319.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Giampaolino P, Della Corte L, Foreste V,
Vitale SG, Chiofalo B, Cianci S, Zullo F and Bifulco G: Unraveling
a difficult diagnosis: The tricks for early recognition of ovarian
cancer. Minerva Med. 110:279–291. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Qi Y, Wang M, Yang Y, Zeng Z and Zhou Y:
Analysis of factors influencing relapse and pregnancy in patients
with borderline ovarian tumors. J Cancer. 12:5275–5285.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fang C, Zhao L, Chen X, Yu A, Xia L and
Zhang P: The impact of clinicopathologic and surgical factors on
relapse and pregnancy in young patients (≤40 years old) with
borderline ovarian tumors. BMC Cancer. 18(1147)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen RF, Li J, Zhu TT, Yu HL and Lu X:
Fertility-sparing surgery for young patients with borderline
ovarian tumors (BOTs): Single institution experience. J Ovarian
Res. 9(16)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sozen H, Vatansever D, Topuz S, Iyibozkurt
C, Kandemir H, Yalçin I, Onder S, Yavuz E and Salihoglu Y:
Clinicopathological analysis of borderline ovarian tumours and risk
factors related to recurrence: Experience of single institution. J
Obstet Gynaecol. 39:253–258. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ayhan A, Guvendag Guven ES, Guven S and
Kucukali T: Recurrence and prognostic factors in borderline ovarian
tumors. Gynecol Oncol. 98:439–445. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Timmerman D, Van Calster B, Testa A,
Savelli L, Fischerova D, Froyman W, Wynants L, Van Holsbeke C,
Epstein E, Franchi D, et al: Predicting the risk of malignancy in
adnexal masses based on the Simple Rules from the International
Ovarian Tumor Analysis group. Am J Obstet Gynecol. 214:424–437.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Watanabe E, Tanaka K, Takeda N, Takayasu
H, Yokota K and Watanabe M: Surgical technique to prevent spillage
of cyst fluid during operation for cystic ovarian tumors. Pediatr
Surg Int. 29:645–649. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gueli Alletti S, Rossitto C, Perrone E,
Cianci S, De Blasis I, Fagotti A and Scambia G: Needleoscopic
conservative staging of borderline ovarian tumor. J Minim Invasive
Gynecol. 24:529–530. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kobayashi H, Sumimoto K, Kitanaka T,
Yamada Y, Sado T, Sakata M, Yoshida S, Kawaguchi R, Kanayama S,
Shigetomi H, et al: Ovarian endometrioma-risks factors of ovarian
cancer development. Eur J Obstet Gynecol Reprod Biol. 138:187–193.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Romagnolo C, Gadducci A, Sartori E, Zola P
and Maggino T: Management of borderline ovarian tumors: Results of
an Italian multicenter study. Gynecol Oncol. 101:255–260.
2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Karataşlı V, Can B, Çakır İ, Erkılınç S,
Karabulut A, Ayaz D, Kuru O, Gökçü M and Sancı M: Effect of tumor
size on the accuracy of frozen section in the evaluation of
mucinous borderline ovarian tumors. J Gynecol Obstet Hum Reprod.
49(101765)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Timmerman D, Testa AC, Bourne T, Ameye L,
Jurkovic D, Van Holsbeke C, Paladini D, Van Calster B, Vergote I,
Van Huffel S and Valentin L: Simple ultrasound-based rules for the
diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 31:681–690.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Franchi D, Boveri S, Fruscio R, Fischerova
D, Guerriero S, Moruzzi MC, Colombo N, Timmerman D, Valentin L and
Testa AC: Imaging in gynecological disease (8): Ultrasound
characteristics of recurrent borderline ovarian tumors. Ultrasound
Obstet Gynecol. 41:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zanetta G, Rota S, Lissoni A, Meni A,
Brancatelli G and Buda A: Ultrasound, physical examination, and CA
125 measurement for the detection of recurrence after conservative
surgery for early borderline ovarian tumors. Gynecol Oncol.
81:63–66. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gizzo S, Berretta R, Di Gangi S, Guido M,
Zanni GC, Franceschetti I, Quaranta M, Plebani M, Nardelli GB and
Patrelli TS: Borderline ovarian tumors and diagnostic dilemma of
intraoperative diagnosis: Could preoperative He4 assay and ROMA
score assessment increase the frozen section accuracy? A
multicenter case-control study. Biomed Res Int.
2014(803598)2014.PubMed/NCBI View Article : Google Scholar
|