Introduction
It has previously been reported that the prevalence
of serum anti-p53 antibodies (s-p53-Abs) is correlated with the
prevalence of p53 mutations in different types of cancers,
including esophageal, colon, lung and uterine cancer (1). The accumulation of p53 in tumors and
the subsequent immune response are attributable to a
self-immunization process linked to the strong immunogenicity of
the p53 protein (2-5).
Although the clinical value of s-p53-Abs remains debatable, several
studies have reported consistent results in colon, esophageal,
breast and gastric cancer types, in which s-p53-Abs have been
associated with high-grade tumors and a poor prognosis (2-5).
Furthermore, the addition of s-p53-Abs may enhance the diagnostic
sensitivity of conventional tumor markers without a decrease in
specificity, suggesting a promising role for s-p53-Abs as part of a
panel of tumor markers (2-5).
For these reasons, the quantitative p53-Abs enzyme-linked
immunosorbent assay (ELISA) Kit II [MESACUP™ anti-p53
test (MESACUP); Medical & Biological Laboratories Co., Ltd.]
for measuring s-p53-Abs was developed, approved by the Japanese
government and covered by national healthcare insurance in 2007
ahead of other countries (6-10).
In patients with various types of cancer, s-p53-Abs can be used for
the diagnosis and monitoring of treatment response and tumor
recurrence (2-5).
In a previous multi-institutional study, a cutoff value of 1.3
U/ml, with >95.5% specificity, was determined and applied in
clinical practice (3). Although
the ELISA method is clinically significant for s-p53-Abs, it is
time-consuming and shows only semiquantitative values.
Compared with manual ELISAs,
electrochemiluminescence immunoassays (ECLIAs) are highly
sensitive, quantitative and quick (11). The s-p53-Abs ECLIA Kit
Elecsys® Anti-p53 (Elecsys) (Roche Diagnostics K.K.) was
developed recently (12) and
approved by the Pharmaceuticals and Medical Devices Agency of
Japan. Results of a clinical study showed that the new s-p53-Abs
assay, Elecsys, was useful in the detection of esophageal and
colorectal cancer, with a specificity of >98.0%. Also, the
addition of s-p53-Abs to conventional tumor markers increased the
positivity rates in these cancer types (12). However, no direct comparison has
been conducted between the clinical performance of Elecsys and
MESACUP.
In the present multi-institutional study, the
clinical performance of the novel Elecsys system was compared with
that of conventional MESACUP for the measurement of s-p53-Abs in
patients with esophageal, colorectal and breast cancer. To the best
of our knowledge, this is the first study to directly compare the
diagnostic utility of these two assay systems, which rely on
distinct technologies.
Patients and methods
Patients and controls
This was a multicenter, prospective study designed
to compare the analytical performance of two diagnostics kits.
Patients with pathologically defined primary esophageal, colorectal
or breast cancer and disease controls were enrolled from seven
hospitals (Chiba Foundation for Health Promotion and Disease
Prevention, Chiba University Hospital, Chiba; Keio University
Hospital, Tokyo; Showa University Hospital, Tokyo; Toho University
Sakura Medical Center, Chiba; Toho University Ohashi Medical
Center, Chiba; Toho University Omori Medical Center, Tokyo; Tokyo
Center Clinic, Tokyo Medical and Dental University, Tokyo, Japan)
(12). All participants were aged
≥20 years, and provided written, informed consent prior to
enrolment. Serum samples from healthy volunteers and patients
(subjects) who met all the following inclusion criteria and did not
meet any of the following exclusion criteria were measured, and
followed by statistical analysis. The subjects who violated the
ethical guidelines were excluded from the study and the remaining
subjects were handled as the full analysis set. The subjects who
met any of the following criteria ii) to v) were excluded from the
full analysis set and the remaining subjects were handled as the
per protocol set. i) Subjects with violation of the ethical
guidelines: Subjects whose serum samples may have been collected
not in compliance with the ethical guidelines, including those for
whom no consent was obtained, those for whom the consent was
obtained in a questionable manner or those whose serum samples were
tested before the consent was obtained. ii) Subjects with
deviations: Subjects in whom designated examinations were not
performed with the procedure or at intervals specified by the
protocol, or those excluded from analysis by the investigator due
to illness or other reasons. iii) Ineligible subjects: Subjects who
should not have been included in the study, as it was found after
registration that they did not meet any of the inclusion criteria
or meet any of the exclusion criteria. iv) Discontinued subjects:
Subjects discontinued from the study by the investigator due to
meeting any of the discontinuation criteria, etc. v) Subjects for
whom no measurement was obtained for either of the test or control
drug. This study was conducted between October 2016 and September
2018. The mean age of healthy subjects, patients with autoimmune
diseases and cancer patients was 40.9 (range, 20-73), 59.6 (range,
22-92) and 64.9 (range, 30-97) years, respectively. A total of 288
patients with esophageal cancer (stage I, n=59; stage II, n=45;
stage III, n=138; stage IV, n=40; recurrent or unknown, n=6), 235
patients with colorectal cancer (stage 0, n=1; stage I, n=50; stage
II, n=70; stage III, n=82; stage IV, n=30; recurrent or unknown,
n=2) (12) and 329 patients with
breast cancer (stage 0, n=65; stage I, n=150; stage II, n=95; stage
III, n=14; stage IV, n=3; recurrent or unknown, n=2) were enrolled
in this multi-institutional study. Control samples were obtained
from 137 healthy volunteers, 105 patients with autoimmune diseases
(rheumatoid arthritis, n=36; polymyalgia rheumatica, n=12; systemic
lupus erythematosus, n=7; adult Still's disease, n=7; eosinophilic
granulomatosis with polyangitis, n=6; Sjögren's syndrome, n=5;
scleroderma, n=5; microscopic polyangiitis, n=5; other diseases,
n=22) and 290 patients with benign diseases, including 100 with a
benign disease of the esophagus (reflux esophagitis, n=91; other
diseases, n=9), 100 with a benign disease/s of the colorectal
system (hemorrhoid, n=45; diverticulosis, n=23; polyp, n=19;
adenoma, n=6; hemorrhoid and diverticulosis, n=2; hemorrhoid,
diverticulosis and polyp, n=1; other diseases, n=4) and 90 with a
benign disease/s of the breast (mastopathy, n=40; fibroadenoma,
n=19; mastitis, n=8; lactocele, n=8; mastopathy and fibroadenoma,
n=1; mastopathy and lactocele, n=1; other diseases, n=13) (12).
From the subjects, 5 ml of blood was drawn for the
study. The blood was held at room temparature until coagulation was
complete and then the serum was separated. Serum samples were
obtained before treatment, divided into two tubes and stored at
-20˚C. Patient recruitment and sample collections were performed
within the guidelines of protocols approved by the Ethics Committee
of Toho University (Tokyo, Japan; approval no. A16049) and the
Institutional Review Boards of each participating hospital. In
addition, written informed consent was obtained from all
participants.
Enzyme immunoassay for s-p53-Abs
s-p53-Abs were assessed via immunoassay for the
in vitro quantitative determination of anti-p53
autoantibodies in human serum using the anti-p53 ECLIA Kit (catalog
no. 07751605174; Elecsys; Roche Diagnostics K.K.) according to the
manufacturer's instructions (12).
In brief, to allow for the formation of complexes of capture
antigen-anti-p53 antibody-detection antigen, the biotinylated
capture antigen, 20 µl of the sample and the ruthenylated detection
antigen were incubated at 37˚C. If anti-p53 antibodies were present
in the sample, they formed a bridge between the capture and
detection antigens, resulting in the formation of a stable complex.
The complexes were immobilized on streptavidin-coated beads that
interacted with the biotin on the capture antigen, and the
chemiluminescence signal detection was performed using the Cobas
6000 analyzer (Roche Diagnostics K.K.). Electrogenerated
chemiluminescence generates species at the electrode surfaces,
which undergo electron-transfer reactions and form excited states
to emit light. The signal output is expressed in arbitrary light
units, which is equivalent to the concentration of the analyte,
providing a fully quantitative result (12). This is a fully automated
immunoassay system with a high throughput of 300 samples/h, and the
reaction time is as short as 18 min. In addition, three different
peptides that can strongly capture the wild-type sequences of the
p53 antibodies were designed and included in the assay, for use as
antigens to maximize the sensitivity and specificity of the
assay.
Simultaneously, samples from the same subjects were
sent to LSI Medience Corporation for assessment by the p53-Abs
ELISA Kit II (MESACUP; RG-7640E; Medical & Biological
Laboratories Co., Ltd.) based on the manufacturer's instructions
(3). The rationale for setting the
cutoff value (Elecsys, 0.05 µg/ml; MESACUP, 1.3 U/ml) and their
package inserts are as previously described (3,12).
Statistical analysis
The correlation between the two assay systems was
evaluated using Pearson's correlation analysis. To compare the
sensitivity and specificity of the two different systems, as well
as the values from patients with cancer and those with benign
disease according to each assay system, an exact McNemar test was
applied. P<0.05 was considered to indicate a statistically
significant difference. R version 3.6.3 (R Foundation for
Statistical Computing; https://www.r-project.org/foundation/) was used for
all the statistical analyses. The P-values were calculated using
Fisher's exact test with JMP version 15.2 (J.M.P, Co., Ltd.).
Results
Correlation between the two assay
systems with regard to s-p53-Ab titer
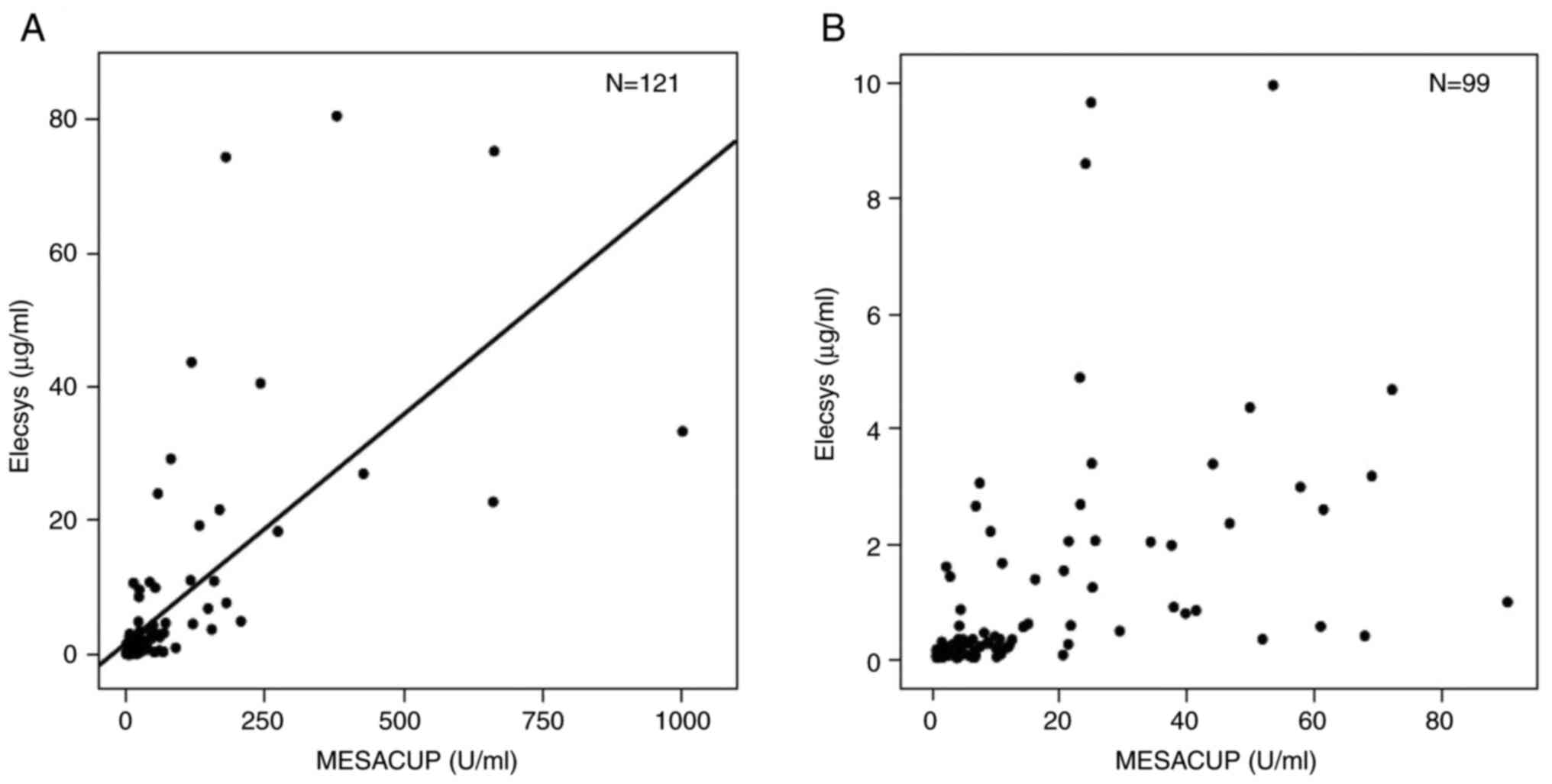
Fig. 1 shows the
correlation between the two assay systems with regard to the
s-p53-Ab titer. The overall correlation was calculated as y=0.068x
+ 1.633, with r=0.674 (Fig. 1A).
To examine the correlation around cut off values, when focusing on
the low-titer group (0.02-10 µg/ml for Elecsys and 0.7-100 U/ml for
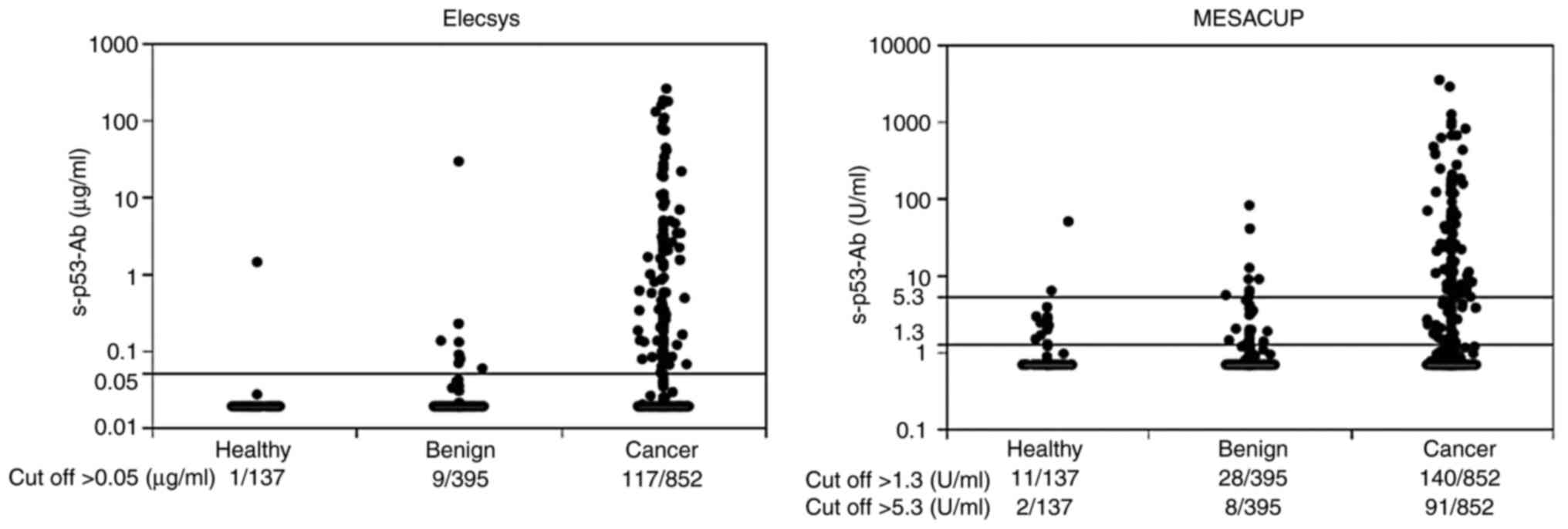
MESACUP), the values were widely spread (n=99; Fig. 1B). Fig. 2 shows the distribution of
measurement values by disease for the two assay systems. The
positive p53 detection rates in the patients with cancer were
higher than those of healthy and benign subjects for both assay
systems. In addition, higher s-p53-Ab titers were observed in the
patients with cancer compared with those in the healthy and benign
subjects for the two assay systems.
Agreement rates and judgments of the
two assay systems for each cancer type
Table I shows the
rates of agreement between the two assay systems for each cancer
type when compared with the cutoff values in the package inserts
(Elecsys: 0.05 µg/ml; MESACUP: 1.3 U/ml). Positive agreement rates
were 58.7% in all samples, 71.2% in esophageal samples, 73.6% in
colorectal samples and 35.1% in breast samples (Table I). Negative agreement rates for
each cancer type were ≥97.1%, and overall agreement rates were
≥92.3% (Table I). Checking the
agreement between the two assay systems, 6 control and 16 cancer
samples were positive by Elecsys only, and 35 control and 39 cancer
samples were positive by MESACUP only (Table II).
 | Table IAgreement rates between Elecsys
(cutoff, 0.05 µg/ml) and MESACUP (cutoff, 1.3 U/ml). |
Table I
Agreement rates between Elecsys
(cutoff, 0.05 µg/ml) and MESACUP (cutoff, 1.3 U/ml).
| A, All
samplesa |
|---|
| | MESACUP, n | |
|---|
| Method | + | - | Total | Positive agreement
rate, % (95% CI) | Negative agreement
rate, (95% CI) | Overall agreement
rate, % (95% CI) |
|---|
| Elecsys, n | | | | 58.7 (51.1-66.0) | 98.2 (97.2-98.9) | 93.1 (91.6-94.3) |
|
+ | 105 | 22 | 127 | | | |
|
- | 74 | 1,183 | 1,257 | | | |
|
Total | 179 | 1,205 | 1,384 | | | |
| B, Esophageal
samplesb |
| | MESACUP, n | |
| Method | + | - | Total | Positive agreement
rate, % (95% CI) | Negative agreement
rate, (95% CI) | Overall agreement
rate, % (95% CI) |
| Elecsys, n | | | | 71.2 (59.4-81.2) | 97.1 (94.6-98.7) | 92.3 (89.1-94.7) |
|
+ | 52 | 9 | 61 | | | |
|
- | 21 | 306 | 327 | | | |
|
Total | 73 | 315 | 388 | | | |
| C, Colorectal
samplesc |
| | MESACUP, n | |
| Method | + | - | Total | Positive agreement
rate, % (95% CI) | Negative agreement
rate, (95% CI) | Overall agreement
rate, % (95% CI) |
| Elecsys, n | | | | 73.6 (59.7-84.7) | 97.5 (95.0-99.0) | 93.7 (90.6-96.1) |
|
+ | 39 | 7 | 46 | | | |
|
- | 14 | 275 | 289 | | | |
|
Total | 53 | 282 | 335 | | | |
| D, Breast
samplesd |
| | MESACUP, n | |
| Method | + | - | Total | Positive agreement
rate, % (95% CI) | Negative agreement
rate, (95% CI) | Overall agreement
rate, % (95% CI) |
| Elecsys, n | | | | 35.1 (20.2-52.5) | 99.0 (97.3-99.7) | 93.3
(90.5-95.5) |
|
+ | 13 | 4 | 17 | | | |
|
- | 24 | 378 | 402 | | | |
|
Total | 37 | 382 | 419 | | | |
 | Table IIBreakdown of agreement between
Elecsys (cutoff, 0.05 µg/ml) and MESACUP (cutoff, 1.3 U/ml). |
Table II
Breakdown of agreement between
Elecsys (cutoff, 0.05 µg/ml) and MESACUP (cutoff, 1.3 U/ml).
| A, Controls |
|---|
| Volunteers and
patients | Elecsys +/ MESACUP
+, n | Elecsys +/ MESACUP
-, n | Elecsys-/ MESACUP
+, n | Elecsys-/ MESACUP
-, n | Total, n |
|---|
| Healthy
volunteers | 1 | 0 | 10 | 126 | 137 |
| Autoimmune
diseases | 0 | 2 | 5 | 98 | 105 |
| Esophageal benign
diseases | 1 | 2 | 6 | 91 | 100 |
| Colorectal benign
diseases | 2 | 2 | 5 | 91 | 100 |
| Breast benign
diseases | 0 | 0 | 9 | 81 | 90 |
| Subtotal | 4 | 6 | 35 | 487 | 532 |
| B, Cancer |
| Volunteers and
patients | Elecsys +/ MESACUP
+, n | Elecsys +/ MESACUP
-, n | Elecsys-/ MESACUP
+, n | Elecsys-/ MESACUP
-, n | Total, n |
| Esophageal
cancer | 51 | 7 | 15 | 215 | 288 |
| Colorectal
cancer | 37 | 5 | 9 | 184 | 235 |
| Breast cancer | 13 | 4 | 15 | 297 | 329 |
| Subtotal | 101 | 16 | 39 | 696 | 852 |
| Total | 105 | 22 | 74 | 1,183 | 1,384 |
Table III shows
the determinations for s-p53-Abs of the two assay systems for each
cancer type when compared with the cutoff values of 0.05 µg/ml for
Elecsys and 1.3 U/ml for MESACUP. Those specimens that were above
the cutoff were defined as positive, and those below the cutoff
were defined as negative. Of the 852 patients with cancer, 117 were
positive by Elecsys and 140 were positive by MESACUP. Of the 532
control subjects, 10 were positive by Elecsys and 39 were positive
by MESACUP. In general, compared with MESACUP, Elecsys exhibited
lower sensitivities and higher specificities. This tendency was
observed in esophageal, colorectal and breast cancer. The two assay
systems significantly discriminated patients with cancer from
control subjects overall (P<0.001), but MESACUP did not find a
significant difference (P=0.3742) between the breast cancer and
control cases.
 | Table IIIDetermination of the serum anti-p53
antibodies of the two assay systems with the cutoff in the package
insert. |
Table III
Determination of the serum anti-p53
antibodies of the two assay systems with the cutoff in the package
insert.
| A, All
samplesa |
|---|
| Method | Cancer, n | Control, n | Total, n | P-value |
|---|
| Elecsys | | | | |
|
+ | 117 | 10 | 127 | |
|
- | 735 | 522 | 1,257 | |
|
Total | 852 | 532 | 1,384 | <0.0001 |
| MESACUP | | | | |
|
+ | 140 | 39 | 179 | |
|
- | 712 | 493 | 1,205 | |
|
Total | 852 | 532 | 1,384 | <0.0001 |
| B, Esophageal
samplesb |
| Method | Cancer, n | Control, n | Total, n | |
| Elecsys | | | | |
|
+ | 58 | 6 | 64 | |
|
- | 230 | 336 | 566 | |
|
Total | 288 | 342 | 630 | <0.0001 |
| MESACUP | | | | |
|
+ | 66 | 23 | 89 | |
|
- | 222 | 319 | 541 | |
|
Total | 288 | 342 | 630 | <0.0001 |
| C, Colorectal
samplesc |
| Method | Cancer, n | Control, n | Total, n | |
| Elecsys | | | | |
|
+ | 42 | 7 | 49 | |
|
- | 193 | 335 | 528 | |
|
Total | 235 | 342 | 577 | <0.0001 |
| MESACUP | | | | |
|
+ | 46 | 23 | 69 | |
|
- | 189 | 319 | 508 | |
|
Total | 235 | 342 | 577 | <0.0001 |
| D, Breast
samplesd |
| Method | Cancer, n | Control, n | Total, n | |
| Elecsys | | | | |
|
+ | 17 | 3 | 20 | |
|
- | 312 | 329 | 641 | |
|
Total | 329 | 332 | 661 | 0.001 |
| MESACUP | | | | |
|
+ | 28 | 25 | 53 | |
|
| | | | |
|
- | 301 | 307 | 608 | |
|
Total | 329 | 332 | 661 | 0.3742 |
Comparison of the sensitivity and
specificity between the two assay systems
As shown in Tables
II and III, when comparing
the assays using the cutoff values of the package inserts, Elecsys
tended to demonstrate a higher specificity, whereas MESACUP tended
to demonstrate a higher sensitivity. To facilitate a head-to-head
comparison, the performance of the two assay systems was compared
using the cutoff values when the specificities were aligned for all
samples (specificity, 98.1%; Elecsys: 0.05 µg/ml; MESACUP: 5.3
U/ml; Tables IV and V), as aligning with the specificity of
MESACUP would have resulted in an Elecsys cutoff value that was
below the lower end of the measuring range. Also, Table IV shows the determinations for
s-p53-Abs of the two assay systems with the aligned cutoff as in
Table III. As shown in Tables III and IV, by setting the cutoff value of
MESACUP higher than the cutoff value in the package insert, the
results of 78 subjects (healthy volunteers, n=9; autoimmune
diseases, n=1; esophageal benign diseases, n=5; colorectal benign
diseases, n=6; breast benign diseases, n=8; esophageal cancer,
n=21; colorectal cancer, n=10; breast cancer, n=18) changed from
positive to negative.
 | Table IVDetermination of the serum anti-p53
antibodies of the two assay systems with the aligned cutoff for
MESACUP of 5.3 U/ml. |
Table IV
Determination of the serum anti-p53
antibodies of the two assay systems with the aligned cutoff for
MESACUP of 5.3 U/ml.
| A, All
samplesa |
|---|
| MESACUP | Cancer, n | Control, n | Total, n |
|---|
| + | 91 | 10 | 101 |
| - | 761 | 522 | 1,283 |
| Total | 852 | 532 | 1,384 |
| B, Esophageal
samplesb |
| MESACUP | Cancer, n | Control, n | Total, n |
| + | 45 | 2 | 47 |
| - | 243 | 98 | 341 |
| Total | 288 | 100 | 388 |
| C, Colorectal
samplesc |
| MESACUP | Cancer, n | Control, n | Total, n |
| + | 36 | 1 | 37 |
| - | 199 | 99 | 298 |
| Total | 235 | 100 | 335 |
| D, Breast
samplesd |
| MESACUP | Cancer, n | Control, n | Total, n |
| + | 10 | 1 | 11 |
| - | 319 | 89 | 408 |
| Total | 329 | 90 | 419 |
 | Table VSensitivity and specificity between
the two assay systems [Elecsys (cutoff, 0.05 µg/ml) and MESACUP
(cutoff, 1.3 U/ml)] with the aligned cutoff. |
Table V
Sensitivity and specificity between
the two assay systems [Elecsys (cutoff, 0.05 µg/ml) and MESACUP
(cutoff, 1.3 U/ml)] with the aligned cutoff.
| A, All
samplesa |
|---|
| Diagnostic
accuracy | Elecsys, % (95%
CI) | MESACUP, % (95%
CI) | P-value |
|---|
| Sensitivity | 13.7
(11.5-16.2) | 10.7
(8.7-13.0) | <0.001 |
| Specificity | 98.1
(96.6-99.1) | 98.1
(96.6-99.1) | 1.000 |
| B, Esophageal
samplesb |
| Diagnostic
accuracy | Elecsys, % (95%
CI) | MESACUP, % (95%
CI) | P-value |
| Sensitivity
(All) | 20.1
(15.7-25.2) | 15.6
(11.6-20.3) | 0.002 |
| Sensitivity at
stage I | 11.9
(4.9-22.9) | 10.2
(3.8-20.8) | >0.999 |
| Sensitivity at
stage II | 24.4
(12.9-39.5) | 15.6
(6.5-29.5) | 0.219 |
| Sensitivity at
stage III | 21.0
(14.5-28.8) | 18.8
(12.7-26.4) | 0.375 |
| Sensitivity at
stage IV | 27.5
(14.6-43.9) | 15.0
(5.7-29.8) | 0.063 |
| Specificity | 97.0
(91.5-99.4) | 98.0
(93.0-99.8) | >0.999 |
| C, Colorectal
samplesc |
| Diagnostic
accuracy | Elecsys, % (95%
CI) | MESACUP, % (95%
CI) | P-value |
| Sensitivity
(All) | 17.9
(13.2-23.4) | 15.3
(11.0-20.6) | 0.210 |
| Sensitivity at
stage I | 10.0
(3.3-21.8) | 12.0
(4.5-24.3) | >0.999 |
| Sensitivity at
stage II | 18.6
(10.3-29.7) | 14.3
(7.1-24.7) | 0.250 |
| Sensitivity at
stage III | 17.1
(9.7-27.0) | 15.9
(8.7-25.6) | >0.999 |
| Sensitivity at
stage IV | 33.3
(17.3-52.8) | 23.3
(9.9-42.3) | 0.250 |
| Specificity | 96.0
(90.1-98.9) | 99.0
(94.6-100.0) | 0.375 |
| D, Breast
samplesd |
| Diagnostic
accuracy | Elecsys, % (95%
CI) | MESACUP, % (95%
CI) | P-value |
| Sensitivity
(All) | 5.2 (3.0-8.1) | 3.0 (1.5-5.5) | 0.039 |
| Sensitivity at
stage I | 3.1 (0.4-10.7) | 1.5 (0.00-8.3) | >0.999 |
| Sensitivity at
stage II | 5.3 (2.3-10.2) | 3.3 (1.1-7.6) | 0.375 |
| Sensitivity at
stage III | 7.4 (3.0-14.6) | 4.2 (1.2-10.4) | 0.250 |
| Sensitivity at
stage IV | 0.0 (0.0-23.2) | 0.0 (0.0-23.2) | 1.00 |
| Specificity | 0.0 (0.0-70.8) | 0.0 (0.0-70.8) | 1.00 |
The sensitivities of Elecsys for all samples (13.7
vs. 10.7%; P<0.001), esophageal samples (20.1 vs. 15.6%;
P=0.002) and breast samples (5.17 vs. 3.04%; P=0.039) were
significantly higher than those of MESACUP (Table V). Although the sensitivity of
Elecsys was higher than that of MESACUP for the colorectal samples
(17.9 vs. 15.3; P=0.210), the difference was not considered
significant. The sensitivities for each stage of cancer tended to
be higher for Elecsys, except for colorectal cancer (stage I);
however, none of the differences were significant. The specificity
adjusted for all samples was not significantly different between
the two assay systems for each sample (Table V).
Discussion
In this study, the sensitivities and specificities
of a new ECLIA-based assay (Elecsys) and an existing ELISA-based
assay (MESACUP) were compared using a large number of cancer
(n=852) and control (n=532) samples, in a multi-institutional
study.
The two assay systems could clearly distinguish
between patients with cancer and those with benign disease, and a
correlation (r=0.674) was found between the two assay systems. The
remaining differences can partially be explained by the
characteristics of the systems, such as the differences in
detection antigens, units of measurement and quantitative factors
(3,12). For example, whereas MESACUP uses
the full-length p53 protein, Elecsys uses three different peptides
containing epitopes of the p53 protein that are recognized through
anti-p53 antibodies (3,12). This can lead to differences in
reactivity to the antibodies in each method (3,12).
Therefore, no conversion factor between the two products can be
provided.
Conversely, Table I
shows a relatively low positive agreement rate for all samples
[58.7%; 95% confidence interval (CI), 51.1-66.0] due to the lower
positive agreement rate observed in the breast cancer group (35.1%;
95% CI, 20.2-52.5). Indeed, Table
II shows that MESACUP detected 9 of the 90 (10%) patients with
benign breast disease as false-positives, whereas Elecsys detected
no patients with benign breast disease. Table I shows that there were relatively
good positive rate agreements between the two methods for the
esophageal (71.2%; 95% CI, 59.4-81.2) and colorectal (73.6%; 95%
CI, 59.7-84.7) cancer types. However, when MESACUP was compared
with Elecsys, higher false-positive rates were observed overall,
including those for healthy volunteers and autoimmune diseases
(Tables II and III). False-positive results may lead to
unnecessary invasive procedures, such as biopsies, to confirm the
diagnosis, and a higher specificity for an assay is desirable for
daily clinical use, especially when tumor markers are used in
combination. Different positivity rates of patients with cancer
could also influence the inconsistency in results between the two
methods. Table II shows that
MESACUP missed a total of 16 patients with cancer, counting them as
false-negatives, whereas the results were positive with Elecsys. In
addition, Elecsys missed 39 patients with cancer, whereas positive
results were obtained using MESACUP. A comparison of the cutoff
values listed in the package inserts may suggest that Elecsys was
developed with a focus on specificity, whereas MESACUP may focus on
sensitivity.
To assess the diagnostic accuracy between the two
methods, the clinical performance (sensitivity and specificity)
after aligning the specificity of the two assay systems was
compared (Fig. 2; Tables III and IV). A total of 49 samples from patients
with cancer and 29 samples from controls changed status from
positive to negative when applying the convetntional MESACUP assay.
The new Elecsys assay was shown to demonstrate significantly higher
sensitivity than MESACUP for esophageal and breast cancer. In
addition, Elecsys was shown to be more sensitive than MESACUP in
colorectal cancer, although the difference was not considered
significant. These results indicate that in daily clinical
practice, Elecsys performs as well as the MESACUP for the detection
of esophageal and colorectal cancer.
Elecsys exhibits low sensitivity as a single-marker
test, but its high specificity (≥96.0%) allows its effective use in
combination with other tumor markers, such as carcinoembryonic
antigen (CEA), cytokeratin 19 fragment and squamous cell carcinoma
antigen. Moreover, when combined with other tumor markers, Elecsys
showed increased sensitivity in esophageal and colorectal cancer
(12). The routine clinical use of
anti-p53 in combination with other tumor markers is facilitated by
its availability on an automated platform that allows s-p53-Abs to
be measured simultaneously with multiple tumor markers vs. the
manual MESACUP. As a successor to MESACUP, the STACIA
MEBLux™ test anti-p53 (catalog no. 2385; Medical &
Biological Laboratories Co., Ltd.) has become commercially
available since starting the present study, and the reagent can be
run on an automated platform with the same cutoff value and the
same clinical performance (e.g., sensitivity and specificity) as
the manual MESACUP. However, only limited parameters are available
for analysis on the same platform. This means that the parallel
measurement of other tumor markers, such as CEA or CA19-9, must
rely on another platform, resulting in additional costs and reduced
testing efficiency.
The present study exhibited several limitations.
First, data on the immunoreactivity of p53 expression in the tumor
tissues were not evaluated, and no data were collected after
treatment. To evaluate tumor recurrence, it may be useful to
monitor the antibody titer changes over time using these two assay
systems. A prospective study should be conducted to confirm the
clinical significance and the practical usefulness of anti-p53
monitoring using Elecsys. Second, as aforementioned, the
accumulation of p53 in tumors and the subsequent immune response
that is associated with the strong immunogenicity of the p53
protein has already been reported (2-5).
Therefore, immunohistochemical staining to confirm the protein
expression status was not conducted in this study.
In conclusion, Elecsys was found to be as useful as
MESACUP and could be used to stratify patients with esophageal,
colorectal and breast cancer. Understanding the diagnostic accuracy
of tumor markers may facilitate the appropriate evaluation and
treatment of patients.
Acknowledgements
The authors would like to thank Dr Shinichi Kawai
(Department of Inflammation and Pain Control Research, School of
Medicine, Toho University, Tokyo, Japan), Dr Toshihiro Nanki and Dr
Sei Muraoka (Division of Rheumatology, Department of Internal
Medicine, School of Medicine, Toho University, Tokyo, Japan), Dr
Yoshihisa Urita (General Medicine and Emergency Center, School of
Medicine, Toho University, Tokyo, Japan), Dr Yoshihisa Saida
(Department of Surgery, Ohashi Medical Center, Toho University,
Tokyo, Japan), Dr Shinichi Okazumi (Department of Surgery, Sakura
Medical Center, Toho University, Tokyo, Japan), Dr Yuko Kitagawa,
Dr Yuki Hirata, Dr Tomoko Seki, Dr Hirotoshi Hasegawa and Dr Koji
Okabayashi (Department of General and Gastroenterological Surgery,
Keio University Hospital, Tokyo, Japan), Dr Masahiko Murakami, Dr
Takeshi Yamashita, Dr Rei Kato and Dr Yoko Kanada (Department of
Surgery, Showa University Hospital, Tokyo, Japan), Dr Goshi Oda and
Dr Yasuaki Nakajima (Esophageal Surgery, Medical Hospital, Tokyo
Medical and Dental University, Tokyo, Japan), and Dr Hisahiro
Matsubara and Dr Kentaro Murakami (Department of Frontier Surgery,
Graduate School of Medicine, Chiba University, Chiba, Japan) for
sample and data collection. The authors would also like to thank
Ms. Seiko Otsuka (Department of Surgery, School of Medicine, Toho
University, Japan) for providing technical assistance. Elecsys is a
trademark of Roche. All other product names and trademarks are the
property of their respective owners.
Funding
Funding: This study was supported by JSPS KAKENHI (grant no.
JP16K10520) and a research grant from Roche Diagnostics.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TS, YO, SY, HO, HSh and HSu were responsible for the
study design, and Hsu was responsible for performing the Elecsys.
SY, FS, MS, HO, TH, SN, TNak, TNan and MU were responsible for
sample data collection and data analysis. ME performed the
statistical data analysis. TS, YO, HSh and ME confirm the
authenticity of all the raw data. TS, YO, and HSh drafted the
initial version of the manuscript. All authors critically reviewed
the manuscript, edited and approved the final version.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee of Toho University Graduate School of Medicine
(Tokyo, Japan; approval no. A16049) and the Institutional Review
Boards of each participating hospital. Serum was collected from
patients who had provided written informed consent.
Patient consent for publication
Written informed consent for publication was
collected.
Competing interests
HSh received research funding from Ono
Pharmaceutical, Taiho Pharmaceutical and Roche Diagnostics K.K. HSu
was an employee of Roche Diagnostics K.K. ME is an employee of
Roche Diagnostics GmbH. The Elecsys assay system is manufactured by
Roche Diagnostics K. K.
References
|
1
|
Soussi T: p53 antibodies in the sera of
patients with various types of cancer: A review. Cancer Res.
60:1777–1788. 2000.PubMed/NCBI
|
|
2
|
Suppiah A and Greenman J: Clinical utility
of anti-p53 auto-antibody: Systematic review and focus on
colorectal cancer. World J Gastroenterol. 19:4651–4670.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shimada H, Ochiai T and Nomura F: Japan
p53 Antibody Research Group. Titration of serum p53 antibodies in
1,085 patients with various types of malignant tumors: A
multiinstitutional analysis by the Japan p53 antibody research
group. Cancer. 97:682–689. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shimada H: p53 molecular approach to
diagnosis and treatment of esophageal squamous cell carcinoma. Ann
Gastroenterol Surg. 2:266–273. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tokunaga R, Sakamoto Y, Nakagawa S,
Yoshida N and Baba H: The utility of tumor marker combination,
including serum P53 antibody, in colorectal cancer treatment. Surg
Today. 47:636–642. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Takashi S, Satoshi Y, Akihiko O, Naoya Y,
Yusuke T, Kentaro M, Yu O, Yasuaki N, Koichi Y, Takashi F, et al:
Clinical impact of preoperative serum p53 antibody titers in 1487
patients with surgically treated esophageal squamous cell
carcinoma: A multi-institutional study. Esophagus. 18:65–71.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ushigome M, Shimada H, Miura Y, Yoshida K,
Kaneko T, Koda T, Nagashima Y, Suzuki T, Kagami S and Funahashi K:
Changing pattern of tumor markers in recurrent colorectal cancer
patients before surgery to recurrence: Serum p53 antibodies, CA19-9
and CEA. Int J Clin Oncol. 25:622–632. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yamashita K, Makino T, Tanaka K, Yamasaki
M, Yamamoto M, Miyazaki Y, Takahashi T, Kurokawa Y, Nakajima K,
Takiguchi S, et al: Peritherapeutic serum p53 antibody titers are
predictors of survival in patients with esophageal squamous cell
carcinoma undergoing neoadjuvant chemotherapy and surgery. World J
Surg. 4:1566–1574. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Suzuki T, Yajima S, Ishioka N, Nanami T,
Oshima Y, Washizawa N, Funahashi K, Otsuka S, Nemoto T and Shimada
H: Prognostic significance of high serum p53 antibody titers in
patients with esophageal squamous cell carcinoma. Esophagus.
15:294–300. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kubota Y, Shimada H, Saito F, Nemoto T,
Ogata H and Kaneko H: Perioperative monitoring of serum p53
antibody titers in Japanese women undergoing surgical treatment
After neoadjuvant chemotherapy for locally advanced breast cancer.
Toho J Med. 3:58–65. 2017.
|
|
11
|
Blackburn GF, Shah HP, Kenten JH, Leland
J, Kamin RA, Link J, Peterman J, Powell MJ, Shah A, Talley DB, et
al: Electrochemiluminescence detection for development of
immunoassays and DNA probe assays for clinical diagnostics. Clin
Chem. 37:534–539. 1991.PubMed/NCBI
|
|
12
|
Yajima S, Suzuki T, Oshima Y, Shiratori F,
Funahashi K, Kawai S, Nanki T, Muraoka S, Urita Y, Saida Y, et al:
New assay system Elecsys anti-p53 to detect serum anti-p53
antibodies in esophageal cancer patients and colorectal cancer
patients: Multi-institutional study. Ann Surg Oncol. 28:4007–4015.
2021.PubMed/NCBI View Article : Google Scholar
|