Introduction
Phytomedicine has been used in chemoprotection and
treatment of several types of cancer, due to the anti-proliferative
activity of numerous plant-based compounds (1). Numerous chemotherapeutics are
frequently obtained as derivatives of biological products used in
traditional medicine (2) (Table SI). Hence, using medicinal plants
with therapeutic properties is a valuable alternative and promising
for pharmaceutical agents (3).
Plants are abundant in a large collection of secondary metabolites
and phytochemical elements such as alkaloids, flavonoids, tannins
and terpenoids useful against various diseases (4-6).
Phytomedicines are proving to be an inexpensive source for
treatment and substantial precision than commercial
chemotherapeutic agents (7). As
the therapeutic options available for chronic and acute illnesses
are significantly reduced, the search for new chemotherapeutic
agents that may be applied as a treatment for these types of acute
and chronic diseases increases (8,9).
According to the reports from WHO, ~80% population from developing
countries have been using herbal products for primary ailments.
Numerous surveys also indicated that around 60% of patients with
cancer have used some form of natural products for treatment
(10-12).
However, only a few medicinal plants applied have been
scientifically assessed for their anticancer properties. The idea
of deriving therapeutic drugs from natural products is therefore
attractive for devising treatment strategies.
Andrographis paniculata (Ap) (Burm.f.) Nees
or ‘King of Bitters, is a small, branched, annual plant belonging
to the family Acanthaceae, has been used extensively in
Unani, Siddha and Ayurveda (Table
SI) (13). It is abundant in a
broad range of phytochemical constituents such as flavonoids,
diterpenes and lactones (12). In
traditional medicine it is used in the treatment of fever, sore
throat, upper respiratory tract and gastrointestinal diseases,
hepatitis and a range of other chronic illnesses as well as
infectious diseases (13,14). The herb and its components
andrographolide, isoandrographolide and neoandrographolide have
been reported to possess anti-inflammatory (15-17),
hepatoprotective, antidiabetic (18), antimalarial (19), antimicrobial (20), anti-HIV, immuno-stimulatory
(21) and anticancer activity
(22,23).
Constant pursuit for natural compounds with the
ability to target cancer cells is presently gathering much interest
in the field of oncology (24).
The andrographolide extracts have been revealed to suppress the
development of cancer cells by inhibiting kinase pathways
NF-kappaB, PI3K/AKT and induce apoptosis (25,26).
The crude dichloromethane extract of the plant inhibits colon
cancer cell proliferation and increased production of human
peripheral blood lymphocytes at smaller concentrations (27). Ap extracts are known to have
potential anti-metastatic and antitumor effect on esophageal cancer
(28). Andrographolide, the key
bioactive component of Ap has been shown to prevent the development
of a diversity of cancer cells corresponding to various types of
human cancer (29), including
leukemia, melanoma, lung and breast cancer (30,31).
Most of the aforementioned studies reported the activity of the
andrographolides components of the AP extracts. In the present
study, the anticancer activity of non-andrographolide components of
Ap extracts was evaluated and the bioactive compounds were
characterized. For the first time, to the best of our knowledge, a
new set of compounds from the Ap extracts whose extracts exhibit
anticancer activity was reported.
Materials and methods
Chemicals
MTT (cat. no. TC191) and dimethyl sulphoxide (DMSO;
cat. no. MB058) were purchased from HiMedia Laboratories, LLC.
RPMI-1640 medium and DMEM were procured from Hyclone; Cytiva.
EZdetect PCR kit for Mycoplasma Detection (cat. no. CCK009) and LPS
(Escherichia coli O55:B5) were obtained from Sigma-Aldrich; Merck
KGaA. Fetal bovine serum (FBS) was purchased from Gibco; Thermo
Fisher Scientific, Inc.) and SYBR Green Master Mix was obtained
from Applied Biosystems; Thermo Fisher Scientific, Inc. Primary
antibodies (Bcl-2, Bax, Caspase-3 and β-actin) were procured from
Cell Signalling Technology, Inc. and polyclonal secondary
antibodies were obtained from Thermo Fisher Scientific, Inc.
Collection of plant material
Ap is known in India as Nelavemu, Kalamegh,
nilavempu, commonly known as King of Bitters and Green chiretta in
English. Finely powdered plant leaves were purchased from Wonder
herbals, Hyderabad, India (Batch number 10, GMP certified). The
components were checked for the presence of any visible
contaminants such as unsafe foreign material, sand, stones and
poisonous chemical residues. The powder was entirely free of moulds
or insects. As microbial contaminants can produce toxins, thin
layer chromatography was performed to detect contamination from
other plants using original plant leaf extract as control. The
plant name has been checked with https://www.theplantlist.org.
Preparation of experimental plant
extracts
A total of 10 g of pulverized leaf powder was
macerated for 72 h in 100 ml of methanol at room temperature in
orbital shaker (80 x g). In the present study, the method used to
extract and identify bio-active compounds from Ap is somewhat
different in terms of using the organic extraction method as this
method could help in extraction of a new set of compounds
previously unknown to be present in the plant. Filtrate was
obtained by Whatman filtration and a crude extract was obtained
after the evaporation of the methanol to complete dryness under
reduced pressure following rotary evaporation at 55˚C, which
yielded a dark green residue of ~5.75 g (26% w/w). The extract was
preserved in sterile tubes under refrigerated conditions (2-8˚C)
until further use. The final concentration of methanol was always
<1% in the experimental solution.
Phytochemical screening
Phytochemical investigation of extracts was
conducted to detect the occurrence of various chemical components.
Air-dried plant extract was examined to check for existence of
tannins, steroids, flavonoids, alkaloids, triterpenoids, saponins,
cardioglycosides and anthraquinones, as previously described
(32).
Gas chromatography-mass spectrometry
(GC-MS) analysis
Gas chromatography is used to identify bioactive
constituents like alkaloids, steroids, ester, short and long-chain
hydrocarbons, acids, alcohols, amino and nitro compounds (33,34).
Samples were analyzed using Gas Chromatograph Mass
Spectrophotometer GC-2010, MS QP2010 (Shimadzu, Japan). Capillary
column used for Chromatographic separation was from Restek Rxi-5MS
(Shimadzu Corporation) was performed via Argon gas as carrier in a
continuous flow rate mode at the rate of 2 ml/min. The GC oven
temperature was progressively raised to 120˚C at the rate of
5˚C/min, then up to 150˚C at the rate of 2˚C/min and 10˚C/min up to
240˚C, with a total run time of 36 min. The ion source, injector,
and interface were maintained at 230, 250 and 280˚C, respectively.
Detection was operated by selected ion monitoring mode and
injection volume was kept at 1 µl.
Cell culture
Human cervical cancer cell line (HeLa-CCL-2; RRID:
CVCL_0030), Human breast adenocarcinoma cell line (MCF7-HTB-22;
RRID: CVCL_0030), Human alveolar basal epithelial cell line
(A549-CCL-185; RRID: CVCL_0023), Human embryonic kidney 293 cell
line (293-CRL-1573; RRID: CVCL_0045), Human invasive ductal
carcinoma cell line (BT549-HTB-122; RRID: CVCL_1092) and a normal
cell line human foreskin fibroblasts (HFF-1-SCRC-1041; RRID:
CVCL_3285) were obtained from National Centre for Cell Science
(NCCS) and were used to test anticancer potential of Ap extracts.
All the cell lines used were cultured in DMEM with 10% FBS in an
incubator containing 5% CO2 at 37˚C. The cells upon
reaching 80% confluency (2-3 days), were rinsed with plain media
and trypsinized, counted using trypan blue (0.4% Trypan Blue
solution; Thermo Fisher Scientific, Inc.) staining method and
seeded into six-well plates for further studies. PCR-based
detection of Mycoplasma was performed for all the cell lines used
in the study using EZdetect (Himedia Laboratories, LLC) as it is
one of the most rapid, sensitive and convenient methods for
detection of mycoplasma contamination. The primer set is specific
to the highly conserved 16S rRNA coding region in the Mycoplasma
genome. The cell lines were authenticated at NCCS using16 Short
Tandem Repeat loci analysis as recommended by the American Type
Culture Collection (ATCC).
Screening of Ap extracts for
cytotoxicity against cancer cells
This bioactive component is extracted using
methanol-based extraction method. In the present study, the leaf
extract was prepared directly using methanol. To check for the
cytotoxicity of the extracts on the different cancer cells while
HFF-1 cells served as a non-cancer control cells, MTT assay was
performed using the following formula: Inhibition (%)=(A570 of
treated cells-blank)/(A570 of control cells-blank) x100-Eq: 1.
Effect of Ap extracts on cytotoxicity against cancer cells was
assessed by MTT assay, which is based on the reduction of MTT into
formazan by the cellular mitochondrial dehydrogenase in viable
cells which yields blue/purple formazan crystals upon cleaving the
tetrazolium ring. Based on the amount of reduction, the level of
cell metabolism can be studied. A-549, MCF-7, HEK, Hela and BT549
cancer cell lines were examined for viability after treatment with
extracts using MTT assay. Briefly, 1x104 cells were
seeded onto a 96-well plate and exposed to 2-100 µg/ml of Ap
extract for 24 h, after which the cells were treated with plain
DMEM medium containing MTT (0.5 mg/ml) and incubated in the dark
for 4 h. The formazan crystals formed after treatment were
dissolved with DMSO and the optical density of the supernatants was
measured at 570 nm (Fig. 2).
Flow cytometry for cell cycle
analysis
Propidium iodide (PI)-based measurement of the DNA
content in cells by flow cytometry. Cell cycle analysis was
performed using Flow Jo software (version 10; Becton, Dickinson and
Company). HeLa cells at a density of 1x106 cells per
well were seeded onto six-well plates and treated with Ap extracts
(25, 50 and 100 µg/ml) for 24 h. After treatment, the cells were
detached using a cell scraper, washed with FACS buffer (PBS
containing 2% FBS) and centrifuged at 801 x g for 10 min at room
temperature. The cell pellet was resuspended and fixed in ice cold
70% ethanol dropwise and incubated at 4˚C for 2 h. The fixed cells
were centrifuged at 961 x g for 8-10 min at room temperature and
the resultant pellet was washed twice with FACS buffer. Finally,
the cell pellet was suspended in 500 µl PBS containing PI (1
mg/ml), RNase A (20 µg/ml) and NP-40 (0.1%) and incubated in the
dark for an additional 30 min. The stained cells were analyzed
using a Guava flow cytometer (MilliporeSigma) to detect changes in
DNA content based on PI staining. A common gating strategy was
followed, wherein cells are distinguished by bivariate analyses of
side scatter vs. forward scatters in terms of height and area of
the scatter, respectively.
Immunoblotting
HeLa cells (2x106) were seeded per well
in a six-well plate and treated with Ap extracts (25, 50 and 100
µg/ml) for 24 h. Treated cells were washed with PBS and lysed in
RIPA buffer (Sigma-Aldrich; Merck KGaA) and the protein
concentration was determined using Bradford's method and ~10 µg of
protein was loaded into each well of a 12% gel and separated.
Samples were immunoblotted onto a nitrocellulose membrane (Bio-Rad
Laboratories, Inc.) via the Criterion blotting system (Bio-Rad
Laboratories, Inc.). Membranes were blocked at 25˚C using 5%
skimmed milk in TBST (20 mM Tris, 150 mM NaCl and 0.1% Tween 20)
for 1 h and were subsequently incubated overnight at 4˚C with
appropriate antibodies diluted (1:1,000) according to the
manufacturer's protocol. The membranes were washed using TBST and
incubated for 1 h at room temperature with appropriate
HRP-conjugated secondary antibody (1:2,000). Bound secondary
antibodies were detected using the enhanced chemiluminescence
system (ECL; Bio-Rad Laboratories, Inc.). Images were acquired
using LAS500 gel documentation System (Bio-Rad Laboratories, Inc.)
and analyzed using ImageJ software (version 1.53a; National
Institutes of Health). The relative intensity of the bands was
calculated and expressed as ratio of treated vs. control. An
average of independent experiments was used for representation.
In vitro cell migration assay
Scratch assay was performed in six-well plates as
previously described (35-37).
HeLa cells were cultured in DMEM medium containing 10% FBS until a
confluent monolayer was formed. The monolayer was scratched with a
sterile 10-µl micropipette tip to create a wound/gap of ~1 mm wide.
Cells were washed twice with PBS to remove detached and loosely
bound cells and the wells were replaced with fresh complete medium
containing 25, 50 and 100 µg/ml of the plant extracts and the cells
were cultured for 24 h. Images of the wounds were captured at 0 and
24 h under a microscope using EVOS XL Core Cell Imaging System.
ImageJ software was used to measure the width of the scratch and
cell migration was assessed. The results are presented as a mean
percentage of inhibition of migration.
Chorioallantoic membrane (CAM)
assay
Ap extracts were tested for anti-angiogenic activity
using CAM of 9-day old fertilized hen eggs (n=64) (Gallus
gallus) (Venkateshwara hatcheries, Hyderabad, Telangana,
India). The shells of fertilized eggs were disinfected and
incubated for 2 days at 37˚C with a relative humidity of 70-75%.
The CAM layers were inoculated with 1x106 (100 µl) of
HeLa cells in absence and presence of plant extracts at
concentrations 25, 50 and 100 µg/ml, (n=4x8 groups) with PBS was
used as a Placebo and the eggs were transferred to the incubator
for 72 h with eggs turned three to five times each over a 24-h
period. After incubation, the embryos were euthanized on the 14th
day in a humane method by placing the eggs in a gas chamber and
filling the chamber with 100% CO2 and maintaining it for
10 min (38), after which the
embryos were inspected by candling to check for any movement. Later
the shells were gradually broken and the embryo and yolk were
removed. The developing vasculature on CAM was imaged and analyzed
followed by manually counting the vessels. The vessels were counted
by the experimenter and two unbiased individuals to authenticate
the data. Changes in angiogenesis were evaluated by observing the
secondary and tertiary blood capillaries. The secondary blood
vessels are the fine blood vessels arising from the larger blood
vessels while the tertiary vessels arise from the secondary
vessels. For measurement of the area covered by different veins,
capillary branching points were used as initiation and termination
markers. All analyses were made in triplicates and any dead embryos
were excluded from the present study. % Angiogenesis=(total number
of Secondary blood vessels in treated-total number secondary blood
vessels in untreated)/(total number of secondary blood vessels in
vehicle control-total number secondary blood vessels in untreated)
x100-Eq:2.
Cell invasion assay
Invasiveness of HeLa cells was tested in presence of
Ap extracts by Transwell migration assay using Matrigel (Thermo
Fisher Scientific, Inc.). Matrigel (20-30 µl) was added to each
insert in a 24-well plate which was incubated in a CO2
incubator at 37˚C for 30 min. After incubation, DMEM with
1x106 HeLa cells in 1% FBS along with Ap extracts was
added to each of the inserts. To the lower chamber, 600 µl of
complete medium (containing 12% FBS) was added as a
chemoattractant. These wells were incubated in an incubator with 5%
CO2 at 37˚C for 24 h. The medium was removed carefully
from the insert and the insert was fixed using 70% ethanol for 10
min at room temperature. After incubation, ethanol was aspirated
carefully and the inserts were allowed to dry at room temperature.
Cells were stained with 0.2% crystal violet for 2 min at room
temperature followed by rinsing with 70% ethanol to remove the
excess stain. Images of were captured using an inverted light
microscope at x40 magnification (Nikon Corporation).
Statistical analysis
All analyses were performed in triplicates and these
values were presented as the mean ± SD and plotted using GraphPad
Prism 7.0 software (GraphPad Software, Inc.). Statistical
comparisons were conducted using analysis of variance (One
way-ANOVA and two way ANOVA) followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Chemical composition of methanolic
extract of Ap
Methanol was used to prepare Ap extracts from dried
and powdered leaves of Ap. The qualitative tests to show the
presence of alkaloids, flavonoids, proteins, terpenoids,
cardioglycosides and steroids were performed, results of which have
been presented in Table I. In the
present study, a method was used to extract and identify bio-active
compounds from Ap which is somewhat different in terms of using the
organic extraction method. This method could help in extraction of
a new set of compounds previously unknown to be present in the
plant. Previous studies have reported biological activity due to
Andrographolides which are terpenoids,
5,7,2',3'-tetramethoxyflavone, an alkaloid and arabinogalactan, a
protein, as certain of the major biologically active components in
the plant. These have been widely studied in their applications in
disease such as fever, infection and even cancers (39). To explore the presence and
identification of more bioactive components, GC-MS was carried
out.
 | Table IPhytochemical screening of Ap
extract. Phytochemicals in Ap comprised alkoloids, flavonoids,
cardioglycosides, terpenoids and steroids, which might be
responsible for the distinct anti-cancer activities of the extract.
Note: (+) positive, (-) negative. |
Table I
Phytochemical screening of Ap
extract. Phytochemicals in Ap comprised alkoloids, flavonoids,
cardioglycosides, terpenoids and steroids, which might be
responsible for the distinct anti-cancer activities of the extract.
Note: (+) positive, (-) negative.
| Phyto-chemical
compounds | Test performed | Methanol extract of
Ap |
|---|
| Alkaloids | Mayer's test | + |
| Flavonoids | Alkaline reagent
test | + |
| Saponins | Froth Test | - |
| Tannins | Braymers's
Test | - |
| Phenols | Ferric Chloride
test | - |
|
Cardioglycosides | Keller-Killani
test | + |
| Terpenoids | Copper acetate
test | + |
| Steroids | Brown ring
test | + |
Identification of components by
GC-MS
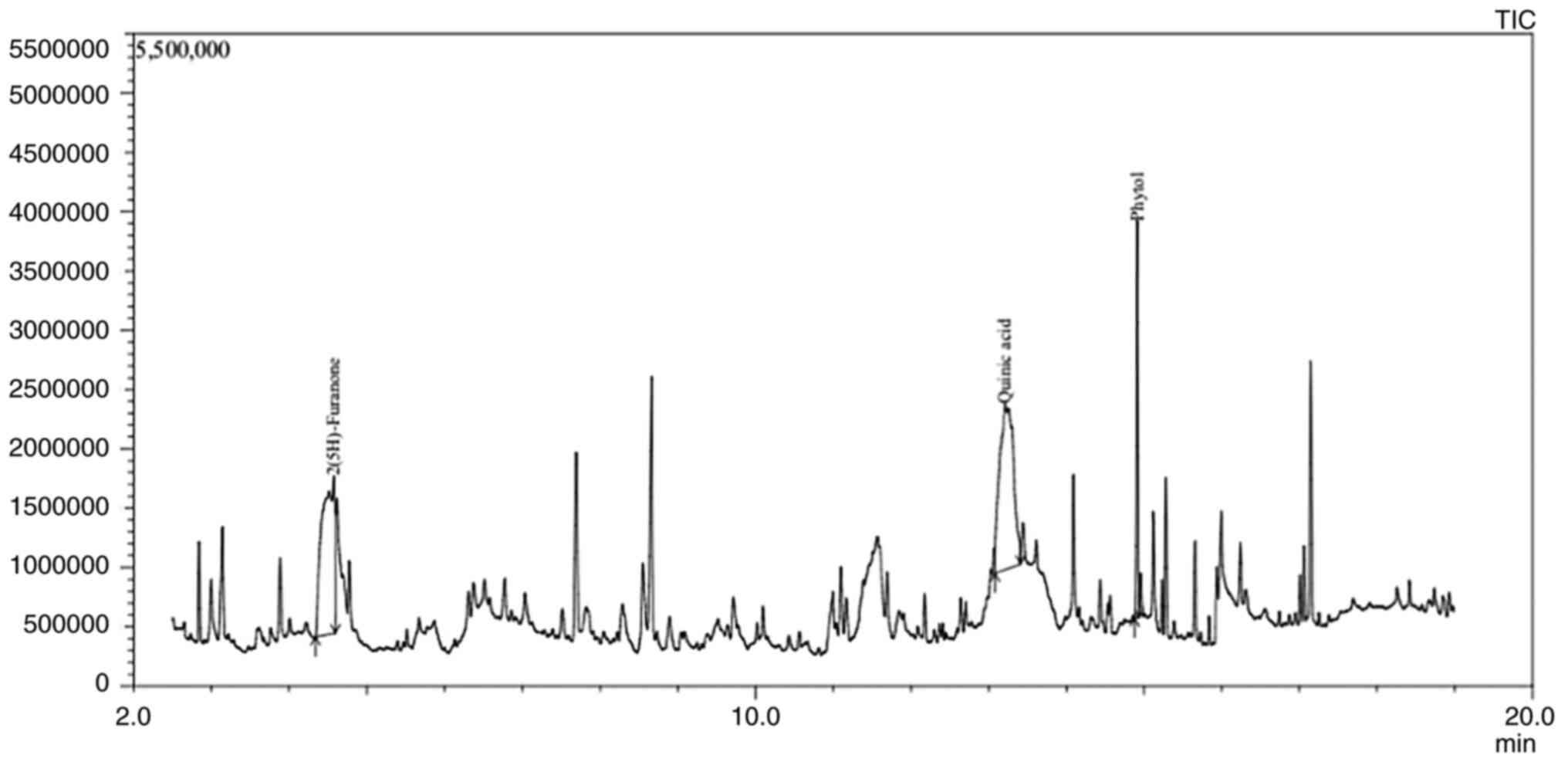
Ap extracts displayed 21 peaks in the GC-MS
chromatogram showing the presence of 21 phytochemical constituents
(Fig. 1). The GC-MS data was
interpreted using National Institute Standard and Techniques (NIST)
database which comprises more than 62,000 patterns. By comparing
the average peak area to the total area the relative percentage of
each component in the mixture was estimated. By comparing the
spectrum of the unknown component with that of a known component
found in the NIST library, the molecular weights, structure and
names of the components in the test materials were determined.
Among all the compounds, 2(5H)-Furanone (14.73%), Quinic acid (QA;
17.32%) and Phytol (11.43%) were found to be the major
phytochemical constituents, the retention time, molecular formula,
molecular weight and concentrations of the compounds are presented
in Table II. Apart from the three
aforementioned components, 5-Hydroxymethylfurfural (4.75%) was also
observed in some abundance, followed by Dimethyl sulfone (3.93%),
3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (3.15%),
1,2-Cyclopentanedione (2.33%), 1-Ascorbic acid 2,6-dihexadecanoate
(2.30%), 2-Propenoic acid (1.76%), Benzofuran, 2,3-dihydro-Coumaran
(1.74%), 3,5-Dimethylanisole (1.23%), Hexadecanoic acid (1.17%) and
3-Furanmethanol (1.03%), 8,11,14-Docosatrienoic acid (0.89%),
Benzenepropanoic acid (0.89%), Dibutyl phthalate (0.87%),
2(4H)-Benzofuranone (0.59%), 9-Heptadecanone (0.57%),
9,12-Octadecadienoicacid (0.57%), 2-Pentadecanone (0.52%),
presented in Fig. S1 and Table SII.
 | Table IIMajor compounds identified in the
crude extract of Ap 2(5H)-Furanone (14.73%), Quinic acid (17.32%)
and Phytol (11.43%) using gas chromatography-mass spectrometry
analysis with its retention time, peak area and reported biological
activities. |
Table II
Major compounds identified in the
crude extract of Ap 2(5H)-Furanone (14.73%), Quinic acid (17.32%)
and Phytol (11.43%) using gas chromatography-mass spectrometry
analysis with its retention time, peak area and reported biological
activities.
| S. No. | Compounds | Formula | Molecular weight
(g/mol) | Retention time | Area% | Biological
activities | (Refs.) |
|---|
| 1. | 2(5H)-Furanone |
C4H4O2 | 84 | 4.591 | 14.73 | Anti-inflammatory,
anticancer, antimicrobial, and antifungal activity | (46-48) |
| 2. | Quinic acid |
C7H12O6 | 192 | 13.21 | 17.32 | Antioxidant
activity, anti-inflammatory | (53,55) |
| 3. | Phytol |
C22H42O2 | 338 | 14.91 | 11.43 | Antimicrobial,
anticancer, anti-inflammatory and diuretic | (56,57,62) |
Methanol extracts of Ap show
cytotoxicity against multiple cancer cell lines
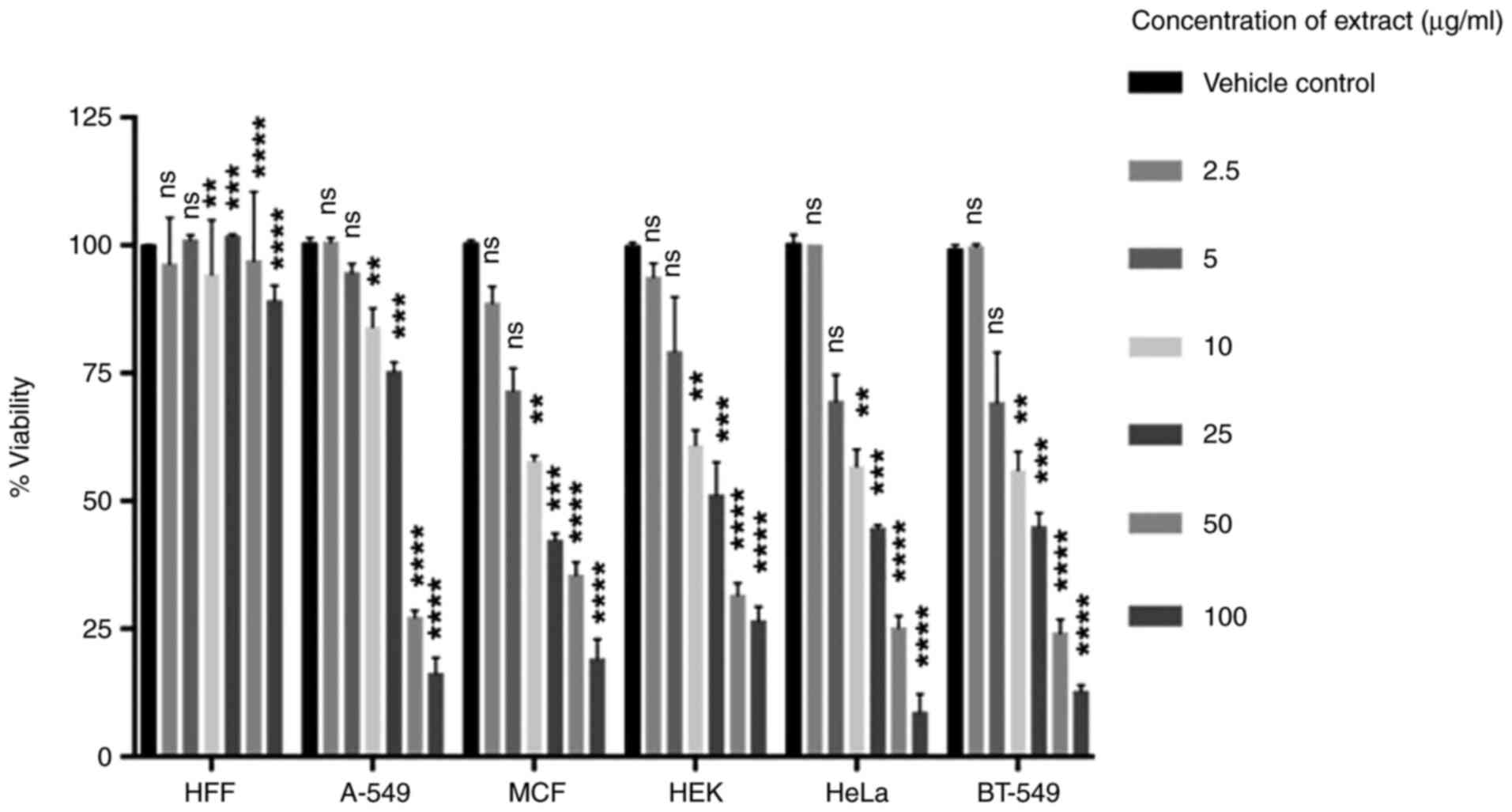
The cytoxicity of the extracts was compared between
different cancer cell lines in the concentration range of 2-100
µg/ml and expressed as percent viability (Fig. 2). A range of 25-100 µg/ml showed
effective inhibition against cancer cell lines, wherein, a dose
dependent increase in cytotoxicity was observed. However, at 25
µg/ml cell viability against MCF-7, Hela, and BT-549 cancer cell
lines was observed to be less than 50%. The extracts demonstrated
strong activity against cancer cell lines in vitro with
IC50 <100 µg/ml. An IC50 of 50.347±1.975,
36.237±1.729, 43.779±5.516, 33.136±2.370, 33.514±2.261 µg/ml was
recorded against A-549, MCF-7, HEK, HeLa and BT-549 cell lines,
respectively. Inhibitory effects were observed from 5 µg/ml itself
in MCF-7, HEK, HeLa and BT-549 cells whereas in A-549 cells,
inhibition was observed from 25 µg/ml. Cell viability decreased
significantly with increasing concentrations and at 25 µg/ml less
than 50% viability was observed, i.e., 42.1, 45, and 43.8% cell
viability in MCF-7, Hela and BT-549 cells respectively with no
cytotoxicity against HFF cells.
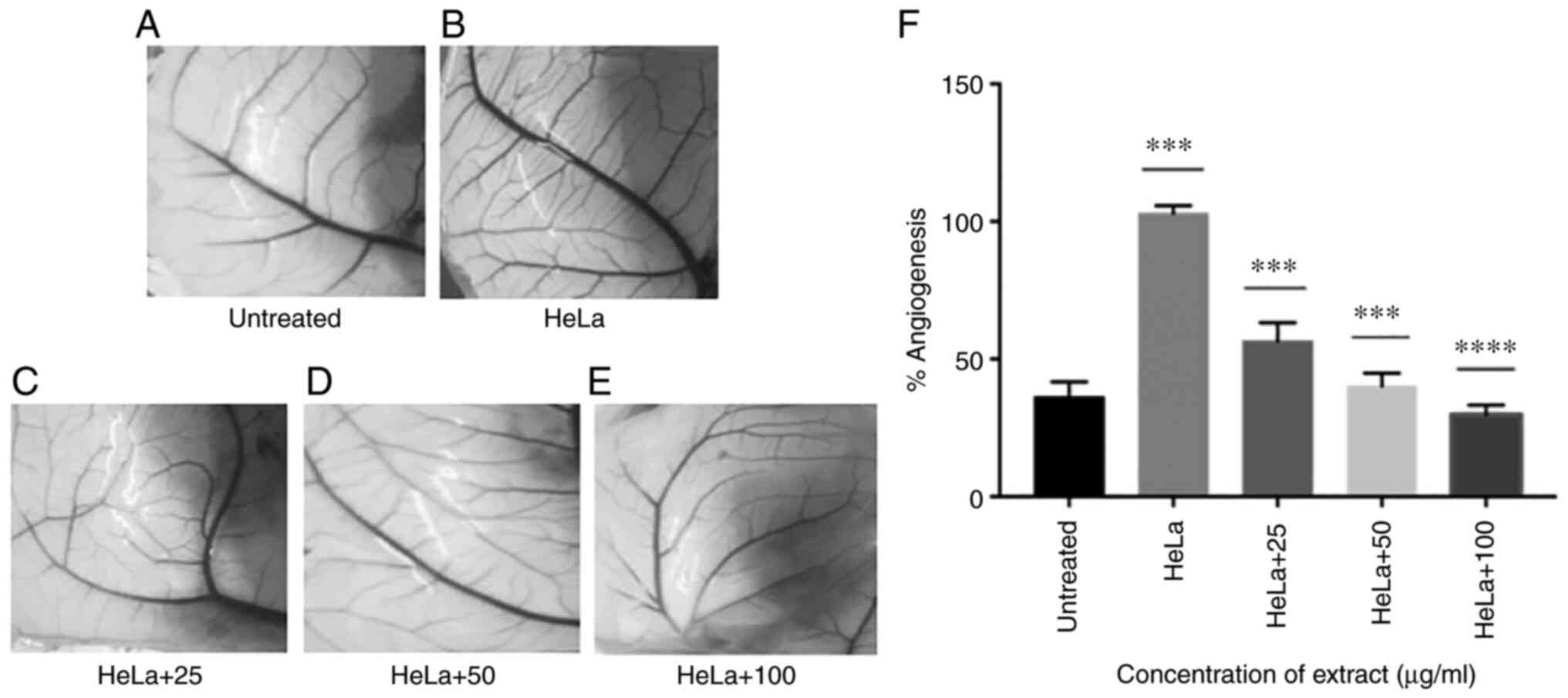
Ap extracts inhibit angiogensis in CAM
layer of eggs co-inoculated with HeLa cells
In order to assess the effect of the Ap extracts on
the angiogeneis and cell migration, HeLa cell line was chosen for
all the further expreriments as Ap extracts appeared to be
exhibiting highest inhibition against HeLa as compared with the
other cell lines examined. Ap extracts were able to reduce the
in vivo angiogenesis induced by HeLa cancer cells on CAM of
embryonated eggs (Fig. 3). In the
CAM of untreated/placebo controls, secondary vessel number in the
area covered did not show much change after 72 h, while in the Hela
injected CAMs, (treated control) number of secondary vessels were
higher after 72 h. In the CAMs injected with Hela and treated with
the Ap extract, anti-angiogenic activity was observed to be
dose-dependent showing a decrease in fresh capillary formation with
increasing concentrations. Reduction in the secondary blood vessel
formation was observed as 38.71, 56.46 and 67.75% with 25, 50 and
100 µg/ml respectively, as compared with CAM of the embryos treated
with only HeLa which showed increased number of capillaries, unlike
untreated CAMs which did not show significant change. These results
supported the anti-antiangiogenic effects of Ap.
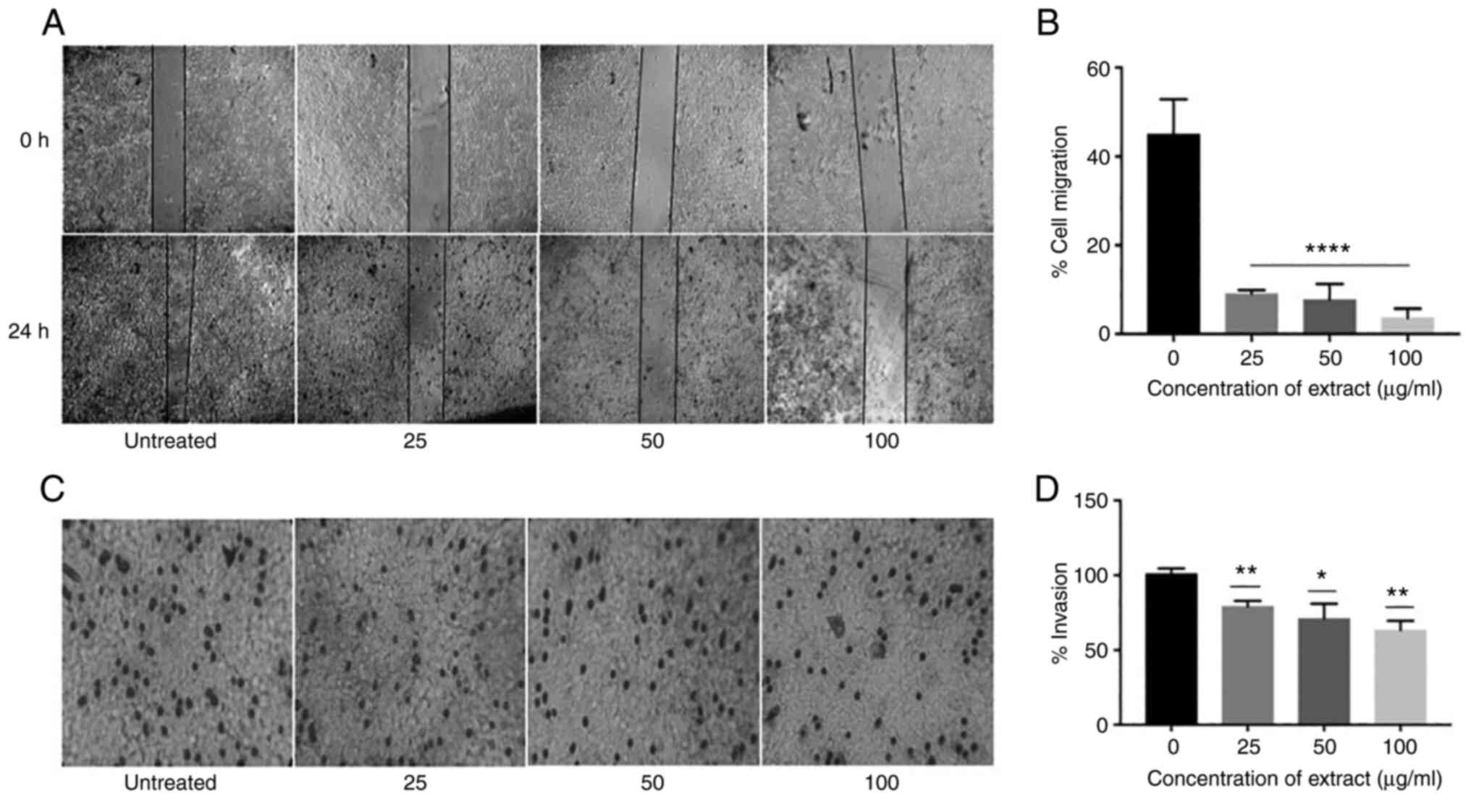
Inhibition of in vitro cell migration
by Ap
Ap extracts were further examined for their ability
to inhibit the migration of HeLa cells, which was tested by
performing an in vitro scratch assay. The doubling time
required for HeLa is 33-35 h, since the experiment was performed
for a period of 24 h which limited the possibility of cells
proliferating into the wound. Furthermore, flow cytometric analysis
revealed that the cells treated with the extract were arrested in
the G2/M phase lowering the possibility that the cells proliferated
into the wound rather than migrating into it (Fig. 4A). After incubation for 24 h with
the extracts, the gap created by the scratch remained fairly intact
and minimum number of cells were observed to fill the wound in the
treated groups (25, 50, and 100 µg/ml) compared with the untreated
group. Percentage of cell migration was significantly reduced with
increasing concentrations of the Ap extract indicating inhibitory
effect of the Ap extract on HeLa cell migration (Fig. 4B).
Ap extracts inhibit the invasiveness
and migration of cervical cancer
Metastasis involves a sequence of steps linking
cancer cell invasion followed by migration. In order to investigate
if Ap extract has any anti-invasion properties, Matrigel-coated
Transwell chambers were used in the presence of different
concentrations (25, 50 and 100 µg/ml) of Ap extracts. The total
number of cells passing through the Matrigel-coated filter reduced
significantly in presence of the extracts (Fig. 4C). Compared with the control group,
the inhibition percentage of invasion was 21.37, 28.2 and 38.47%
for 25, 50 and 100 µg/ml of extracts, respectively (Fig. 4D). Thus, in addition to its
inhibitory effect on cell viability, Ap extracts also inhibited the
cell invasion potential of HeLa cells in vitro. This effect
of the extracts could be due to cytotoxicity leading to cell death,
thereby, preventing cell migration and invasion by tumor cells.
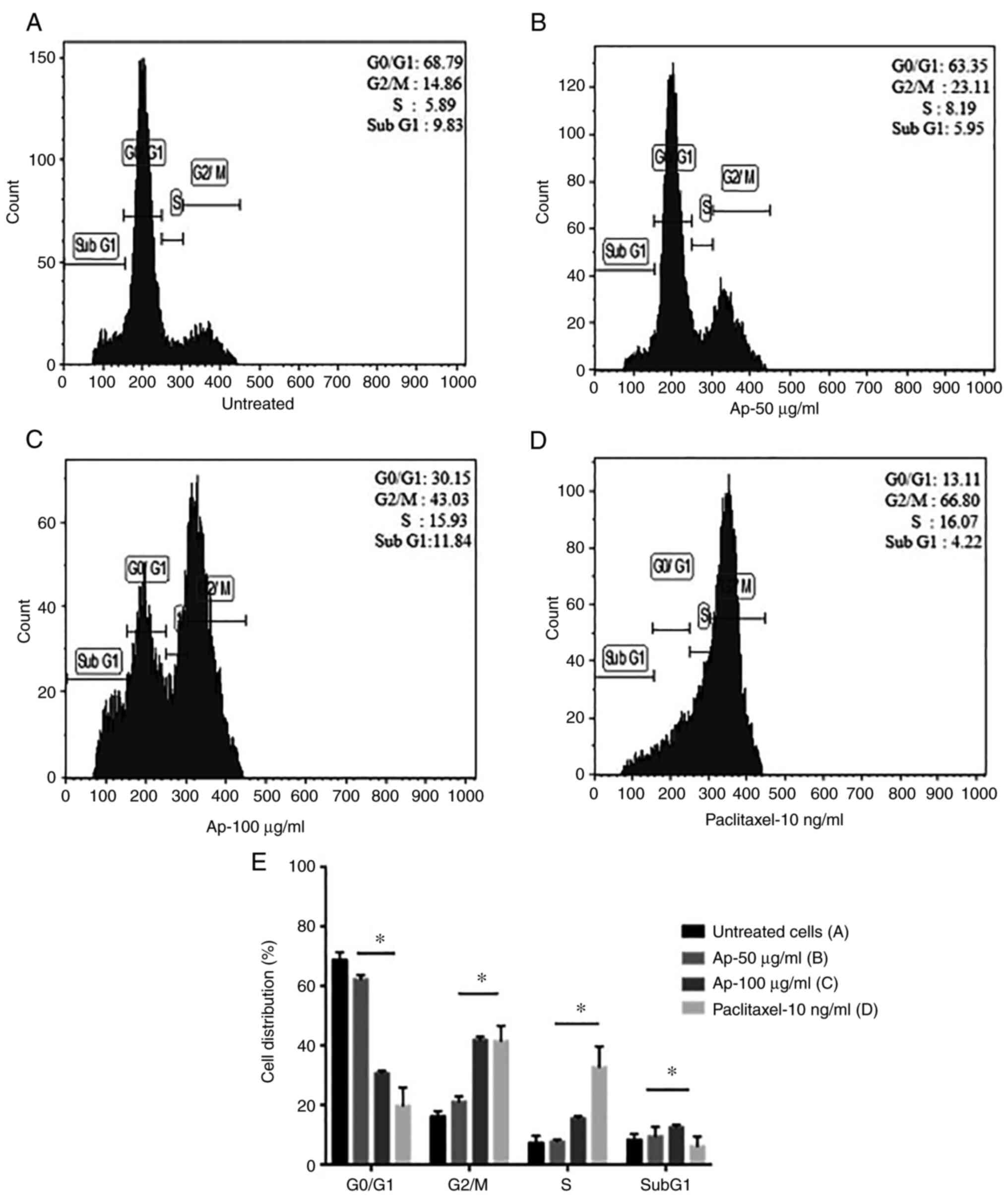
Ap extract arrests cell cycle
progression in cancer cells
To investigate the mechanism behind growth
inhibition, the effect of the Ap extracts on cell cycle
distribution of HeLa cells was analyzed after 24 h of treatment by
flow cytometry. An alteration in the distribution of different
phases of the cell cycle was clearly evident (Fig. 5A-D). Paclitaxel was used as a
positive control for cell cycle arrest. In the treated cells,
increase in cell population in the G2/M phase was observed. A surge
in the percentage of cells accumulating in the G2/M phase with
increasing concentrations of the extract (Fig. 5E) was identified. While the
untreated cells showed lower percentage at 16.18% in the G2/M
phase, Ap extract-treated cells showed a considerable increase in
percentage of accumulated cells at 41.93% at a concetration of 100
µg/ml. No significant change in all other phases was observed.
These findings suggested induction of apopotic cell death in HeLa
cells upon treatment with Ap extracts via G2/M phase arrest of cell
cycle progression.
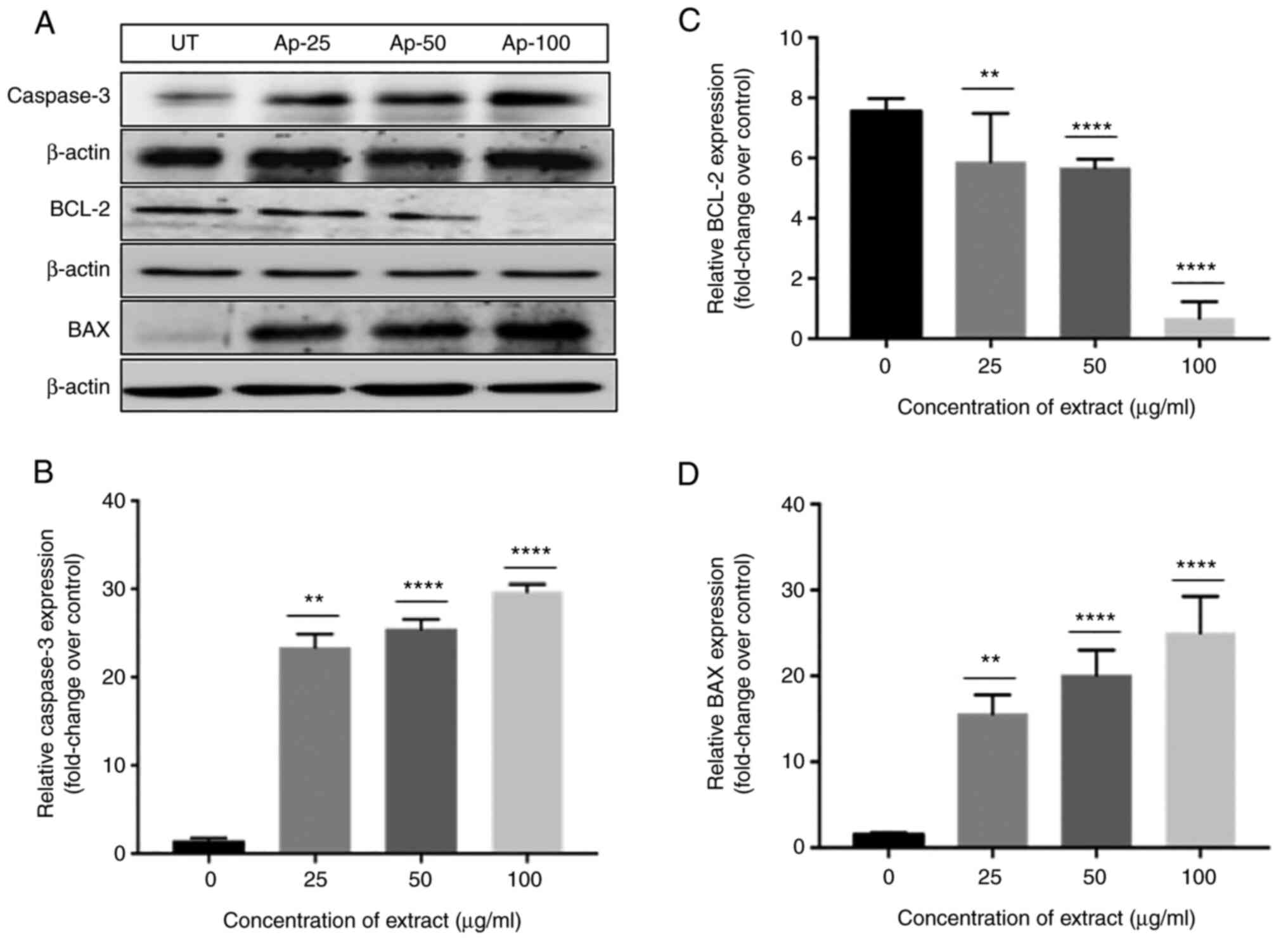
Ap extracts induce apoptosis in cancer
cells
Cell death has been observed in human cervical
carcinoma (HeLa) induced by treatment with methanolic extract
prepared from Ap. To validate aforementioned data from flow
cytometry, an attempt was made to understand the mechanism leading
to apoptotic cell death. Cells treated with the extracts showed
significant increase of ~20 fold in the activity of caspase-3, a
crucial mediator of apoptosis with increasing concentrations when
compared with the untreated cells (Fig. 6B). The extracts were also able to
increase the levels of Bax, a pro-apoptotic marker, by 15 and 20
fold when exposed to 25 and 50 µg/ml of the extract while a maximum
of 30 fold increase was observed when exposed to 100 µg/ml
(Fig. 6D). By contrast, a
simultaneous downregulation by 8 fold in the expression of the
anti-apopotic marker Bcl-2 was observed for 25 and 50 µg/ml which
was completely inhibited when treated with 100 µg/ml of the extract
(Fig. 6C), confirming induction of
apoptotic cell death in Ap-treated HeLa cells (40).
Discussion
Medicinal plants and their activities are of immense
interest due to their unique properties including significant
anticancer activity and milder or negligible side effects. Recent
investigations have involved the identification and separation of
new and beneficial compounds of therapeutic importance from higher
plants for specific diseases. As per the WHO report published in
the year 2002, 60% of medicinal drugs including anticancer drugs
are derived from natural sources (41). HeLa cells are highly invasive and
robust cells with shorter multiplication time and simple growth
requirements and hence were chosen for the present study. After
complete analysis of the Ap methanolic extracts, it was estimated
that the anti-proliferative activity of the extracts could be due
to the presence of 2(5H)-Furanone, QA and Phytol which share a
major percentage of the components even when concentrations as low
as 25 µg/ml were used in the assay thus suggesting that the
activity could be due to the major components of the extract, while
the remaining components accounted for a markedly lower share.
Notably, these compounds have not been previously reported in Ap.
Most of the previous studies used dichloromethane with methanol
(1:1) while fractionation by cold maceration method was used for
extraction of bioactive components (27,42-44).
In the present study, only methanol was used during extraction and
it could be an important factor in extraction of tough to isolate
ingredients from the extracts. Methanol is suitable for extraction
of primary and secondary metabolites since it has a higher
dielectric constant and can strongly penetrate the cell to extract
even polar contents. Due to its high polarity, methanol has higher
extraction yields and can extract both hydrophilic and lipophilic
molecules from plant parts. After extraction, methanol can be
removed by distillation at lower temperatures as methanol is highly
volatile. Moreover, the isolation of andrographolides which form
the major component in Ap, involves extraction of the leaf powder
by cold maceration in a 1:1 mixture of dichloromethane and methanol
and recrystallisation of andrographolide directly from the
resulting extract (42). The
presented method of extraction with methanol did not help to
isolate the main andrographolides and other bioactive components
previously reported by other groups. Hence, it can be suggested as
an adjunct to other methods for maximum extraction of bioactive
components from the plant, as it was mainly possible to isolate the
active non-andrographolide components using methanol-based
extraction.
2(5H)-Furanone, also known as γ-crotonolactone, is a
part of a large number of biologically active compounds, comprising
natural products and pharmaceutical agents. It belongs to the group
of α, β-unsaturated lactones, which are observed in numerous
natural products. 2(5H)-furanone derivatives are known to have good
pharmacological activities; particularly 2(5H)-furanone skeleton
are known for their extensive biological activity, together with
antitumor (45), antibacterial
(46), antifungal (44), antiviral (48), anti-inflammatory (38,49)
and antioxidant effects. QA is an important natural cyclitol found
in tea, coffee, fruits and vegetables (50,51).
This bioavailable, non-toxic, natural polyol was found to be
exhibiting potent antioxidant, anti-inflammatory (52) and anti-mutagenic activities
(38) and was also revealed to
chelate transition metals (53).
Hence this versatile parent chiral compound is used in the
synthesis of new drugs (54) QA
has also been studied as a potent drug for treatment of prostate
cancer (55).
Phytol, is a chlorophyll-derived acyclic diterpene
alcohol and a scented ingredient used in numerous fragrant
compounds abundantly present in nature which is utilized in
cosmetic and non-cosmetic products (52). It exhibits various pharmacological
activities such as antimicrobial (55), anticancer (56), immunoadjuvant, antidiabetic and
lipid lowering (57), anxiolytic
(58), antinociceptive (59), anti-teratogenic (60) antioxidant, anti-inflammatory
(61) as well as anti-allergic
effects (40,62). Phytol also functions as
immuno-stimulant and is superior to several commercial adjuvants in
terms of immunological memory induction over a longer-term and
activation of innate and acquired immunity (61). Phytol and its byproducts have no
collective inflammatory or toxic effects even in immuno-compromised
mice (63). It is also known to
inhibit the growth of pathogens such as Mycobacterium
tuberculosis (62,64) and Staphylococcus aureus
(65). Apart from the
aforementioned effects, phytol also showed anti-angiogenic activity
by inducing apoptosis in A549 cells by depolarizing the
mitochondrial membrane potential (66).
In addition, certain minor components in the extract
such as 5-Hydroxymethylfurfural are known to exhibit
anti-inflammatory and antitumor effects (67). For example, dimethyl sulfone has
antimicrobial, anti-inflammatory and anticancer properties
(68), while
3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one is considered to
exhibit free radical scavenging activity (69). Similarly, 1,2-cyclopentanedione was
reported to be effective in prevention of gastrointestinal tumor
growth (70) and 1-Ascorbic acid
2,6-dihexadecanoate was found to have antiproliferative efficacy
(71). 2-Propenoic acid is said to
have antibacterial properties (72) and 2,3-dihydro-Coumaran was reported
to express anti-inflammatory effects (73). 3,5-Dimethylanisole has
antimicrobial, anti-inflammatory and antioxidant effects (74). Unlike other components,
Hexadecanoic acid has potent antimicrobial activity along with
antifungal, antitumor, antioxidant, chemo-preventive, antioxidant
and immuno-stimulant effects (75). 3-Furanmethanol is known to possess
anticancer, anti-inflammatory and antimicrobial activities
(76); 8,11,14-Docosatrienoic acid
is cardioprotective (77) and
benzenepropanoic acid was reported to possess antifungal,
antioxidant activities (78);
Dibutyl phthalate was found to express antimicrobial and antitumor
(79) properties. Furthermore,
2(4H)-Benzofuranone has been revealed to possess analgesic,
antidiabetic, antibacterial and antifungal properties (72) whereas 9-Heptadecanone expressed
antibacterial (80) activity.
Similarly, 9,12-Octadecadienoicacid was found to have anticancer
properties (81) while
2-Pentadecanone (0.52%) was reported for hypocholesterolemic,
antioxidant properties (82). It
is definitely possible that all the aforementioned compounds,
though present in minor proportions may act along with phytol, QA
and (5H)-furanone. However, it has been revealed that the 3 major
aforementioned components showed activity at very low
concentrations of 50 and 100 µg. In such low concentrations it is
expected that the predominant components can exhibit apparent
biological activity.
Cell division is an intricate phenomenon regulated
by various protein kinases which oversee the gene transcription
necessary for progression into subsequent phases of the cell cycle.
The mitogen-activated protein kinases play a crucial role in the
initiation, advancement and coupling of these phases. Ap extracts
screened for effect on cell cycle progression were observed to
cause buildup of cells in the G2/M phase, reflecting increase in
apoptotic cell death. This outcome was also dose-dependent,
indicating that Ap extracts work by arresting the cell cycle at
G2/M phase. Evasion of malignant cells from apoptotic cell death is
crucial in tumor formation. Most anticancer therapies currently in
use such as chemotherapy and γ-irradiation and immunotherapy
involve killing tumor cells by activation of the intrinsic and/or
extrinsic apoptotic pathways in cancer cells. Bcl-2 and Bax are an
important signaling proteins in the mitochondria-mediated apoptotic
pathways that regulate the release of cytochrome c, DNA
fragmentation and activation of caspases. The ratio of Bcl-2 and
Bax determines the sensitivity of cancer cells to death signals.
Bcl-2, is an anti-apoptotic protein which promotes cancer cell
growth and is a member of the Bcl-2 family while Bax, another
component of the Bcl-2 family, induces apoptosis (83). Expression of pro-apoptotic Bax is
required to induce apoptosis and to correct uncontrolled cell
proliferation (84). Caspases are
proteases that are essential mediators of apoptosis and
inflammation. HeLa cells exposed to Ap extracts showed increased
Caspase-3 and Bax and lowered Bcl-2 levels, indicating activation
of intrinsic apoptotic pathway. This indicated that Ap extracts can
induce the intrinsic apoptotic pathway in HeLa cells.
The anti-angiogenesis concept has been an important
component in cancer treatment and it is considered that blocking
angiogenesis could be a strategy to arrest tumor growth and
metastasis (85). Angiogenesis
leads to development of tumors leading to invasion and metastasis.
Anti-angiogenesis is a strategy that prevents tumor progression and
can be contemplated as one of the targets for anticancer therapy,
since the tumor growth can be controlled if oxygen and nutrient
supply to a tumor could be reduced. New blood vessel formation
(angiogenesis) involves a multistep process involving cell
proliferation, migration and remodeling. Suppression of any step
may result in inhibition of new blood vessel formation. Using the
chick embryo CAM model, it could be demonstrated that Ap extracts
inhibit angiogenesis in CAM even at minimum concentrations,
indicating potent anti-angiogenic activity. Numerous flavonoids,
terpenoids and polyphenols, are known to inhibit carcinogenesis and
tumorigenesis in vivo and in vitro. Among the
identified compounds 2(5H)-Furanone (86-89)
along with phytol (90,91) and QA (92,93)
are used in the treatment of several types of cancer. Since it is
the predominant component identified by GC-MS, it could be the
predominant anticancer and anti-inflammatory component of the
extracts. With the existence of other bioactive components in the
extract, it is also possible that the antiangiogenic activity of
the extracts is due to the synergistic effect of the predominant
compounds; 2(5H)-furanone, phytol and QA augmenting the
antiangiogenic effect. Anti-migratory activity, motility and
invasion of cervical cancer cells after Ap treatment were evaluated
by scratch wound and Transwell invasion assays. Our results
suggested that Ap significantly inhibited the motility and
invasiveness of cervical cancer cells. Cervical cancer accounts for
6-29% of cancers in India and is one of the most common forms of
cancer in women worldwide. Therefore, the present study
concentrated on utilization of HeLa cells for establishing the
mechanism of action of the extracts. However, it is recommended
that testing multiple cell lines derived from cervical cancer or
other cancers will be extremely useful in establishing the
mechanism. It is a limitation to the present study that the
findings could not be confirmed in other cell lines, but since the
main goal was to characterize the mechanism of action of Ap
extracts, it could be stated that, some new previously unreported
components from Ap which have strong anti-proliferative activity
were identified by mainly suppressing cell cycle progression and
promoting intrinsic apoptotic pathway. In conclusion, the
non-andrographolide components of Ap appear to have significant
anticancer and anti-angiogenic activity. While the anticancer
properties of andrographolides are well established, to the best of
our knowledge, this is the first investigation reporting the
anticancer effects of the non-andrographolide components of Ap.
Though in the present study the activity of the total methanol
extract, 2(5H)-furanone, phytol and QA were revealed to be the
major components, the anticancer properties could not be attributed
to these compounds. 2(5H)-furanone, phytol and QA are known
separately for having anti-inflammatory and antitumor activity and
these compounds appear to target cell cycle arrest and induce the
intrinsic apoptotic pathways. It is possible that their
combinations in crude extract have synergistic activity causing
significant reduction in invasiveness and metastatic properties of
malignant cells. Further studies using purified compounds would
help in specifically identifying the role of each of these
components.
Supplementary Material
Gas chromatograms for of crude
methanolic extracts of Andrographis paniculata showing the
21 identified components.
Traditional usesof the plant Ap
Total compounds identified in the
crude methanolic extract of Andrographis paniculata using
gas chromatography-mass spectrometryanalysis with its retention
time, peak area, and reported biological activities. Of the 21
compounds identified, 4 of them are known to exhibited antioxidant
capacity and 5 of them possessed anti-inflammatory potential, 6
compounds had anti-cancer and anti-tumor potential while
antimicrobial activities were determined in 9 of the
compounds.
Acknowledgements
The authors would like to thank Sreeram Kaveti,
(Senior Research Fellow, Applied Biology, CSIR-IICT, Hyderabad,
Telangana 500007) for helping with experimentation and Dr Gopal
Reddy, (Professor (Retd) Department of Microbiology, Osmania
University, Hyderabad, Telangana 500007) for providing logistic
support during the study.
Funding
Funding: The present study was supported by UGC-BSR Fellowship
(grant nos. F4-1/2006(BSR)7-369/2012(BSR)/2013-2014/4) and
122/C/BB/MICRO/HOD/2015). The APC was supported by Rashtriya
Uchchatar Shiksha Abhiyan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PKA conceptualized the study, provided methodology
and software, validated the data and prepared the original draft.
ANY peformed investigation, data curation and contributed to
preparation of the original draft. KR conducted investigation,
software and formal analysis. RK performed investigation and formal
analysis. CT conducted investigation, validation, wrote, reviewed
and edited the manuscript. KSN performed validation, formal
analysis, wrote, reviewed and edited the manuscript. SB
conceptualized and supervised the study, provided resources,
performed project administration, prepared the original draft,
wrote, reviewed and edited the manuscript. All authors read and
approved the final version of the manuscript. PKA and SB confirm
the authenticity of all the raw data provided in the present
article.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ouhtit A, Gaur RL, Abdraboh M, Ireland SK,
Rao PN, Raj SG, Al-Riyami H, Shanmuganathan S, Gupta I, Murthy SN,
et al: Simultaneous inhibition of cell-cycle, proliferation,
survival, metastatic pathways and induction of apoptosis in breast
cancer cells by a phytochemical super-cocktail: Genes that underpin
its mode of action. J Cancer. 4:703–715. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Sukanya SL, Sudisha J, Hariprasad P,
Niranjana SR, Prakash HS and Fathima SK: Antimicrobial activity of
leaf extracts of Indian medicinal plants against clinical and
phytopathogenic bacteria. Afr J Biotechnol. 8:6677–6682. 2009.
|
|
3
|
Abutbul S, Golan-Goldhirsh A, Barazani O,
Ofir R and Zilberg D: Screening of desert plants for use against
bacterial pathogens in fish. Isr J Aquacult-Bamid. 57:71–80.
2005.
|
|
4
|
Pandey G and Madhuri S: Significance of
fruits and vegetables in malnutrition cancer. Plant Arch.
10:517–22. 2010.
|
|
5
|
Pandey G, Madhuri S and Mandloi AK:
Medicinal plants useful in fish diseases. Plant Arch. 12:1–4.
2012.
|
|
6
|
Ravikumar S, Selvan GP and Gracelin AA:
Antimicrobial activity of medicinal plants along Kanyakumari coast,
Tamil Nadu, India. Afr J Basic Appl Sci. 2:153–157. 2010.
|
|
7
|
Umamaheswari S and Mainzen Prince PS:
Antihyperglycaemic effect of ‘ilogen-excel’, an ayurvedic herbal
formulation in streptozotocin-induced diabetes mellitus. Acta Pol
Pharm. 64:53–61. 2007.PubMed/NCBI
|
|
8
|
Misra P, Pal NL, Guru PY, Katiyar JC,
Srivastava V and Tandon JS: Antimalarial activity of
Andrographis paniculata (Kalmegh) against plasmodium berghei
NK 65 in mastomys natalensis. Int J Pharmacogn. 30:263–274.
1992.
|
|
9
|
Singha PK, Roy S and Dey S: Antimicrobial
activity of Andrographis paniculata. Fitoterapia.
74:692–694. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ernst E and Cassileth BR: The prevalence
of complementary/alternative medicine in cancer: A systematic
review. Cancer. 83:777–782. 1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the nearly four decades from 01/1981
to 09/2019. J Nat Prod. 83:770–803. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
See D, Mason S and Roshan R: Increased
tumor necrosis factor alpha (TNF-alpha) and natural killer cell
(NK) function using an integrative approach in late stage cancers.
Immunol Invest. 31:137–153. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ahilan B, Nithiyapriyatharshini A and
Ravaneshwaran K: Influence of certain herbal additives on the
growth, survival and disease resistance of goldfish, carassius
auratus (Linnaeus). Tamilnadu J Vet Ani Sci. 6:5–11. 2010.
|
|
15
|
Zilberberg MD, Shorr AF, Micek ST,
Vazquez-Guillamet C and Kollef MH: Multi-drug resistance,
inappropriate initial antibiotic therapy and mortality in
Gram-negative severe sepsis and septic shock: A retrospective
cohort study. Crit Care. 18(596)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Runyoro DK, Matee MI, Ngassapa OD, Joseph
CC and Mbwambo ZH: Screening of Tanzanian medicinal plants for
anti-Candida activity. BMC Complement Altern Med.
6(11)2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shukla B, Visen PKS, Patnaik GK and Dhawan
BN: Choleretic effect of andrographolide in rats and guinea pigs1.
Planta Med. 58:146–149. 1992.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Srinivasa Reddy P, Jamil K, Madhusudhan P,
Anjani G and Das B: Antibacterial activity of isolates from piper
longum and taxus baccata. Pharm Biol. 39:236–238. 2001.
|
|
19
|
Prajapati ND, Purohit SS, Sharma AK and
Kumar T: A handbook of medicinal plants: A complete source book.
Agrobios, Rajasthan, p554, 2003.
|
|
20
|
Chang HM and But PP: Pharmacology and
Applications of Chinese Materia Medica. Vol 1. World Scientific,
Singapore, p304, 1986.
|
|
21
|
Liu J, Wang ZT and Ji LL: In vivo and in
vitro anti-inflammatory activities of neoandrographolide. Am J Chin
Med. 35:317–328. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Islam MT, Ali ES, Uddin SJ, Shaw S, Islam
MA, Ahmed MI, Chandra Shill M, Karmakar UK, Yarla NS, Khan IN, et
al: Phytol: A review of biomedical activities. Food Chem Toxicol.
121:82–94. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Suriyo T, Pholphana N, Rangkadilok N,
Thiantanawat A, Watcharasit P and Satayavivad J: Andrographis
paniculata extracts and major constituent diterpenoids inhibit
growth of intrahepatic cholangiocarcinoma cells by inducing cell
cycle arrest and apoptosis. Planta Med. 80:533–543. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fulda S: Modulation of apoptosis by
natural products for cancer therapy. Planta Med. 76:1075–1079.
2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mishra T: Ethno-botanical and therapeutic
importance of sacred plants of Terai region of Gorakhpur division.
Eur J Pharm Med Res. 3:534–540. 2016.
|
|
26
|
Plubrukarn A, Pinsuwan S, Ingkatawornwong
S and Supavita T: Stability of andrographolide in powdered
Andrographis herb under accelerated conditions. Planta Med.
72:954–956. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kumar RA, Sridevi K, Kumar NV, Nanduri S
and Rajagopal S: Anticancer and immunostimulatory compounds from
Andrographis paniculata. J Ethnopharmacol. 92:291–295.
2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li L, Yue GG, Lee JK, Wong EC, Fung KP, Yu
J, Lau CB and Chiu PW: The adjuvant value of Andrographis
paniculata in metastatic esophageal cancer treatment-from
preclinical perspectives. Sci Rep. 7(854)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chiou WF, Chen CF and Lin JJ: Mechanisms
of suppression of inducible nitric oxide synthase (iNOS) expression
in RAW 264.7 cells by andrographolide. Br J Pharmacol.
129:1553–1560. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rajagopal S, Kumar RA, Deevi DS,
Satyanarayana C and Rajagopalan R: Andrographolide, a potential
cancer therapeutic agent isolated from Andrographis
paniculata. J Exp Ther Oncol. 3:147–158. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nanduri S, Nyavanandi VK, Thunuguntla SS,
Kasu S, Pallerla MK, Ram PS, Rajagopal S, Kumar RA, Ramanujam R,
Babu JM, et al: Synthesis and structure-activity relationships of
andrographolide analogues as novel cytotoxic agents. Bioorg Med
Chem Lett. 14:4711–4717. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Khandelwal KR: Practical Pharmacognosy.
Pragati Books Pvt. Ltd., Pune, 2008.
|
|
33
|
Razack S, Kumar KH, Nallamuthu I, Naika M
and Khanum F: Antioxidant, biomolecule oxidation protective
activities of Nardostachys jatamansi DC and its phytochemical
analysis by RP-HPLC and GC-MS. Antioxidants (Basel). 4:185–203.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Konappa N, Udayashankar AC, Krishnamurthy
S, Pradeep CK, Chowdappa S and Jogaiah S: GC-MS analysis of
phytoconstituents from Amomum nilgiricum and molecular docking
interactions of bioactive serverogenin acetate with target
proteins. Sci Rep. 10(16438)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tsai HC, Li YC, Hsu SH, Young TH and Chen
MH: Inhibition of growth and migration of oral and cervical cancer
cells by citrus polyphenol. J Formos Med Assoc. 115:171–185.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ganguly A, Yang H, Sharma R, Patel KD and
Cabral F: The role of microtubules and their dynamics in cell
migration. J Biol Chem. 287:43359–43369. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nikolova I, Marinov L, Georgieva A,
Toshkova R, Malchev M, Voynikov Y and Kostadinova I: Metamizole
(dipyrone)-cytotoxic and antiproliferative effects on HeLa, HT-29
and MCF-7 cancer cell lines. Biotechnol Biotechnol Equip.
32:1327–1337. 2018.
|
|
38
|
American Veterinary Medical Association
(AVMA): AVMA Guidelines for the Euthanasia of Animals: 2020
Edition. AVMA, Schaumburg, IL, 2020.
|
|
39
|
Okhuarobo A, Falodun JE, Erharuyi O,
Imieje V, Falodun A and Langer P: Harnessing the medicinal
properties of Andrographis paniculata for diseases and
beyond: A review of its phytochemistry and pharmacology. Asian Pac
J Trop Dis. 4:213–222. 2014.
|
|
40
|
Oskoueian E, Abdullah N, Ahmad S, Saad WZ,
Omar AR and Ho YW: Bioactive compounds and biological activities of
Jatropha curcas L. kernel meal extract. Int J Mol Sci.
12:5955–5970. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Özçelik B, Kartal M and Orhan I:
Cytotoxicity, antiviral and antimicrobial activities of alkaloids,
flavonoids, and phenolic acids. Pharm Biol. 49:396–402.
2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Pejin B, Savic A, Sokovic M, Tesevic V,
Savic A, Radotic K and Mojovic M: Further in vitro evaluation of
antiradical and antimicrobial activities of phytol. Nat Prod Res.
28:372–376. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sareer O, Ahmad S and Umar S:
Andrographis paniculata: A critical appraisal of extraction,
isolation and quantification of andrographolide and other active
constituents. Nat Prod Res. 28:2081–2101. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rajani M, Shrivastava N and Ravishankara
MN: A rapid method for isolation of andrographolide from
Andrographis paniculata nees (Kalmegh). Pharm Biol.
38:204–209. 2000.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Redza-Dutordoir M and Averill-Bates DA:
Activation of apoptosis signalling pathways by reactive oxygen
species. Biochim Biophys Acta. 1863:2977–2992. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang CZ, Calway T and Yuan CS: Herbal
medicines as adjuvants for cancer therapeutics. Am J Chin Med.
40:657–669. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wu YC, Luo SH, Mei WJ, Cao L, Wu HQ and
Wang ZY: Synthesis and biological evaluation of
4-biphenylamino-5-halo-2(5H)-furanones as potential anticancer
agents. Eur J Med Chem. 139:84–94. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lattmann E, Sattayasai N, Schwalbe CS,
Niamsanit S, Billington DC, Lattmann P, Langley CA, Singh H and
Dunn S: Novel anti-bacterials against MRSA: Synthesis of focussed
combinatorial libraries of tri-substituted 2(5H)-furanones. Curr
Drug Discov Technol. 3:125–134. 2006.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zapf S, Anke T and Sterner O:
Incrustoporin, a new antibiotic from Incrustoporia carneola (Bres.)
Ryv. (Basidiomycetes). Acta Chem Scand (Cph). 49:233–234.
1995.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hashem AI, Youssef AS, Kandeel KA and
Abou-Elmagd WS: Conversion of some 2(3H)-furanones bearing a
pyrazolyl group into other heterocyclic systems with a study of
their antiviral activity. Eur J Med Chem. 42:934–939.
2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Weber V, Coudert P, Rubat C, Duroux E,
Vallée-Goyet D, Gardette D, Bria M, Albuisson E, Leal F, Gramain
JC, et al: Novel 4,5-diaryl-3-hydroxy-2(5H)-furanones as
anti-oxidants and anti-inflammatory agents. Bioorg Med Chem.
10:1647–1658. 2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Cinkilic N, Cetintas SK, Zorlu T, Vatan O,
Yilmaz D, Cavas T, Tunc S, Ozkan L and Bilaloglu R: Radioprotection
by two phenolic compounds: Chlorogenic and quinic acid, on X-ray
induced DNA damage in human blood lymphocytes in vitro. Food Chem
Toxicol. 53:359–363. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Pero RW, Lund H and Leanderson T:
Antioxidant metabolism induced by quinic acid. Increased urinary
excretion of tryptophan and nicotinamide. Phytother Res.
23:335–346. 2009.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Inbathamizh L and Padmini E: Quinic acid
as a potent drug candidate for prostate cancer-a comparative
pharmacokinetic approach. Asian J Pharm Clin Res. 6:106–112.
2013.
|
|
55
|
Jang SA, Park DW, Kwon JE, Song HS, Park
B, Jeon H, Sohn EH, Koo HJ and Kang SC: Quinic acid inhibits
vascular inflammation in TNF-α-stimulated vascular smooth muscle
cells. Biomed Pharmacother. 96:563–571. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang W, Zhu XL, Ding W and Shi XX: A
novel stereoselective synthesis of (-)-quinic acid starting from
the naturally abundant (-)-shikimic acid. Tetrahedron Asymmetry.
26:1375–1381. 2015.
|
|
57
|
Soh Y, Kim JA, Sohn NW, Lee KR and Kim SY:
Protective effects of quinic acid derivatives on
tetrahydropapaveroline-induced cell death in C6 glioma cells. Biol
Pharm Bull. 26:803–807. 2003.PubMed/NCBI View Article : Google Scholar
|
|
58
|
de Moraes J, de Oliveira RN, Costa JP,
Junior AL, de Sousa DP, Freitas RM, Allegretti SM and Pinto PL:
Phytol, a diterpene alcohol from chlorophyll, as a drug against
neglected tropical disease Schistosomiasis mansoni. PLoS Negl Trop
Dis. 8(e2617)2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Guo J, Yuan Y, Lu D, Du B, Xiong L, Shi J,
Yang L, Liu W, Yuan X, Zhang G and Wang F: Two natural products,
trans-phytol and (22E)-ergosta-6,9,22-triene-3β,5α,8α-triol,
inhibit the biosynthesis of estrogen in human ovarian granulosa
cells by aromatase (CYP19). Toxicol Appl Pharmacol. 279:23–32.
2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Takahashi N, Kawada T, Goto T, Yamamoto T,
Taimatsu A, Matsui N, Kimura K, Saito M, Hosokawa M, Miyashita K
and Fushiki T: Dual action of isoprenols from herbal medicines on
both PPARgamma and PPARalpha in 3T3-L1 adipocytes and HepG2
hepatocytes. FEBS Lett. 514:315–322. 2002.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Costa JP, de Oliveira GA, de Almeida AA,
Islam MT, de Sousa DP and de Freitas RM: Anxiolytic-like effects of
phytol: Possible involvement of GABAergic transmission. Brain Res.
1547:34–42. 2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Santos CC, Salvadori MS, Mota VG, Costa
LM, de Almeida AA, de Oliveira GA, Costa JP, de Sousa DP, de
Freitas RM and de Almeida RN: Antinociceptive and antioxidant
activities of phytol in vivo and in vitro models. Neurosci J.
2013(949452)2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Arnhold T, Elmazar MM and Nau H:
Prevention of vitamin A teratogenesis by phytol or phytanic acid
results from reduced metabolism of retinol to the teratogenic
metabolite, all-trans-retinoic acid. Toxicol Sci. 66:274–282.
2002.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ryu KR, Choi JY, Chung S and Kim DH:
Anti-scratching behavioral effect of the essential oil and phytol
isolated from Artemisia princeps Pamp. in mice. Planta Med.
77:22–26. 2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chowdhury RR and Ghosh SK: Phytol-derived
novel isoprenoid immunostimulants. Front Immunol.
3(49)2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Rajab MS, Cantrell CL, Franzblau SG and
Fischer NH: Antimycobacterial activity of (E)-phytol and
derivatives: A preliminary structure-activity study. Planta Med.
64:2–4. 1998.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Saikia D, Parihar S, Chanda D, Ojha S,
Kumar JK, Chanotiya CS, Shanker K and Negi AS: Antitubercular
potential of some semisynthetic analogues of phytol. Bioorg Med
Chem Lett. 20:508–512. 2010.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Inoue Y, Hada T, Shiraishi A, Hirose K,
Hamashima H and Kobayashi S: Biphasic effects of geranylgeraniol,
teprenone, and phytol on the growth of Staphylococcus
aureus. Antimicrob Agents Chemother. 49:1770–1774.
2005.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Sakthivel R, Malar DS and Devi KP: Phytol
shows anti-angiogenic activity and induces apoptosis in A549 cells
by depolarizing the mitochondrial membrane potential. Biomed
Pharmacother. 105:742–752. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Zhao Q, Ou J, Huang C, Qiu R, Wang Y, Liu
F, Zheng J and Ou S: Absorption of
1-dicysteinethioacetal-5-hydroxymethylfurfural in rats and its
effect on oxidative stress and gut microbiota. J Agric Food Chem.
66:11451–11458. 2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Silva Ferreira AC, Rodrigues P, Hogg T and
Guedes de Pinho P: Influence of some technological parameters on
the formation of dimethyl sulfide, 2-mercaptoethanol, methionol,
and dimethyl sulfone in port wines. J Agric Food Chem. 51:727–732.
2003.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Yoshiki Y and Okubo K: Active oxygen
scavenging activity of DDMP (2,3-dihydro-2,5-dihydroxy-6-methyl-4
H-pyran-4-one) saponin in soybean seed. Biosci Biotechnol Biochem.
59:1556–1557. 1995.
|
|
73
|
Nair SC, Kurumboor SK and Hasegawa JH:
Saffron chemoprevention in biology and medicine: A review. Cancer
Biother. 10:257–264. 1995.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Begum SFMM, Priya S, Sundararajan R and
Hemalatha S: Novel anticancerous compounds from Sargassum wightii:
In silico and in vitro approaches to test the antiproliferative
efficacy. J Adv Pharm Educ Res. 7:272–276. 2017.
|
|
75
|
Mujeeb F, Bajpai P and Pathak N:
Phytochemical evaluation, antimicrobial activity, and determination
of bioactive components from leaves of Aegle marmelos. Biomed Res
In. 2014(497606)2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Inagaki M, Jyoyama H, Ono T, Yamada K,
Kobayashi M, Baba T, Touchi A, Iwatani K, Ohkawa T, Matsumoto S and
Tsuri T: Synthesis and activities of oxidative metabolites of the
anti-arthritic drug candidate S-2474. Bioorg Med Chem.
11:2415–2419. 2003.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Abdullah BM, Mehdi MAH, Khan AR and Pathan
JM: Gas chromatography-mass spectrometry (GC-MS) analysis of ajwain
(Trachyspermum ammi) seed extract. Int J Pharm Qual Assur.
11:228–231. 2020.
|
|
78
|
Rahbar N, Shafaghat A and Salimi F:
Antimicrobial activity and constituents of the hexane extracts from
leaf and stem of Origanum vulgare L. ssp. Viride (Boiss.) Hayek.
growing wild in Northwest Iran. J Med Plants Res. 6:2681–2685.
2012.
|
|
79
|
Zekeya N, Chacha M, Shahada F and Kidukuli
A: Analysis of phytochemical composition of Bersama abyssinica by
gas chromatography-mass spectrometry. J Pharmacogn Phytochem.
3:246–252. 2014.
|
|
80
|
Huang CB and Ebersole JL: A novel
bioactivity of omega-3 polyunsaturated fatty acids and their ester
derivatives. Mol Oral Microbiol. 25:75–80. 2010.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Ahmad B, Khan I, Bashir S and Azam S:
Chemical composition and antifungal, phytotoxic, brine shrimp
cytotoxicity, insecticidal and antibacterial activities of the
essential oils of Acacia modesta. J Med Plants Res. 6:4653–4659.
2012.
|
|
82
|
Roy RN, Laskar S and Sen SK: Dibutyl
phthalate, the bioactive compound produced by Streptomyces
albidoflavus 321.2. Microbiol Res. 161:121–126. 2006.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Sunita A, Ganesh K and Sonam M: Screening
and evaluation of bioactive components of Cenchrus ciliaris L. by
GC-MS analysis. Int Res J Pharm. 8:69–76. 2017.
|
|
84
|
Yu FR, Lian XZ, Guo HY, McGuire PM, Li RD,
Wang R and Yu FH: Isolation and characterization of methyl esters
and derivatives from euphorbia kansui (euphorbiaceae) and their
inhibitory effects on the human SGC-7901 cells. J Pharm Pharm Sci.
8:528–535. 2005.PubMed/NCBI
|
|
85
|
Siyumbwa SN, Ekeuku SO, Amini F, Emerald
NM, Sharma D and Okechukwu PN: Wound healing and antibacterial
activities of 2-Pentadecanone in streptozotocin-induced Type 2
diabetic rats. Pharmacogn Mag. 15:71–77. 2019.
|
|
86
|
Gupta S, Afaq F and Mukhtar H: Involvement
of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle
arrest and apoptosis by apigenin in human prostate carcinoma cells.
Oncogene. 21:3727–3738. 2002.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Tian Z, Shen J, Moseman AP, Yang Q, Yang
J, Xiao P, Wu E and Kohane IS: Dulxanthone A induces cell cycle
arrest and apoptosis via up-regulation of p53 through mitochondrial
pathway in HepG2 cells. Int J Cancer. 122:31–38. 2008.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Yue GG, Lee JK, Kwok HF, Cheng L, Wong EC,
Jiang L, Yu H, Leung HW, Wong YL, Leung PC, et al: Novel PI3K/AKT
targeting anti-angiogenic activities of 4-vinylphenol, a new
therapeutic potential of a well-known styrene metabolite. Sci Rep.
5(11149)2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Cimmino A, Scafato P, Mathieu V, Ingels A,
D'Amico W, Pisani L, Maddau L, Superchi S, Kiss R and Evidente A:
Natural and synthetic furanones with anticancer activity. Nat Prod
Commun. 11:1471–1474. 2016.PubMed/NCBI
|
|
90
|
Ma F, Zhang J, Li M, Yu J, Luo W, Li X and
Wei M: Synthesis of novel matrine derivatives containing furanone
skeleton and preliminary evaluation of their anticancer activity in
vitro. Chin J Org Chem. 38:2633–2638. 2018.
|
|
91
|
de Alencar MVOB, Islam MT, de Lima RMT,
Paz MFCJ, Dos Reis AC, da Mata AMOF, Filho JWGO, Cerqueira GS,
Ferreira PMP, E Sousa JMC, et al: Phytol as an anticarcinogenic and
antitumoral agent: An in vivo study in swiss mice with DMBA-Induced
breast cancer. IUBMB Life. 71:200–212. 2019.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Murugesan A, Holmstedt S, Brown KC,
Koivuporras A, Macedo AS, Nguyen N, Fonte P, Rijo P, Yli-Harja O,
Candeias NR and Kandhavelu M: Design and synthesis of novel quinic
acid derivatives: In vitro cytotoxicity and anticancer effect on
glioblastoma. Future Med Chem. 12:1891–1910. 2020.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Singh A, Chauhan SS and Tripathi V: Quinic
acid attenuates oral cancer cell proliferation by downregulating
cyclin D1 Expression and Akt signaling. Pharmacogn Mag. 14:14–19.
2018.
|