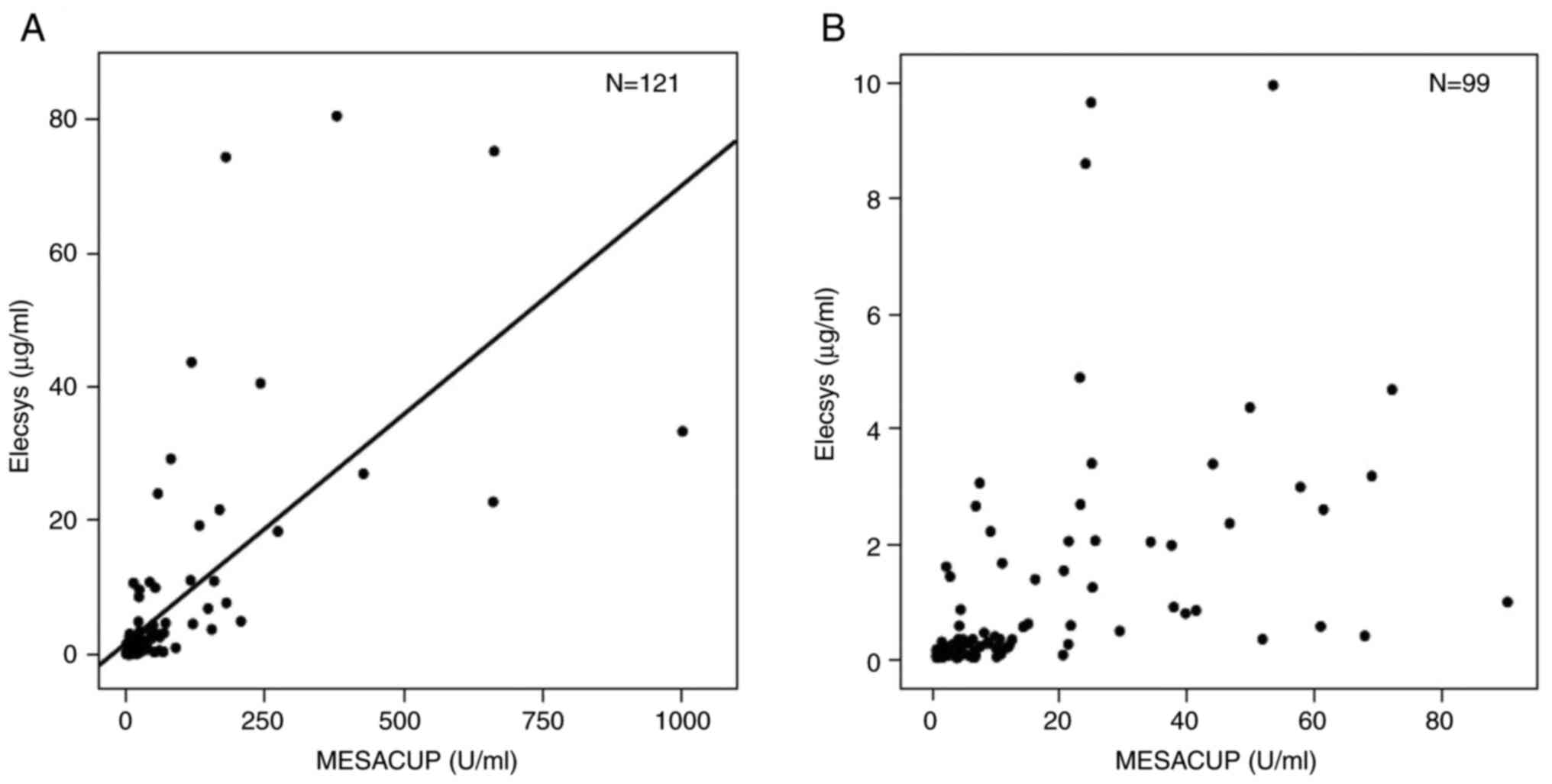
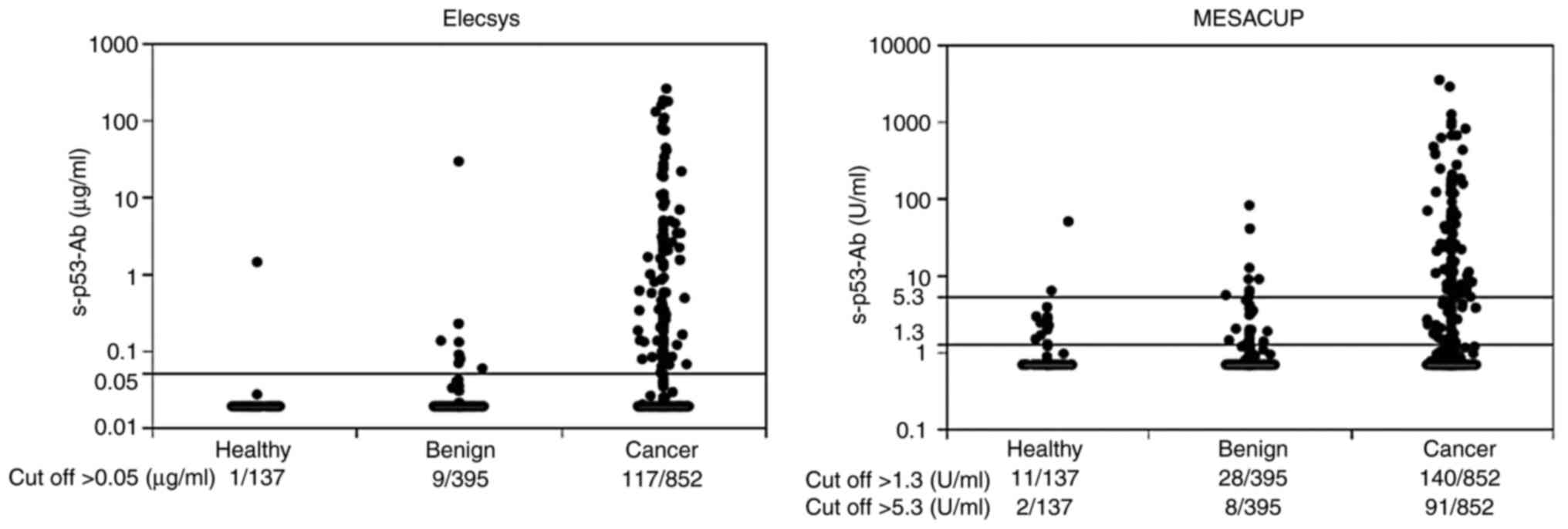
Spandidos Publications style
Suzuki T, Oshima Y, Shiratori F, Nanami T, Yajima S, Sumazaki M, Ushigome M, Sugita H, Eberl M, Ogata H, Ogata H, et al: Comparison between a new assay system, Elecsys® Anti‑p53, and conventional MESACUP™ for the detection of serum anti‑p53 antibodies: A multi‑institutional study. Mol Clin Oncol 17: 130, 2022.
APA
Suzuki, T., Oshima, Y., Shiratori, F., Nanami, T., Yajima, S., Sumazaki, M. ... Shimada, H. (2022). Comparison between a new assay system, Elecsys® Anti‑p53, and conventional MESACUP™ for the detection of serum anti‑p53 antibodies: A multi‑institutional study. Molecular and Clinical Oncology, 17, 130. https://doi.org/10.3892/mco.2022.2563
MLA
Suzuki, T., Oshima, Y., Shiratori, F., Nanami, T., Yajima, S., Sumazaki, M., Ushigome, M., Sugita, H., Eberl, M., Ogata, H., Hayashida, T., Nakamura, S., Nakagawa, T., Shimada, H."Comparison between a new assay system, Elecsys® Anti‑p53, and conventional MESACUP™ for the detection of serum anti‑p53 antibodies: A multi‑institutional study". Molecular and Clinical Oncology 17.2 (2022): 130.
Chicago
Suzuki, T., Oshima, Y., Shiratori, F., Nanami, T., Yajima, S., Sumazaki, M., Ushigome, M., Sugita, H., Eberl, M., Ogata, H., Hayashida, T., Nakamura, S., Nakagawa, T., Shimada, H."Comparison between a new assay system, Elecsys® Anti‑p53, and conventional MESACUP™ for the detection of serum anti‑p53 antibodies: A multi‑institutional study". Molecular and Clinical Oncology 17, no. 2 (2022): 130. https://doi.org/10.3892/mco.2022.2563