Introduction
Infantile hemangioma is a benign vascular neoplasm
with abnormal proliferation of vascular endothelial cells. It is
the most common benign tumor in infants and children, with an
incidence of 4-5% (1,2). The development of infantile
hemangioma can generally be divided in two phases, namely, the
proliferative phase and the involuting phase. The majority of
hemangiomas can naturally subside after 1-5 years without the need
for intervention (3); however,
~10% of infantile hemangiomas with a particular location and large
size may develop functional or life-threatening complications
(4). Treatment of extensive
hemangioma is diverse and difficult, and is accompanied with a risk
of scar formation, organ dysfunction or tumor recurrence after
medical intervention (5). With the
continuous development and progress of gene therapy research,
numerous studies have focused on the exploration of target genes,
and various genes associated with hemangioma proliferation and
differentiation have been identified (6,7).
However, the molecular mechanism that controls the proliferation
and differentiation of hemangioma is not well understood.
Annexin A1 (ANX A1) is a 37-kDa calcium and
phospholipid-binding protein involved in a variety of biological
processes, including inflammation, cell proliferation and apoptosis
(8). ANX A1 was initially widely
studied in inflammatory reactions, but subsequent studies found
that the change in the expression level of the protein was
associated with the formation and development of different types of
malignant tumors (8,9). The expression changes of the ANX A1
gene in different cell lines vary. It has been reported that the
expression of ANX A1 was significantly increased in colorectal
cancer, melanoma, pancreatic cancer and hepatocellular carcinoma
(10-13),
while it was reduced in esophageal and prostate cancer (14). ANX A1 was reported to play a key
role in the differentiation of various types of cells. During the
differentiation of oral squamous cell carcinoma, it has been
demonstrated that the expression of ANX A1 increases, while
downregulation of ANX A1 significantly inhibits the differentiation
process (15). Upregulation of ANX
A1 was observed in the differentiation process of C2C12 myoblasts,
which induces the transforming growth factor-β signaling pathway
in-turn promoting differentiation (16). However, Vishwanatha et al
(17) reported that knocking down
the ANX A1 gene promoted B lymphocytes to differentiate into
non-Hodgkin's lymphoma-like cells. The aforementioned studies have
confirmed that ANX A1 plays an important role in regulating
tumorigenesis.
However, the study of ANX A1 protein in infantile
hemangioma has not been reported. Whether the proliferation and
differentiation of infantile hemangioma are associated with ANX A1
is worthy of investigation. In the present study, proliferating and
involuting infantile hemangioma tissues were collected to detect
the expression of ANX A1, and to explore the significance of ANX A1
in the process of proliferation or differentiation of
hemangioma.
Materials and methods
Reagents and antibodies
The following antibodies were used for
immunohistochemical and western blot analyses in the present study:
Anti-hypoxia-inducible factor (HIF)-1α antibody (product code
ab51608; Abcam), anti-ANX A1 antibody (product code ab88865;
Abcam), anti-phosphorylated (p)-44/42 mitogen-activated protein
kinase (MAPK) (Thr202/Tyr204) rabbit monoclonal antibody (mAb)
(product no. 4370; Cell Signaling Technology, Inc.), anti-p44/42
MAPK rabbit mAb (product no. 4695; Cell Signaling Technology,
Inc.), anti-acetyl-α-tubulin rabbit mAb (product no. 5335; Cell
Signaling Technology, Inc.), goat anti-rabbit IgG H&L
(DyLight® 488; product code ab150077; Abcam), goat
anti-mouse (DyLight® 647; product code ab150115; Abcam)
and goat anti-rabbit/mouse IRDye-800CW secondary antibodies
(product nos. 926-32211 and 926-32210, respectively; LI-COR
Biosciences). The immunohistochemical staining kit (cat. no.
D01-18) was purchased from OriGene Technologies, Inc.
Collection of clinical specimens
A total of 30 patients with hemangioma admitted to
Minzu Hospital of Guangxi Zhuang Autonomous Region (Nanning, China)
between March 2019 and October 2020 were selected as research
subjects. The age of the patients ranged between 6 months and 5
years. The cohort consisted of male and female patients (13 males
and 17 females) of Asian ethnicity and different body weights. None
of the patients had received any treatment before the surgery.
Pathological examination was used to confirm the diagnosis of
hemangioma after surgery. In addition, normal skin tissues of
patients with cleft lip were excised during cheiloplasty and used
as a negative control in the present study.
The use of tissue samples in the present study was
conducted with the authorization of the patients or their
relatives. The present study was approved by the Bioethics
Committee of Minzu Hospital of Guangxi Zhuang Autonomous Region
[approval no. (2018)12; Nanning, China].
Hematoxylin and eosin (H&E)
staining to identify hemangioma pathologically
Hemangioma tissues were preserved in a 10% neutral
buffered formalin at room temperature for 24 h. The tissues were
embedded in paraffin and cut into slices with a thickness of 5 µm.
After permeabilization and dehydration, the slices were washed with
PBS three times, and then incubated with a hematoxylin dye solution
at room temperature for 5 min. Subsequently, the tissue sections
were stained with eosin at room temperature for 2 min. The
H&E-stained sections of hemangioma tissues were examined under
a light microscope (BX53; Olympus Corporation).
Immunohistochemical analysis
Hemangioma specimens were fixed in 10% neutral
buffered formalin at room temperature for 24 h, and then were
embedded in paraffin and cut into slices of 5 µm in thickness.
Paraffin sections were deparaffinized and incubated with 5% normal
goat serum (product no. PH0424; Phygene Scientific) for 10 min at
room temperature. After blocking and incubation with a mouse
anti-ANX A1 mAb (at 1:500 dilution; product no. ab88865; Abcam) at
4˚C overnight, the sections were washed three times with PBS and
then incubated with streptavidin-peroxidase-conjugated secondary
antibodies (product no. D01-18; OriGene Technologies, Inc.) at room
temperature for 10 min. The specimens were incubated for 5 min with
a 3,3'-diaminobenzidine solution. Positively stained cells were
detected under a light microscope. The expression levels of ANX A1
were quantified by determining the integral optical density (IOD)
using Image Pro Plus 6.0 (Media Cybernetics, Inc.).
Double-label immunofluorescence
The paraffin sections were prepared as previously
described for immunohistochemistry. Paraffin sections were
thoroughly washed in PBS and blocked with 1% BSA/10% normal goat
serum/0.3 M glycine in 0.1% PBS-Tween-20 at room temperature for 1
h. Sections were then incubated overnight at 4˚C with mouse
polyclonal to ANX A1 (product code ab88865; Abcam) and rabbit
polyclonal to HIF-1α (cat. no. ab51608; Abcam) antibodies at a
dilution of 1:200. The secondary antibodies used were goat
anti-rabbit IgG H&L (DyLight® 488) and goat
anti-mouse (DyLight® 647) at a dilution of 1:500, at
room temperature for 1 h. Nuclei were counterstained with 5 µg/ml
of DAPI for 5 min at room temperature,. Protein expression was
observed using a fluorescence microscope.
Western blot analysis
Proteins were extracted from the hemangioma tissues
using a lysis buffer containing 0.5% Nonidet P-40, 10 mM Tris (pH
7.4), 150 mM NaCl, 1 mM EDTA and 1 mM Na3VO4.
Protein concentrations were determined using a BCA protein assay
kit. Total proteins (30-40 µg/lane) were separated by 10% SDS-PAGE
and then transferred onto nitrocellulose membranes for 1.5 h at 100
mA. Next, the membranes were blocked with BSA-Tween-20 (containing
3% TBS and 0.05% Tween-20) for 1 h at room temperature. Appropriate
dilutions of the primary antibodies against ANX A1 (1:1,000
dilution; product code ab88865; Abcam), HIF-1α (1:2,000 dilution;
product no. 5335), p-ERK1/2 (1:1,000 dilution; product no. 4370)
and total-ERK1/2 (1:1,000 dilution; product no. 4695; all from Cell
Signaling Technology, Inc.) were used to incubate the
nitrocellulose membranes at 4˚C overnight, respectively.
Subsequently, the membranes were incubated with a fluorescent
dye-conjugated secondary antibody IRDye-800CW (1:10,000 dilution;
product no. 926-32211 or 926-32210; LI-COR Biosciences) at room
temperature for 1 h. Blots were visualized using the Odyssey
Imaging System (LI-COR Biosciences). Densitometric analysis was
performed using Quantity One 4.6.2 software (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All experiments were repeated three times for data
analysis. The results are presented as the mean ± SD. An unpaired
Student's t-test was used to compare differences between two
groups, and an one-way ANOVA followed by a Tukey's post hoc test
was used to compare differences between three groups. All analyses
were performed using SPSS 25.0 software (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Histopathological analysis of the
hemangioma tissues
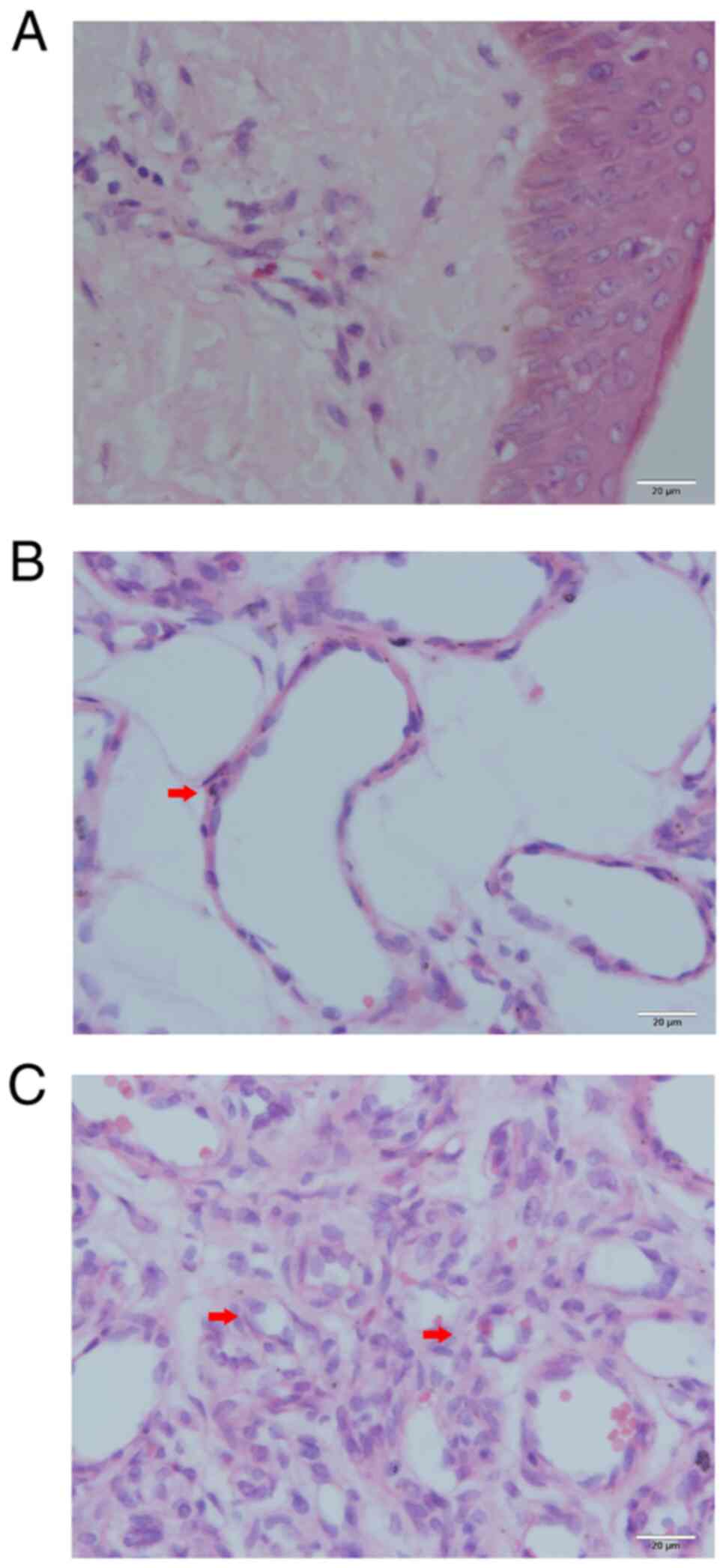
To observe the histopathological morphology of
hemangioma tissue sections, they were stained with H&E.
According to the morphology of vascular endothelial cells, the
hemangiomas were divided into a proliferative phase group and an
involution phase group. A total of 15 samples were classified as
proliferative hemangioma. Microscopic observation of proliferative
hemangioma revealed that the vascular endothelial cells were
densely packed and formed small capillaries (Fig. 1C). These endothelial cells
exhibited enlarged nuclei and an abundance of clear cytoplasm. The
proliferative capillaries formed a lobulated arrangement and were
separated by slender fibrous septa. The other 15 cases were
classified as the involution phase. The numbers of capillaries and
pericytes had decreased, which was accompanied by enlargement of
the vascular lumen (Fig. 1B). In
addition, apoptotic bodies were observed in the endothelial cells
and pericytes. By contrast, the normal skin of the control group
had few blood vessels with a small lumen (Fig. 1A).
Western blot analysis of ANX A1
expression
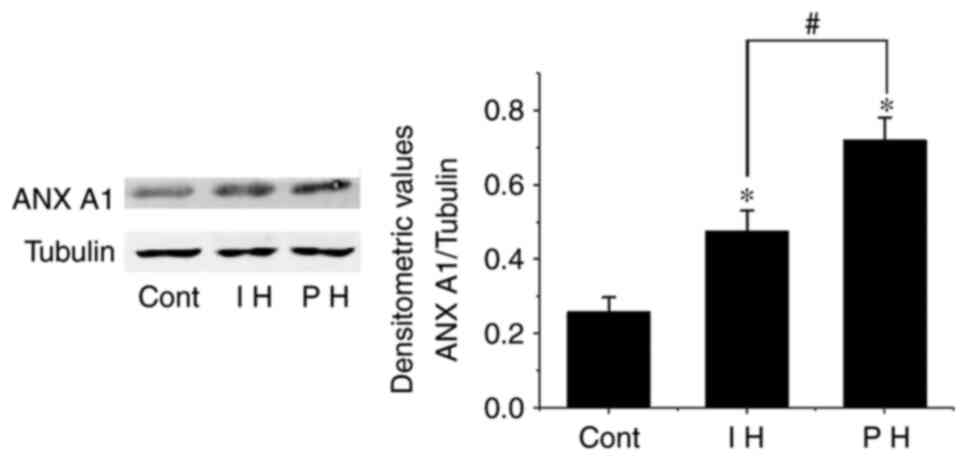
To detect the expression levels of ANX A1 in
hemangioma tissues in an accurate manner, western blotting was used
to detect the changes in the expression levels of ANX A1 in the
proliferative and involuting hemangioma tissues. The results
demonstrated that the expression levels of ANX A1 in both the
proliferating and involuting hemangioma tissues were higher than
those in the control group (normal skin tissue; P<0.01).
However, the expression levels of ANX A1 in involuting hemangioma
were lower than those in proliferating hemangioma (P<0.01;
Fig. 2). These data suggested that
increased ANX A1 expression may be associated with the development
of hemangioma.
Localization of ANX A1 in hemangioma
tissues
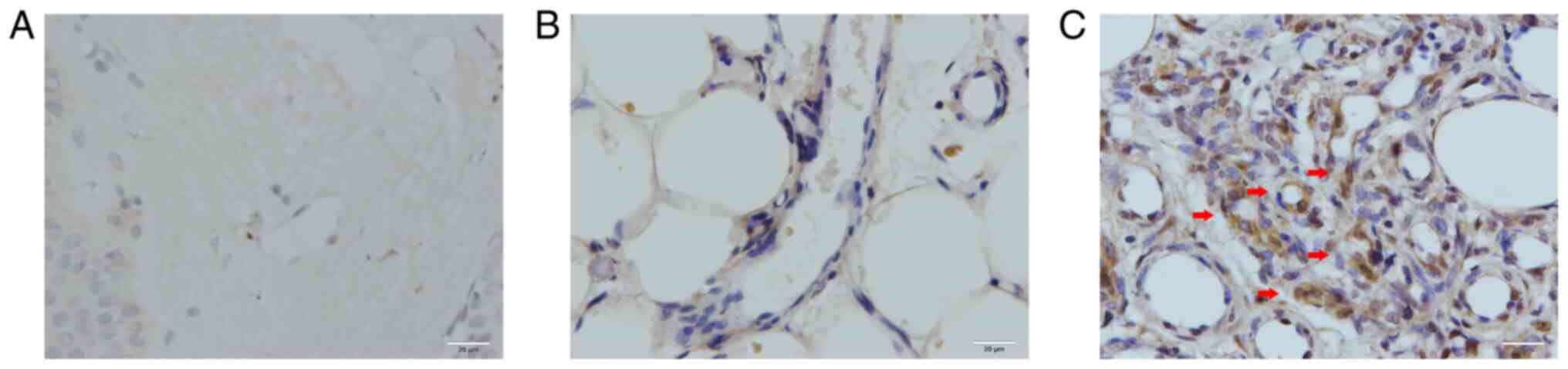
To determine the intracellular localization of ANX
A1, immunohistochemical analysis of hemangioma tissues was
performed. A total of 6 samples were randomly selected from each
group for immunohistochemical analysis. In the control group
(normal skin tissues), only a small number of fibroblasts were
positive for ANX A1 (Fig. 3A). As
revealed in Fig. 3B and C, ANX A1 was primarily expressed in the
nucleus and cytoplasm of endothelial cells at both the
proliferative and involution phases of hemangioma. In the
involution phase of hemangioma, ANX A1 was mainly expressed in the
endothelial cells, and its expression was markedly lower than that
in the proliferative phase. Of note, in the proliferative phase of
hemangioma, ANX A1 showed a strong positive reaction in the
endothelial cells of newly born capillaries. With the continuous
expansion of the capillary lumen, ANX1 A1 expression gradually
decreased. These results were consistent with those observed by
western blotting. The IOD data of ANX A1 are presented in Table I.
 | Table IExpression of Annexin A1 in hemangioma
and normal skin tissue. |
Table I
Expression of Annexin A1 in hemangioma
and normal skin tissue.
| Groups | IOD | No. of cases |
|---|
| Normal skin | 24.59±8.37 | 6 |
| Involuting
hemangioma |
55.82±9.80a | 6 |
| Proliferating
hemangioma |
126.09±20.65a,b | 6 |
HIF-1α expression in hemangioma
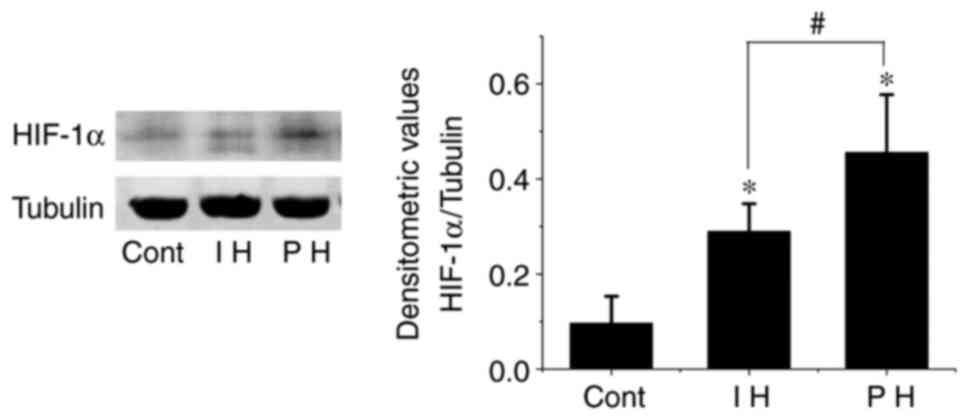
It has been demonstrated that hypoxia is one of the
mechanisms that induces proliferation of hemangioma endothelial
cells (18). HIF-1α is a key
regulator of hypoxia signal transduction (19). Therefore, HIF-1α expression in
hemangioma was investigated. It was observed that the expression
levels of HIF-1α in both proliferative and involuting hemangiomas
were elevated compared with those in the control group (P<0.01).
However, the expression levels of HIF-1α in the involuting phase
were significantly lower than those in the proliferative phase
(P<0.01; Fig. 4).
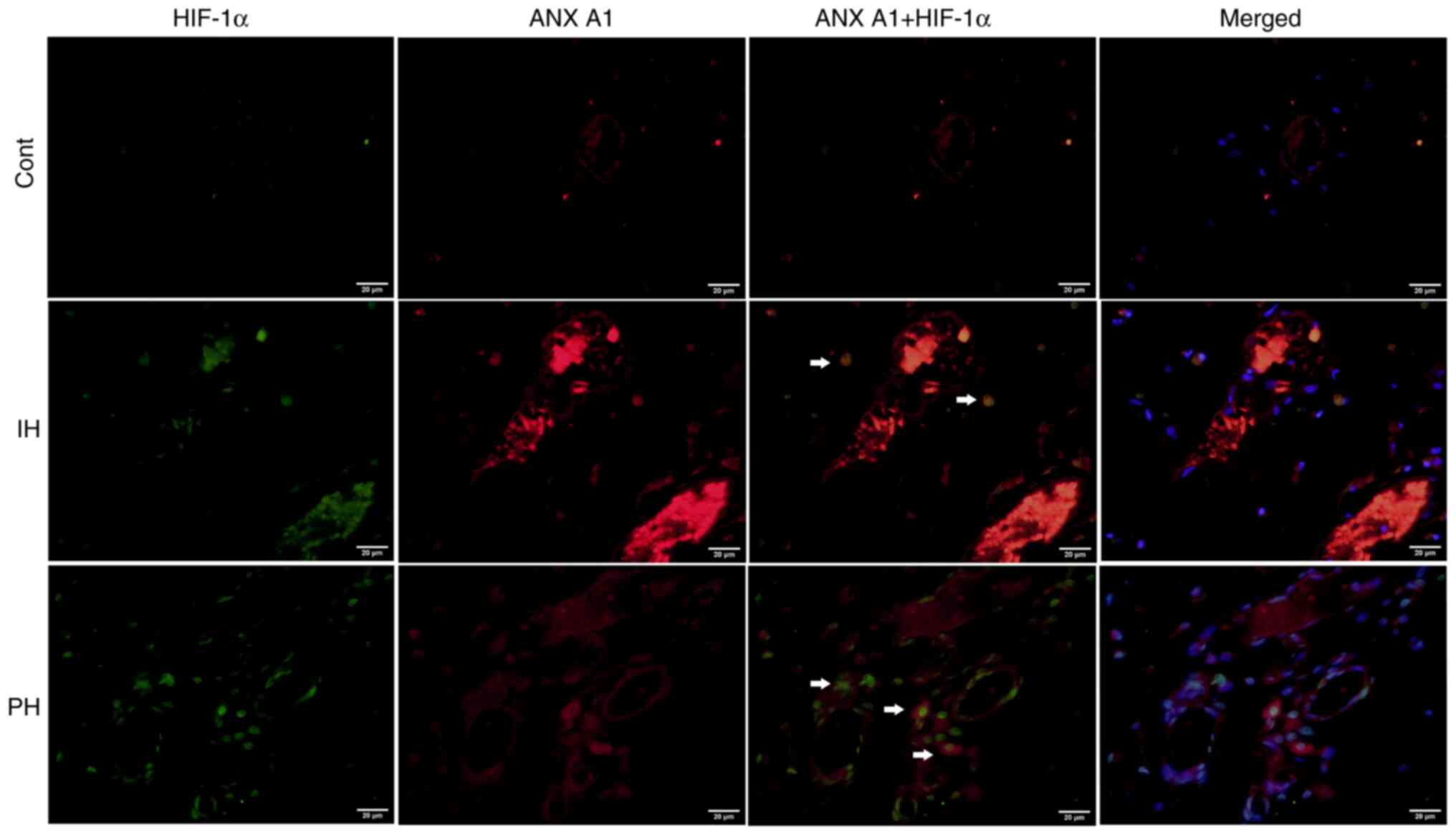
In order to determine whether ANX A1 expression is
associated to HIF-1α during the proliferation stage of hemangioma,
the double-label immunofluorescence method was used to detect the
localization of HIF-1α and ANX A1 expression in hemangioma tissues
simultaneously. As revealed in Fig.
5, HIF-1α was predominantly localized to the nucleus of
endothelial cells, and positive cells for both HIF-1α and ANX A1
were frequently observed in proliferative hemangiomas. Overall, the
number of double-labeled cells was significantly greater in
proliferative hemangioma tissues than in involuting hemangiomas
(proliferative hemangioma, 51.46±9.24% vs. involuting hemangioma,
6.51±3.75%; P<0.01). HIF-1α and ANX A1 co-labeled cells were not
detected in the control tissues. This phenomenon indicated that
high ANX A1 expression may be associated to the increase of
HIF-1α.
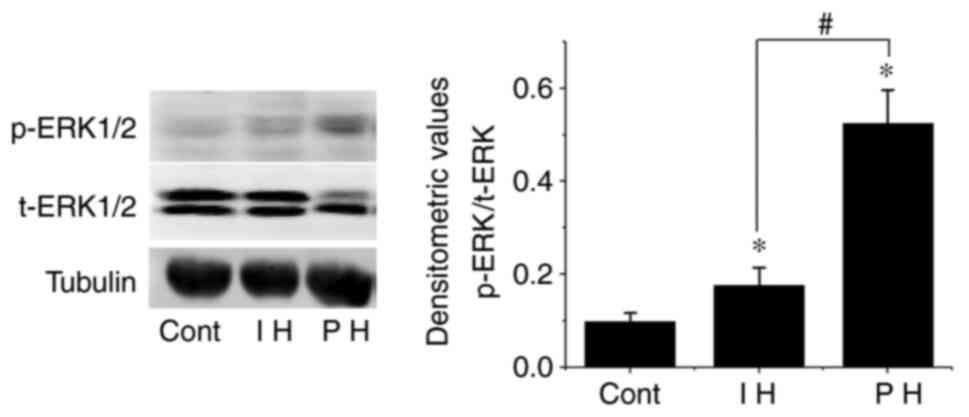
Phosphorylation of ERK1/2 in
hemangioma
Previous studies have reported that ANX A1 regulates
cell proliferation, apoptosis and differentiation by inducing the
activation of the ERK1/2 signaling pathway (8). Therefore, the present study further
explored the phosphorylation levels of the ERK1/2 pathway in
samples exhibiting high expression levels of ANX A1. The results
demonstrated that the phosphorylation levels of ERK1/2 in the
proliferative and involuting hemangioma tissues were significantly
higher than those in the control group, and the difference was
statistically significant (P<0.01). In addition, the
phosphorylation levels of ERK1/2 in involuting hemangiomas were
lower than those in proliferative hemangioma (P<0.01; Fig. 6).
Discussion
Endothelial cell proliferation and apoptosis are
considered to be associated with the pathogenesis of hemangioma
(20). The mechanism that
regulates the proliferation and migration of vascular endothelial
cells is complex and involves the regulation of multiple cytokines.
In previous studies, VEGF, basic fibroblast growth factor (bFGF),
glucose transporter 1 and MMP were found to be closely associated
with proliferation of hemangiomas (21-23).
There are numerous methods to detect the expression of these
factors in patients with infantile hemangiomas, e.g., by urine,
serum, or hemangioma tissue. The method of urine and serum
detection is non-invasive; however, the accuracy remains to be
verified. For example, serum VEGF and bFGF may exhibit different
trends in different research (24-26).
It is more direct and accurate to analyze gene or protein
expression in surgically removed hemangioma tissues. However, as a
popular protein in tumor research, ANX A1 has not yet been detected
in infantile hemangiomas. The present study revealed abnormal ANX
A1 expression in proliferating and involuting hemangiomas using
western blotting and immunohistochemical methods.
ANX A1 is one of the 13 members of the Annexin
superfamily. As an epidermal growth factor receptor substrate, ANX
A1 is involved in the processes of cell proliferation and migration
(8). ANX A1 is widely expressed in
multiple tissues, including epithelial and endothelial cells. A
previous study demonstrated that treatment with ANX
A12-26 increased angiogenesis and migration of
fibroblasts on a heterologous skin scaffold transplantation model.
Furthermore, ANX A12-26 was demonstrated to increase
endothelial cell migration and actin polymerization in vitro
(27). Yi and Schnitzer (28) found that absence of ANX A1
prevented the formation of blood vessels in tumors, and thus
inhibited the growth of tumors. These studies suggest that ANX A1
may be a key regulator in modulating the balance of pathological
and physiological angiogenesis. These findings suggest that ANX A1
may be involved in the proliferation or apoptosis of endothelial
cells in hemangiomas. In the present study, human hemangioma
tissues were collected to investigate whether there is a change in
ANX A1 expression in infantile hemangioma. First, ANX A1 expression
was examined by western blotting in different phases of hemangioma
development/progression. The results demonstrated that the highest
expression levels of ANX A1 were detected in proliferating
hemangioma tissues, and involuting hemangioma was associated with
lower expression levels of ANX A1, while normal skin tissues
exhibited the lowest expression levels of ANX A1. However,
hemangioma tissue contains a variety of cells, and western blotting
can only detect total tissue proteins. Therefore, to confirm the
observations from western blotting of hemangioma tissues and to
investigate the intracellular localization of ANX A1,
immunohistochemical analysis was performed to detect ANX A1
expression in different phases of hemangioma and in normal skin
tissues. It was revealed that infantile hemangiomas stained
positively for ANX A1 during both the proliferative and involution
phases, while the normal skin tissues exhibited barely detectable
staining of ANX A1. Notably, in the proliferative phase, the
strongest staining of ANX A1 was observed in newly born
capillaries, and the staining of ANX A1 became weaker in enlarged
vessels. This phenomenon suggested that ANX A1 may serve an
important role in promoting the formation of capillaries during the
proliferation of hemangioma. Taken together, the present results
provide original evidence for the increased expression of ANX A1 in
the development of infantile hemangiomas, indicating that the
higher the expression of this protein, the faster the hemangioma
grows.
It has been hypothesized that hypoxia may be a
pathogenesis of infantile hemangioma (18). When tissues suffer from hypoxia,
the expression of hypoxia-induced factors induced by vascular
endothelial cells, including MMP-9, VEGF-A and HIF-1α, promotes the
proliferation of vessels, thereby improving the blood supply of
hypoxic tissues (29,30). Under hypoxic conditions, the
protein levels of HIF-1α, which is one of the main transcription
factors, can rapidly increase and regulate the expression of
downstream hypoxia-responsive genes. It was revealed that hypoxia
induced ANX A1 protein expression, and ANX A1 expression decreased
when HIF-1α was inhibited, indicating that ANX A1 is a direct
regulatory target of HIF-1α (31,32).
The present study demonstrated that HIF-1α expression in
proliferating hemangioma was markedly higher than that in normal
skin tissues, and that HIF-1α expression was positively associated
with the expression trend of ANX A1. Furthermore,
immunofluorescence results revealed that ~51% of the HIF-1α cells
were co-labeled with ANX A1 in proliferating hemangioma. Therefore,
it was hypothesized that the upregulation of ANX A1 in the
proliferative phase of infantile hemangioma may be associated with
the increase in HIF-1α expression induced by hypoxia.
The present study revealed that the activation
levels of ERK1/2 in proliferating hemangiomas were markedly higher
than those in normal skin. As hemangiomas entered the involuting
phase, the activation of ERK1/2 decreased. MAPKs serve an important
role in cell proliferation and angiogenesis. ERKs are important
members of the MAPK family (33).
It has been demonstrated that ERK1/2 can regulate transcription
factors such as c-Jun and activator protein-1, which serve a key
role in regulating angiogenesis factors and inhibitors (34,35).
EGF receptor activation and internalization can lead to the
tyrosine phosphorylation of ANX A1, thereby targeting PI3K and
ERK/MAPK signaling, and exerting important signaling functions in
cell proliferation (36). Pin
et al (37) reported that
the proliferation and differentiation of vascular endothelial cells
were markedly inhibited after the application of microRNA-196a to
specifically inhibit ANX A1 expression, and this process was also
closely associated with the activation of the MAPK signaling
pathway. ANX A1 expression was upregulated in glioma cells, and
downregulation of ANX A1 inhibited glioma cell proliferation via
negative regulation of the activation of the PI3K/AKT signaling
pathway (38). The present study
revealed that the activation levels of ERK1/2 in proliferating
hemangiomas were markedly higher than those in normal skin. As
hemangiomas entered the involuting phase, the activation of ERK1/2
decreased. However, it has been revealed that hypoxia induced
phosphorylation of ERK1/2, and ERK1/2 inhibitors inhibited
hypoxia-induced HIF-1α expression or phosphorylation (39,40).
Therefore, whether the increase of ERK1/2 activity in proliferating
hemangiomas is involved in ANX A1 signaling or in hypoxia-induced
HIF-1 expression remains to be further investigated.
In summary, the results of the present study
demonstrated that the expression levels of ANX A1 were increased in
proliferating hemangioma tissues, and the high expression levels of
ANX A1 may be closely associated with the formation of capillaries
in infantile hemangioma. ANX A1 may become a marker for the
occurrence and development of infantile hemangioma in the future
and is expected to become a novel target for the combined genetic
treatment of infantile hemangioma.
Acknowledgements
Not applicable.
Funding
Funding: This article was supported mainly by the Natural
Science Foundation of Guangxi, China (grant no.
2018GXNSFBA138001).
Availability of data and materials
The data presented in this study are available on
request from the corresponding author.
Authors' contributions
XP conceived the present study. XP, HH and KW
performed the experiments and data collection. XT contributed to
the data analysis. XP was a major contributor to the preparation of
the manuscript. XP and HH confirm the authenticity of all the raw
data. All authors have read and agreed to the published version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Bioethics
Committee of Minzu Hospital of Guangxi Zhuang Autonomous Region
[approval no. (2018)12].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kanada KN, Merin MR, Munden A and
Friedlander SF: A prospective study of cutaneous fifindings in
newborns in the United States: Correlation with race, ethnicity,
and gestational status using updated classifification and
nomenclature. J Pediatr. 161:240–245. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kilcline C and Frieden IJ: Infantile
hemangiomas: How common are they? A systematic review of the
medical literature. Pediatr Dermatol. 25:168–173. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Couto RA, Maclellan RA, Zurakowski D and
Greene AK: Infantile hemangioma: Clinical assessment of the
involuting phase and implications for management. Plast Reconstr
Surg. 130:619–624. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nguyen HP, Pickrell BB and Wright TS:
Beta-blockers as therapy for infantile hemangiomas. Semin Plast
Surg. 28:87–90. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Priya C, Varshini C and Biswakumar B: Case
report: A rare case of infantile hemangioma, treated in a private
clinic as out patient. Prim Health Care. 9(321)2019.
|
|
6
|
Wu Y, Li H, Xie J, Wang F, Cao D and Lou
Y: miR-139-5p affects cell proliferation, migration and
adipogenesis by targeting insulin-like growth factor 1 receptor in
hemangioma stem cells. Int J Mol Med. 45:569–577. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jin W, Chen L, Gao F, Yang M, Liu Y and
Wang B: Down-regulation of miR-556-3p inhibits hemangioma cell
proliferation and promotes apoptosis by targeting VEGFC. Cell Mol
Biol (Noisy-le-grand). 66:204–207. 2020.PubMed/NCBI
|
|
8
|
Lim LH and Pervaiz S: Annexin 1: The new
face of an old molecule. FASEB J. 21:968–975. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Biaoxue R, Xiguang C and Shuanying Y:
Annexin A1 in malignant tumors: Current opinions and controversies.
Int J Biol Markers. 29:e8–e20. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rubinstein MR, Baik JE, Lagana SM, Han RP,
Raab WJ, Sahoo D, Dalerba P, Wang TC and Han YW: Fusobacterium
nucleatum promotes colorectal cancer by inducing Wnt/β-catenin
modulator Annexin A1. EMBO Rep. 20(e47638)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Boudhraa Z, Rondepierre F, Ouchchane L,
Kintossou R, Trzeciakiewicz A, Franck F, Kanitakis J, Labeille B,
Joubert-Zakeyh J, Bouchon B, et al: Annexin A1 in primary tumors
promotes melanoma dissemination. Clin Exp Metastasis. 31:749–760.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pessolano E, Belvedere R, Bizzarro V,
Franco P, Marco I, Porta A, Tosco A, Parente L, Perretti M and
Petrella A: Annexin A1 may induce pancreatic cancer progression as
a key player of extracellular vesicles effects as evidenced in the
in vitro MIA PaCa-2 model system. Int J Mol Sci.
19(3878)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lin Y, Lin G, Fang W, Zhu H and Chu K:
Increased expression of annexin A1 predicts poor prognosis in human
hepatocellular carcinoma and enhances cell malignant phenotype. Med
Oncol. 31(327)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Paweletz CP, Ornstein DK, Roth MJ, Bichsel
VE, Gillespie JW, Calvert VS, Vocke CD, Hewitt SM, Duray PH,
Herring J, et al: Loss of annexin 1 correlates with early onset of
tumorigenesis in esophageal and prostate carcinoma. Cancer Res.
60:6293–6297. 2000.PubMed/NCBI
|
|
15
|
Zhu DW, Yang X, Yang CZ, Ma J, Liu Y, Yan
M, Wang LZ, Li J, Zhang CP, Zhang ZY and Zhong LP: Annexin A1
down-regulation in oral squamous cell carcinoma correlates to
pathological differentiation grade. Oral Oncol. 49:542–550.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ge Y, Li S, Hu XY, Tong HL, Li SF and Yan
YQ: TCEA3 promotes differentiation of C2C12 cells via an Annexin
A1-mediated transforming growth factor-β signaling pathway. J Cell
Physiol. 234:10554–10565. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vishwanatha JK, Salazar E and
Gopalakrishnan VK: Absence of annexin I expression in B-cell
non-Hodgkin's lymphomas and cell lines. BMC Cancer.
4(8)2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
de Jong S, Itinteang T, Withers AH, Davis
PF and Tan ST: Does hypoxia play a role in infantile hemangioma?
Arch Dermatol Res. 308:219–227. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Adams JM, Difazio LT, Rolandelli RH, Luján
JJ, Haskó G, Csóka B, Selmeczy Z and Németh ZH: HIF-1: A key
mediator in hypoxia. Acta Physiol Hung. 96:19–28. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Marchuk DA: Pathogenesis of hemangioma. J
Clin Invest. 107:665–666. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Aydin Köker S, Kömüroğlu AU, Köksoy AY,
Şiraz ÜG, Tekin E and Köker A: Evaluation of GLUT1, IGF-2, VEGF,
FGF 1, and angiopoietin 2 in infantile hemangioma. Arch Pediatr.
28:296–300. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yin RR, Hao D and Chen P: Expression and
correlation of MMP-9, VEGF, and p16 in infantile hemangioma. Eur
Rev Med Pharmacol Sci. 22:4806–4811. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Thaivalappil S, Bauman N, Saieg A, Movius
E, Brown KJ and Preciado D: Propranolol-mediated attenuation of
MMP-9 excretion in infants with hemangiomas. JAMA Otolaryngol Head
Neck Surg. 139:1026–1031. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Şen HS, Yalçın B, Canpınar H, Ocak S and
Akyüz C: Serum levels of VEGF and bFGF in infantile hemangiomas
treated with propranolol. Turk J Pediatr. 62:979–985.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Park M, Jung HL, Shim YJ, Kim HS, Yoon HS,
Park SK, Cheuh HW, Lee MJ, Lee JM, Park ES, et al: Serum cytokine
profiles in infants with infantile hemangiomas on oral propranolol
treatment: VEGF and bFGF, potential biomarkers predicting clinical
outcomes. Pediatr Res. 88:749–755. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rotter A, Lima XT and Oliveira ZNP:
Evaluation of plasma and urinary levels of vascular endothelial
growth factor and matrix metalloproteinase-9 in patients with
infantile hemangioma. Int J Dermatol. 60:1263–1269. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lacerda JZ, Drewes CC, Mimura KKO, Zanon
CF, Ansari T, Gil CD, Greco KV, Farsky SHP and Oliani SM: Annexin
A12-26 treatment improves skin heterologous
transplantation by modulating inflammation and angiogenesis
processes. Front Pharmacol. 9(1015)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yi M and Schnitzer JE: Impaired tumor
growth, metastasis, angiogenesis and wound healing in annexin
A1-null mice. Proc Natl Acad Sci USA. 106:17886–17891.
2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ramakrishnan S, Anand V and Roy S:
Vascular endothelial growth factor signaling in hypoxia and
inflammation. J Neuroimmune Pharmacol. 9:142–160. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang X, Tang G and Sun H: Effect of
hypoxia on the proliferation and expressions of hypoxia-inducible
factor-1α, vascular endothelial growth factor and matrix
metalloproteinase-9 in keratinocytes obtained from oral lichen
planus lesions. Zhonghua Kou Qiang Yi Xue Za Zhi. 50:89–94.
2015.PubMed/NCBI(In Chinese).
|
|
31
|
Liao SH, Zhao XY, Han YH, Zhang J, Wang
LS, Xia L, Zhao KW, Zheng Y, Guo M and Chen GQ: Proteomics-based
identification of two novel direct targets of hypoxia-inducible
factor-1 and their potential roles in migration/invasion of cancer
cells. Proteomics. 9:3901–3912. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang F, Cai J, Zhan H, Situ J, Li W, Mao Y
and Luo Y: Suppression of TRPM7 inhibited hypoxia-induced migration
and invasion of androgen-independent prostate cancer cells by
enhancing RACK1-mediated degradation of HIF-1α. Oxid Med Cell
Longev. 2020(6724810)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Roskoski R Jr: ERK1/2 MAP kinases:
Structure, function, and regulation. Pharmacol Res. 66:105–143.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee CC, Chen SC, Tsai SC, Wang BW, Liu YC,
Lee HM and Shyu KG: Hyperbaric oxygen induces VEGF expression
through ERK, JNK and c-Jun/AP-1 activation in human umbilical vein
endothelial cells. J Biomed Sci. 13:143–156. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Catar R, Moll G, Hosp I, Simon M, Luecht
C, Zhao H, Wu D, Chen L, Kamhieh-Milz J, Korybalska K, et al:
Transcriptional regulation of thrombin-induced endothelial VEGF
induction and proangiogenic response. Cells. 10(910)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Poeter M, Radke S, Koese M, Hessner F,
Hegemann A, Musiol A, Gerke V, Grewal T and Rescher U: Disruption
of the annexin A1/S100A11 complex increases the migration and
clonogenic growth by dysregulating epithelial growth factor (EGF)
signaling. Biochim Biophys Acta. 1833:1700–1711. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pin AL, Houle F, Fournier P, Guillonneau
M, Paquet ÉR, Simard MJ, Royal I and Huot J: Annexin-1-mediated
endothelial cell migration and angiogenesis are regulated by
vascular endothelial growth factor (VEGF)-induced inhibition of
miR-196a expression. J Biol Chem. 287:30541–30551. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wei L, Li L, Liu L, Yu R, Li X and Luo Z:
Knockdown of Annexin-A1 Inhibits growth, migration and invasion of
glioma cells by suppressing the PI3K/Akt signaling pathway. ASN
Neuro. 13(17590914211001218)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Minet E, Arnould T, Michel G, Roland I,
Mottet D, Raes M, Remacle J and Michiels C: ERK activation upon
hypoxia: Involvement in HIF-1 activation. FEBS Lett. 468:53–58.
2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu L, Ning X, Han S, Zhang H, Sun L, Shi
Y, Sun S, Guo C, Yin F, Qiao T, et al: Hypoxia induced HIF-1
accumulation and VEGF expression in gastric epithelial mucosa
cells: Involvement of ERK1/2 and PI3K/Akt. Mol Biol (Mosk).
42:459–469. 2008.PubMed/NCBI(In Russian).
|