Introduction
According to the definition by Gluckman (1), ‘synchronous carcinomas’ include
carcinomas that present either simultaneously or within six months
of identifying the original tumor.
Colorectal lymphoma is rare, with less than 0.5%
incidence of all colorectal malignancies (2), and follicular lymphoma (FL) is less
common (3). In addition, it is
rarer for colorectal cancer to co-occur with colorectal lymphoma
synchronously, and there was a report that the estimated incidence
is ~0.0002% (4). The etiology is
often unclear, and there is no standard treatment strategy. As
histopathological subtypes of malignant lymphoma with colorectal
cancer synchronously, diffuse large B-cell lymphoma (DLBCL)
(5), mantle cell lymphoma
(6), mucosa-associated lymphoid
tissue (MALT) lymphoma (7), and
extranodal natural killer/T-cell lymphoma (8) have been reported previously. More
recently, it has also been reported in FL (9,10).
We report a case of sigmoid colon cancer that was found 6 months
after endoscopic resection of rectal FL, was resected curatively
and survived for more than 3 years without recurrence.
Case report
A 71-year-old male patient with diabetes mellitus
and hypertension was aware of lower abdominal discomfort and
underwent colonoscopy at a nearby hospital (Otaru General Hospital,
Otaru, Japan). A neoplastic lesion was found in his rectum, and a
biopsy suggested malignant lymphoma, so he was referred to our
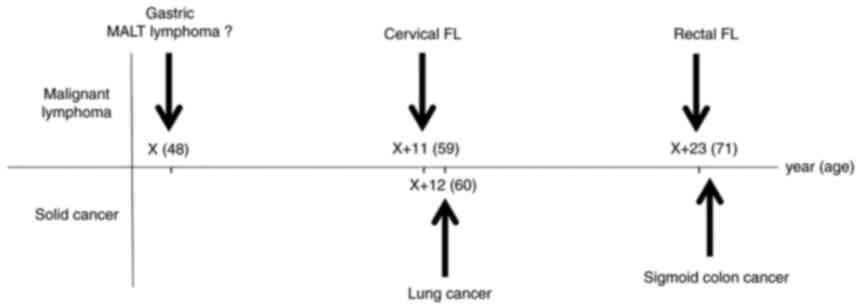
hospital. This patient has a medical history described below
(Fig. 1). In June 1995 (at the age
of 48), this patient underwent a total gastrectomy with primary
gastric MALT lymphoma at another hospital (Otaru Kyokai Hospital,
Otaru, Japan). In April 2006 (at the age of 59), a tumor developed
at the hard palate, right parotid gland and right submandibular
lymph nodes. A biopsy was performed at the department of
otorhinolaryngology in Hokkaido University Hospital (Sapporo,
Japan), and the pathological diagnosis was consistent with MALT
lymphoma. This patient received chemotherapy [3 cycles of rituximab
+ THP-COP (cyclophosphamide, pirarubicin, vincristine and
prednisone)] and radiation (40 Gy) at our hospital and was
evaluated for complete remission (CR). The following year,
September 2007 (at the age of 60), lung cancer (2 cm in size,
poorly differentiated adenocarcinoma) was found during his regular
follow-up, and curative resection was performed at our hospital.
After that, the visit to our hospital was discontinued.
In April 2018 (at the age of 71), he was admitted to
our hospital for the first time in 11 years and re-examined the
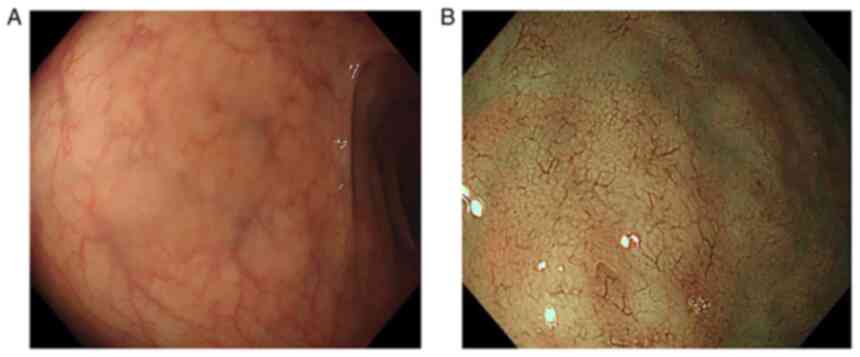
colonoscopy. As a finding, a flat elevated lesion with a diameter
of ~2 cm was confirmed in the rectum (Fig. 2), and endoscopic submucosal
dissection (ESD) was performed for the purpose of complete biopsy.
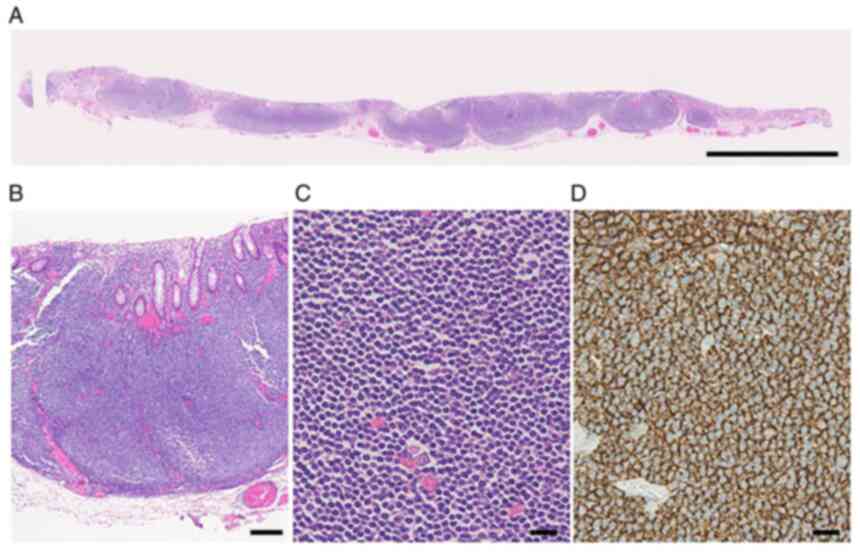
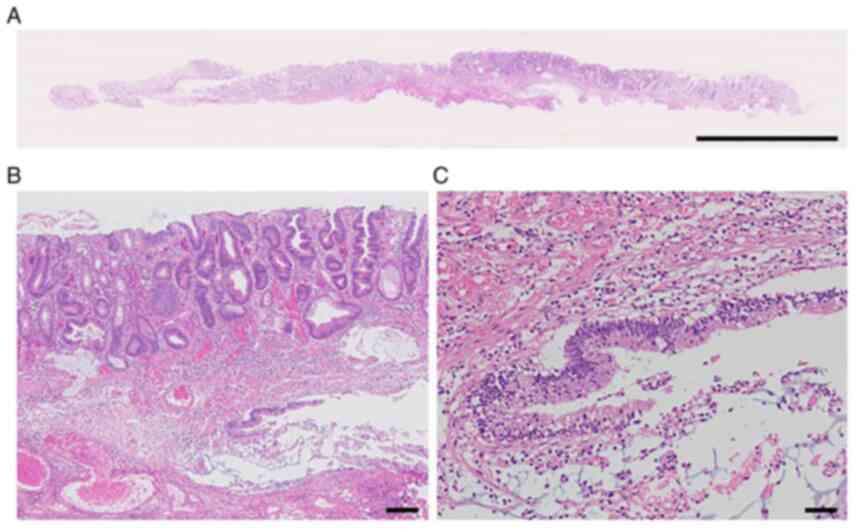
Histopathologically, a dense collection of small lymphocytes with
positive CD20, mainly from the lamina propria to the submucosa, was
confirmed (Fig. 3). The
pathological diagnosis was consistent with FL equivalent to Grades
1-2. IgH/BCL2 was positive (86.1%) in fluorescence in situ
hybridization (FISH), confirming the diagnosis of this patient as
FL. On the other hand, a tumor in the cervical region 12 years
previously was also positive for IgH/BCL2 in FISH, and the
diagnosis was corrected as FL rather than MALT lymphoma. The
specimen of gastric lymphoma 23 years ago was no longer left, and
its histopathological details were unknown. Regarding this time,
small lymph nodes near the lower esophagus and in the diaphragmatic
leg were accumulated on the positron emission tomography (PET)-CT
(standardized uptake value max 3, not shown), and they were
consistent with FL lesions. Rituximab monotherapy was administered
3 times every 2 months, but the treatment was terminated, because
he had to continue his daily work.
The patient occasionally continued to have lower
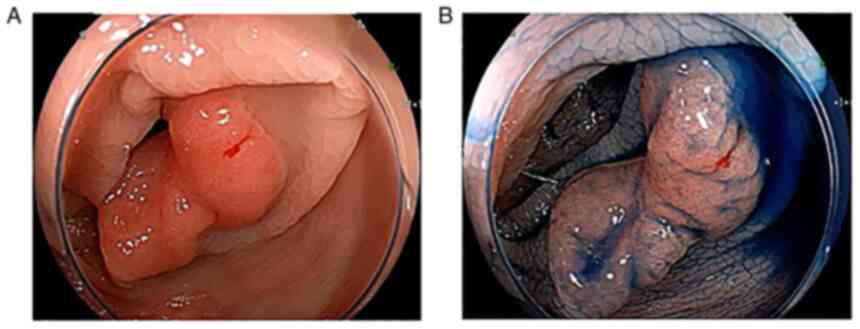
abdominal discomfort. In October 2018, a follow-up colonoscopy 6
months after the above endoscopic procedure confirmed a 15-20 mm
flat elevated lesion in the sigmoid colon (Fig. 4). A pathological diagnosis of colon
cancer was made by biopsy. No abnormalities except for the lymph
nodes mentioned above were found on CT, and ESD was performed to
completely resect the lesion as stage I colon cancer.
Histopathologically, the tumor infiltrated the submucosa, and
well-differentiated adenocarcinoma was predominant, but some
components of mucinous carcinoma were found (Fig. 5). Mucus components were found in
the vertical stump, and additional local resection was performed at
a later date. However, no residual tumor was observed, and as a
result, it was determined that the tumor was almost resected
endoscopically. After more than 3 years with no treatment, and now
at the age of 75, the patient can lead a normal daily life with no
recurrence of both colorectal lymphoma and cancer.
Discussion
As a subtype of FL, it was referred to as
‘duodenal-type FL’ in the revised 4th edition (11). Epidemiologically, D-FL has been
recognized as a rare entity that accounts for ~4% of primary
gastrointestinal lymphomas (12).
Colorectal involvement of FL is thought to be even less frequent in
cases of FL (3). Takata et
al reported that only 4%, that is, 5 cases, originated from the
large intestine (cecum 2, colon 1, rectum 2) among the 125 cases of
intestinal FL (13).
In the past, two patients developed FL and
adenocarcinoma synchronously in the large intestine (9,10).
Both cases were collision tumors that developed in the right large
intestine (cecum and hepatic flexure). One patient died of rapid
progression of FL after treatment with FOLFOX chemotherapy (folinic
acid, fluorouracil and oxaliplatin) for adenocarcinoma with a
predominant tumor volume (9). The
other case was carcinoma in adenoma with FL (10). In our case, the initial colonoscopy
diagnosed with rectal FL could have overlooked this sigmoid colon
cancer; in particular, precancerous mucosal changes may have
already existed. This is the first report of a case in which
colorectal FL and adenocarcinoma were resected at an early
stage.
After the diagnosis of this rectal FL was confirmed,
it became clear that the lymphoma in the cervical region that was
treated as MALT lymphoma 11 years previously was actually FL.
Meanwhile, the patient underwent total gastrectomy for primary
gastric lymphoma 23 years previously. Regarding the treatment of
gastric MALT lymphoma, in 1995, it was allowed to be treated by
surgical resection instead of Helicobacter pylori
eradication, and sometimes by total gastrectomy for the multiple
and/or spreading lesions. However, gastric MALT lymphoma at that
time may actually have been FL. In either case, our patient had a
long-term recurrence of indolent lymphoma from 48 years of age for
23 years in other regions approximately every 10 years and had two
solid cancers (lung and colon) in the short term, only six months
to one year after the onset of lymphoma. We hypothesize that
curative resection of these cancers was possible because we were
able to detect them at an early stage by closely following up on
the lymphomas. Indolent B-cell lymphoma has repeatedly recurred in
different regions without transformation to DLBCL over numerous
years. It can be inferred that some of this patient's
immunosurveillance mechanisms may be failing. Moreover, a
definitive relationship between the onset of lymphoma and carcinoma
has not been established. It may be a coincidence that FL and
adenocarcinoma developed in the same large intestine synchronously.
It is unlikely that administration of rituximab monotherapy after
endoscopic excision of lymphoma induced carcinogenesis. According
to Haddadi et al, the development of lymphoma may accelerate
malignant changes in existing precancerous lesions (14).
This study has several limitations. Firstly, the
cervical tumor at age 59 was FL, not MALT Lymphoma. However, since
both are in the category of indolent lymphomas, the delay in
diagnosis at that time is not considered to have had a negative
effect on the subsequent clinical course. Secondly, there is a lack
of data related to carcinogenesis, such as immune function at the
onset of sigmoid colon cancer, especially natural killer cell
activity, or CD8/regulatory T-cells (Treg). In our case, it is
speculated that lymphoma developed due to immune dysfunction, such
as proliferation of Treg, which may have led to the activation of
oncogenes or the inactivation of tumor suppressor genes in the
precancerous component.
In conclusion, a patient who was first affected 23
years previously and repeatedly developed indolent lymphoma in
another region approximately every 10 years suffered from rectal FL
this time. Six months after endoscopic resection, he had sigmoid
colon cancer. As a mechanism of this carcinogenesis, it was
speculated that the genes involved in cancer development were
changed due to the decrease in immune function associated with the
onset of lymphoma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HE and TK were involved in endoscopic procedure, and
ZIT was involved in the pathological procedure. MS and HE confirm
the authenticity of all the raw data. MS, HE, TK, EY, KI, AM, MM,
TK and ZIT made substantial contributions to conception and design,
acquisition of data or analysis and interpretation of data; took
part in drafting the article or revising it critically for
important intellectual content; gave final approval of the version
to be published; and agree to be accountable for all aspects of the
work.
Ethics approval and consent to
participate
This study was conducted in accordance with the
World Medical Association Declaration of Helsinki, and the Aiiku
Hospital Clinical Research Review Board does not require ethical
approval for reporting a case report.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and the accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Makoto Saito, ORCID: 0000-0002-2683-9475.
References
|
1
|
Gluckman JL, Crissman JD and Donegan JO:
Multicentric squamous-cell carcinoma of the upper aerodigestive
tract. Head Neck Surg. 3:90–96. 1980.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zighelboim J and Larson MV: Primary
colonic lymphoma. Clinical presentation, histopathologic features,
and outcome with combination chemotherapy. J Clin Gastroenterol.
18:291–297. 1994.PubMed/NCBI
|
|
3
|
LeBrun DP, Kamel OW, Cleary ML, Dorfman RF
and Warnke RA: Follicular lymphomas of the gastrointestinal tract.
Pathologic features in 31 cases and bcl-2 oncogenic protein
expression. Am J Pathol. 140:1327–1335. 1992.PubMed/NCBI
|
|
4
|
Barron BA and Localio SA: A statistical
note on the association of colorectal cancer and lymphoma. Am J
Epidemiol. 104:517–522. 1976.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang W and Li P: Coexistence of colon
adenocarcinoma, diffuse large B-cell lymphoma, and myelodysplastic
syndrome: A case report. Medicine (Baltimore).
98(e16742)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Padmanabhan V and Trainer TD: Synchronous
adenocarcinoma and mantle cell lymphoma of the colon. Arch Pathol
Lab Med. 127:E64–E66. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wagle SD, Mohandas KM, Vazifdar KF, Dhir
V, Swaroop VS, Jagannath P and Desouza LJ: Synchronous
adenocarcinoma and lymphoma of the colon. Indian J Gastroenterol.
16:28–29. 1997.PubMed/NCBI
|
|
8
|
Tseng CE, Shu TW, Lin CW and Liao KS:
Synchronous adenocarcinoma and extranodal natural killer/T-cell
lymphoma of the colon: A case report and literature review. World J
Gastroenterol. 19:1850–1854. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kus T, Aktas G, Kalender ME, Sari I, Ulker
E and Camci C: Collision tumor consisting of primary follicular
lymphoma and adenocarcinoma in the cecum: A case report and
literature review. Oncol Lett. 11:2801–2805. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin YS, Hamilton AER, Henderson C and
Farzin M: Tiny but mighty: Collision tumor of a superficial
adenocarcinoma arising from a tubulovillous adenoma with associated
low-grade follicular lymphoma. Int J Surg Pathol. 29:759–763.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jaffe ES, Harris NL, Swerdlow SH, Ott G
and Nathwani BN: Follicular lymphoma. In: WHO Classification of
Tumours of Haematopietic and Lymphoid Tissues. Revised 4th edition.
IARC Press, Lyon, pp266-277, 2017.
|
|
12
|
Marks E and Shi Y: Duodenal-Type
follicular lymphoma: A clinicopathologic review. Arch Pathol Lab
Med. 142:542–547. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Takata K, Okada H, Ohmiya N, Nakamura S,
Kitadai Y, Tari A, Akamatsu T, Kawai H, Tanaka S, Araki H, et al:
Primary gastrointestinal follicular lymphoma involving the duodenal
second portion is a distinct entity: A multicenter, retrospective
analysis in Japan. Cancer Sci. 102:1532–1536. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Haddadi S, Touati R, Graidia N, Ourdane R,
Yahia-Messaoud Y and Namaoui Y: Synchronous adenocarcinoma and
marginal zone B-cell lymphoma of the colon. A case report. Int J
Surg Case Rep. 84(106025)2021.PubMed/NCBI View Article : Google Scholar
|