Introduction
Throughout human history, infectious diseases have
been the leading cause of death, especially during pandemics
(1). As expected, the COVID-19
pandemic had a major impact on humanity. Vaccination has been shown
to reduce morbidity and mortality, particularly among vulnerable
populations; cancer patients are one of the most vulnerable groups
both due to the disease itself and more frequent hospital
presentations. They also have an increased risk of developing
severe complications and even die due to the infection (2).
Cancer patients have a higher risk of mortality as
compared to non-cancer patients. Consequently, mortality rates were
high among patients with active cancer who developed COVID-19
during the first wave of the pandemic. In the United Kingdom,
mortality in hospitalized cancer patients with COVID-19 was 40.5%
vs. 28.5% for non-cancer patients (HR 1.62; p <0.001) (3), while in the German LEOSS registry,
COVID-19-related mortality in cancer patients was 22.5% vs. 14% for
non-cancer patients (p <0.001) (4).
Vaccination campaigns were launched less than a year
after the pandemic outbreak. In studies, the vaccine demonstrated
more than 90% efficacy in the general population (5). Cancer patients were shown to have a
variable, higher risk of developing COVID-19, and were considered a
target population for vaccination (6). Therefore, COVID-19 prevention is
crucial for cancer patients, with vaccination being the most
effective method to achieve this goal.
Vaccines have always been surrounded by controversy
and polarized the wide public into two opposing camps: those in
favor of vaccination and those against it. The most common reasons
for vaccination refusal include negative opinions about the need of
vaccination, fear of side effects and fear of needles (7,8).
Although the infection caused by SARS-CoV-2 spread around the
world, in Romania a large part of the population denied the very
existence of the disease, the need of vaccination and the use of
prevention methods such as wearing a mask or social distancing
(9). Immediately after the
beginning of the pandemic, major research centers focused on the
discovery of a vaccine. The relatively brief time interval needed
for the development of vaccines raised suspicions among the general
population, while doubts about the long-term side effects,
effectiveness and safety of the vaccine were the main concerns of
cancer patients (10).
Furthermore, the use of non-scientific sources of information led
to misconceptions about vaccination (11).
The main purpose of our study was to assess the
acceptance of the COVID-19 vaccine among cancer patients undergoing
immunosuppressive therapy from 12 oncology centers in Western and
Northwestern Romania by means of administration of an adapted
questionnaire and to understand which factors are associated with
COVID-19 vaccine acceptance.
Since according to GLOBOCAN 2020(12), there were approximately 260,000
cancer patients in Romania, we calculated a statistically
representative sample for this population of 384 respondents by
sample size determination test. Normality analyses were performed
and the results and statistical tests were adjusted accordingly.
This means at least 384 measurements/surveys were needed to have a
confidence level of 95% that the real value is within +/- 5% of the
measured/surveyed value.
To the best of our knowledge, no study has been
performed to date to investigate the perception regarding COVID-19
vaccination among cancer patients considering their reason for
acceptance, educational level and source of information. In our
opinion, such an assessment is of extreme importance in this
specific group of patients, therefore we conducted this pilot study
in Romania.
Materials and methods
Patients
This is a pilot cross-sectional study to investigate
the attitudes of Romanian cancer patients towards the COVID-19
vaccination, based on a self-administered questionnaire. The survey
questions were validated by the Institutional Ethics Committee of
the ‘Prof Dr Octavian Fodor’ Regional Institute of Gastroenterology
and Hepatology in Cluj-Napoca, Romania. The target group included
patients meeting the following criteria:
a) >18 years of age;
b) Oncology patient defined as a patient with solid
or hematologic tumours undergoing active systemic treatment or
follow-up;
c) Any level of education;
d) Any status of COVID-19 vaccination;
e) Any history of SARS-CoV-2 infection.
Patients were selected upon presentation to the
oncologist from 12 hospitals (out of 14) in Northwestern Romania.
We targeted these 12 hospitals because they include the largest and
most representative oncology departments that provide treatment and
follow-up to most cancer patients in Northwestern Romania. We
enrolled patients who consented to enter the study on a
presentation to their medical oncologist during active treatment or
follow-up. The recruitment period lasted between December
2021-January 2022, during the fourth wave of the COVID-19 pandemic
in Romania. We avoided a selection bias by indiscriminately
offering the patients the opportunity to take part in the study and
complete the questionnaire. The selection was based on their
consent and all patients provided a written informed consent.
During the mentioned period, 932 patients agreed to complete the
survey.
The details were collected by means of an anonymous
questionnaire including questions about age, the cancer diagnosis,
disease stage and type of cancer treatment, place of residence
(rural or urban) and formal education level (primary, secondary or
university), history of COVID-19 infection, influenza vaccination
status and COVID-19 vaccination status. Patients were further
guided based on the answer to the question: ‘Are you vaccinated
against COVID-19?’ Patients who answered ‘yes’ continued to
questions regarding the type of vaccine chosen, side effects
related to the first and second dose, drivers of the vaccination
acceptance and the source of information about the vaccine.
Patients who answered ‘no’ completed questions regarding the
intention to get vaccinated, why they refused the vaccination and
their main source of information about the vaccination.
Statistical analysis
This a cross-sectional study, so we selected a
number of 932 patients out of the total 260,884 cancer patients in
Romania (GLOBOCAN 2020) (12). To
provide our study with statistical validity or significance/power
(95% confidence level), we calculated that a sample of at least 384
patients was necessary. Vaccinated and non-vaccinated cancer
patients were compared using Pearson χ2 test.
Results
Patient population
A total of 932 cancer patients undergoing active
treatment or follow-up who completed the questionnaire were
included in the study. The analyzed patient population
characteristics are listed in Table
I. The age of the patients ranged between 25 to 82 years; 541
(58.05%) accepted vaccination while 391 (41.95%) rejected it. Only
22 patients were under the age of 30; of these, 8 (36.36%) were
vaccinated and 14 (63.64%) were not vaccinated. The age group where
the vaccination rate was highest ranged between 61-70 years and
included 361 patients, 221 (61.22%) of which were vaccinated and
140 (38.78%) not vaccinated.
 | Table ICharacteristics of patient
population. |
Table I
Characteristics of patient
population.
|
Characteristics | All patients, n
(%) | Vaccinated, n (% of
all patients) | Not vaccinated, n
(% of all patients) | P-value |
|---|
| Number of
patients | 932 | 541 (58.05) | 391 (41.95) | |
| Age group,
years | | | | 0.001 |
|
<30 | 22 (2.36) | 8 (0.85) | 14 (1.51) | |
|
31-40 | 37 (3.97) | 15 (1.60) | 22 (2.37) | |
|
41-50 | 124 (13.30) | 73 (7.84) | 51 (5.47) | |
|
51-60 | 200 (21.46) | 118 (12.67) | 82 (8.79) | |
|
61-70 | 361 (38.74) | 221 (23.72) | 140 (15.02) | |
|
>71 | 188 (20.17) | 106 (11.37) | 82 (8.79) | |
| Residence | | | | 0.001 |
|
Rural | 382 (40.97) | 178 (19.09) | 204 (21.88) | |
|
Urban | 550 (59.00) | 363 (38.94) | 187 (20.06) | |
|
Education | | | | 0.001 |
|
Elementary
school | 416 (44.64) | 193 (20.71) | 223 (23.93) | |
|
High
school | 303 (32.51) | 184 (19.74) | 119 (12.77) | |
|
University | 213 (22.85) | 164 (17.59) | 49 (5.26) | |
| Primary tumor | | | | 0.001 |
|
Breast
cancer | 210 (22.53) | 116 (12.44) | 94 (10.09) | |
|
Gastrointestinal
cancera | 277 (29.72) | 179 (19.21) | 98 (10.51) | |
|
Genitourinary
cancerb | 109 (11.70) | 68 (7.30) | 41 (4.40) | |
|
Gynecological
cancer | 77 (8.26) | 35 (3.75) | 42 (4.51) | |
|
Hematologyc | 119 (12.77) | 68 (7.29) | 51 (5.48) | |
|
Lung
cancer | 85 (9.12) | 45 (4.83) | 40 (4.29) | |
|
Skin
cancerd | 19 (2.04) | 13 (1.40) | 6 (0.64) | |
|
Othere | 36 (3.86) | 17 (1.71) | 19 (2.15) | |
| Cancer treatment
phase | | | | 0.03 |
|
Active
treatment primaryf | 444 (47.64) | 250 (26.83) | 194 (20.81) | |
|
Active
treatment metastaticg | 347 (37.23) | 195 (20.93) | 152 (16.30) | |
|
Follow-up | 141 (15.13) | 96 (10.31) | 45 (4.82) | |
| Previous COVID-19
infections | 294 (31.53) | 163 (17.48) | 131 (14.05) | 0.27 |
| Previous influenza
vaccine | 384 (41.20) | 278 (29.82) | 106 (1.00) | 0.001 |
Place of residence
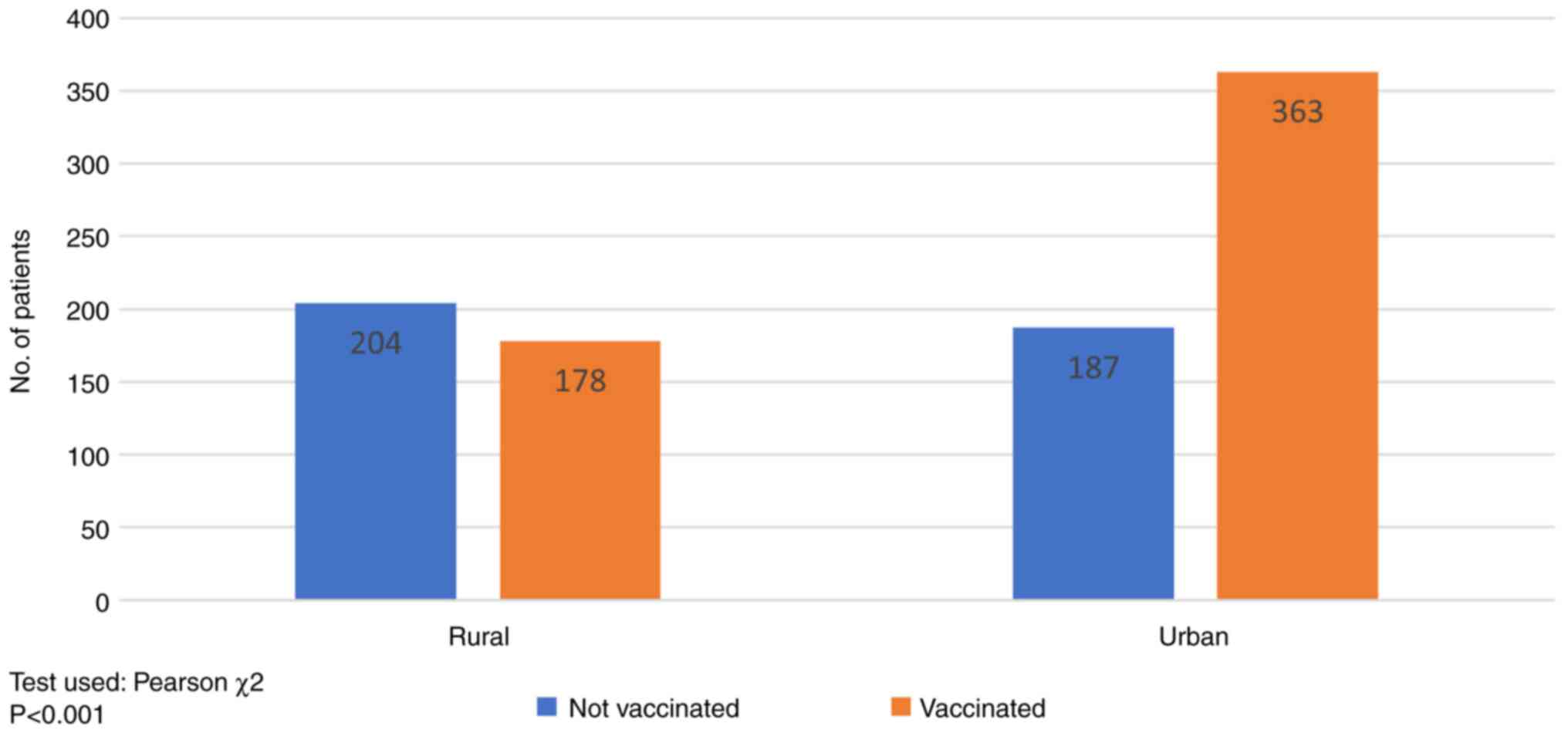
According to the place of residence (Fig. 1), in patients from rural areas the
immunization rate reached 46.06% (178/382), well below the urban
population rate of 66% (363/550). This difference was statistically
significant (P<0.001).
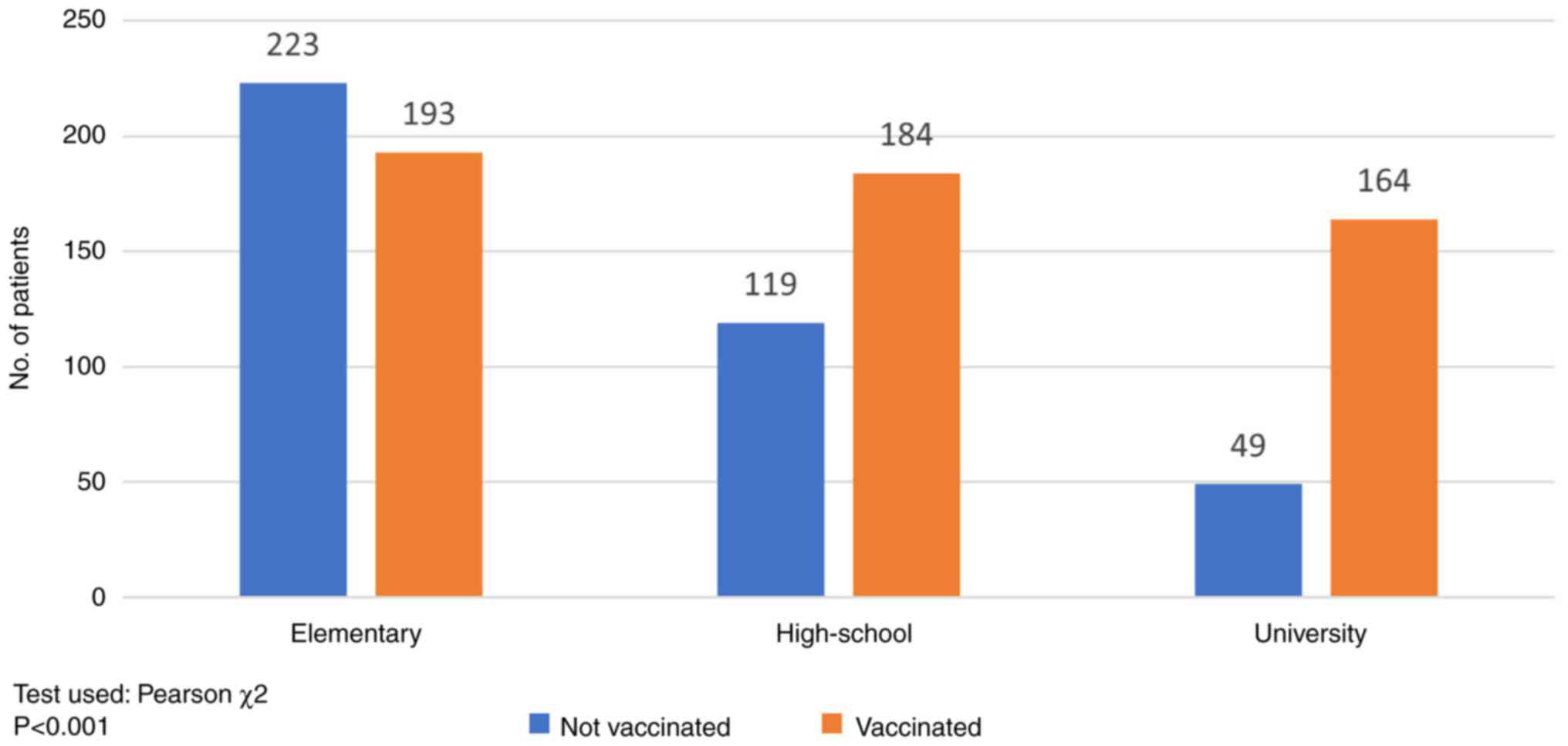
Level of education
The impact of the level of formal education on the
vaccination status is shown in Fig.
2. 416 of the total number of patients had primary education;
193 (46.39%) were vaccinated and 223 (53.61%) non-vaccinated. The
rate of vaccination was higher in the secondary education group
(60.72%, 184/303) and in the post-secondary (university) education
group (76.99%, 164/213) (P<0.001).
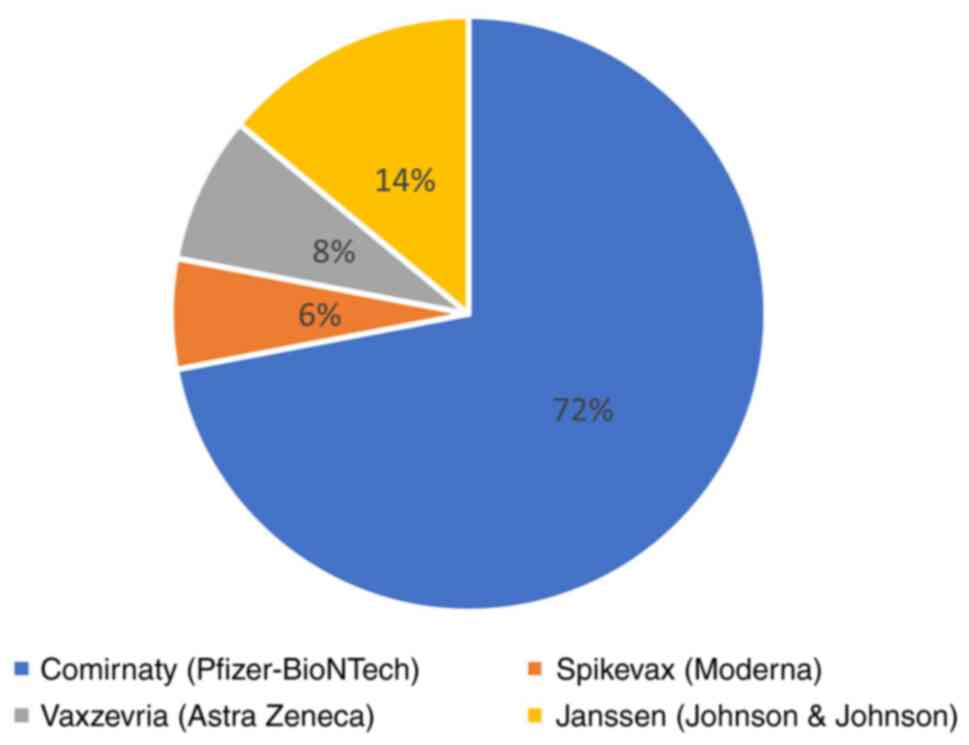
Vaccine distribution
In this cancer population, the most widely used
vaccine was Pfizer-BioNTech (72%, 329 patients), followed by
Johnson & Johnson, (14%, 75 patients), AstraZeneca (8%, 43
patients), and Moderna (6%, 33 patients) (Fig. 3).
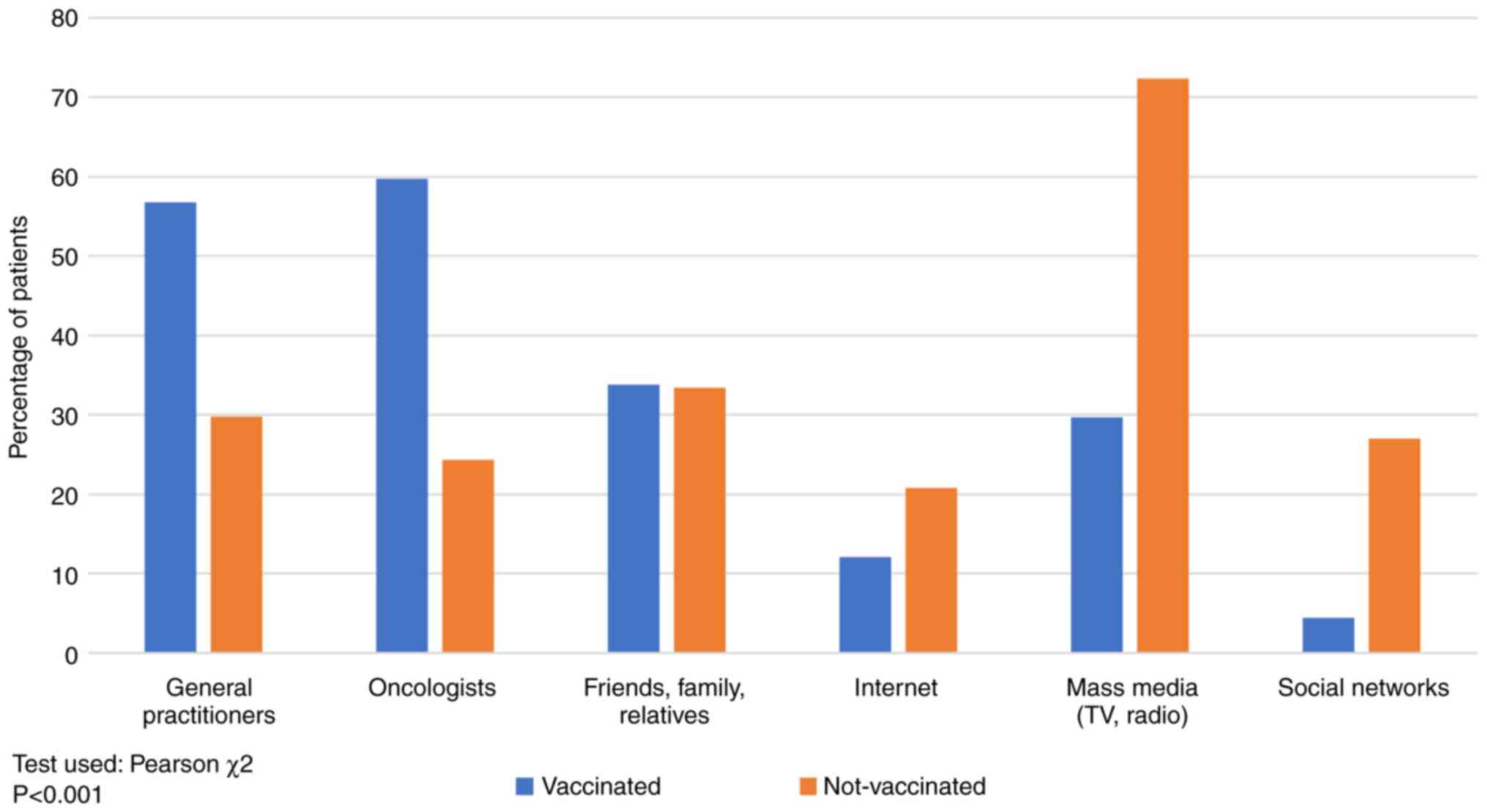
Source of information
As shown in Fig. 4,
the main source of information in non-vaccinated patients was the
mass-media (TV, radio), 72.38% vs. 29.67% in vaccinated patients,
respectively, while for vaccinated patients the primary source of
information was the recommendation by the oncologist (59.70%) and
the general practitioner (56.76%). Recommendations by general
practitioners and oncologists are considered in medical sources,
while mass-media, internet, social platforms and
friends'/family/relatives' advice are non-medical sources.
Since statistically significant differences were
observed in terms of the patients' place of residence and
vaccination status, we further investigated the main source of
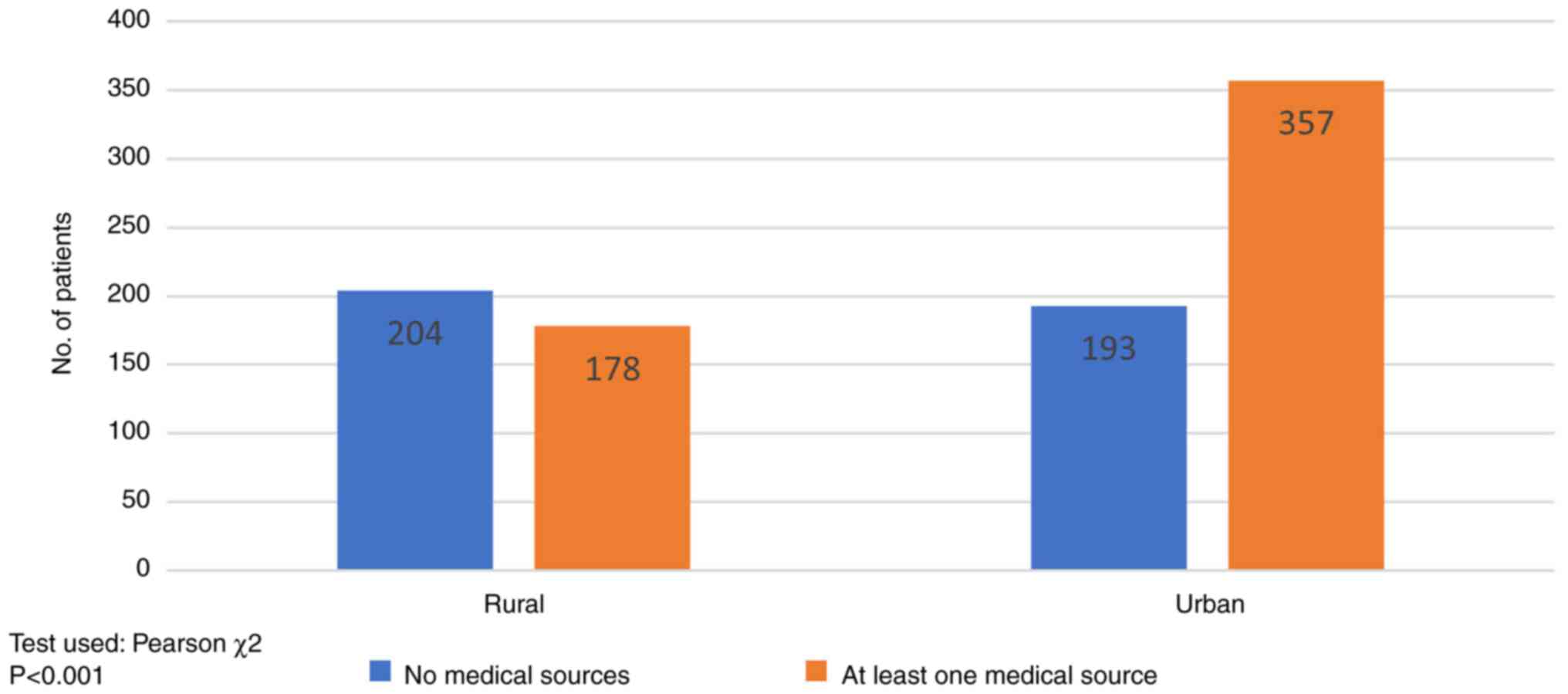
information based on the former. As shown in Fig. 5, patients (53.40%, 204/382) from
rural areas relied on a non-medical main source of information
(mass-media, social platforms, friends'/family advice), while in
urban areas medical sources of information predominated (64.09%,
357/550) (recommendation by the oncologist and general
practitioner) (P<0.001).
A statistically significant association between the
level of education and the rate of vaccination was also noticed,
therefore we explored the preferred sources of information
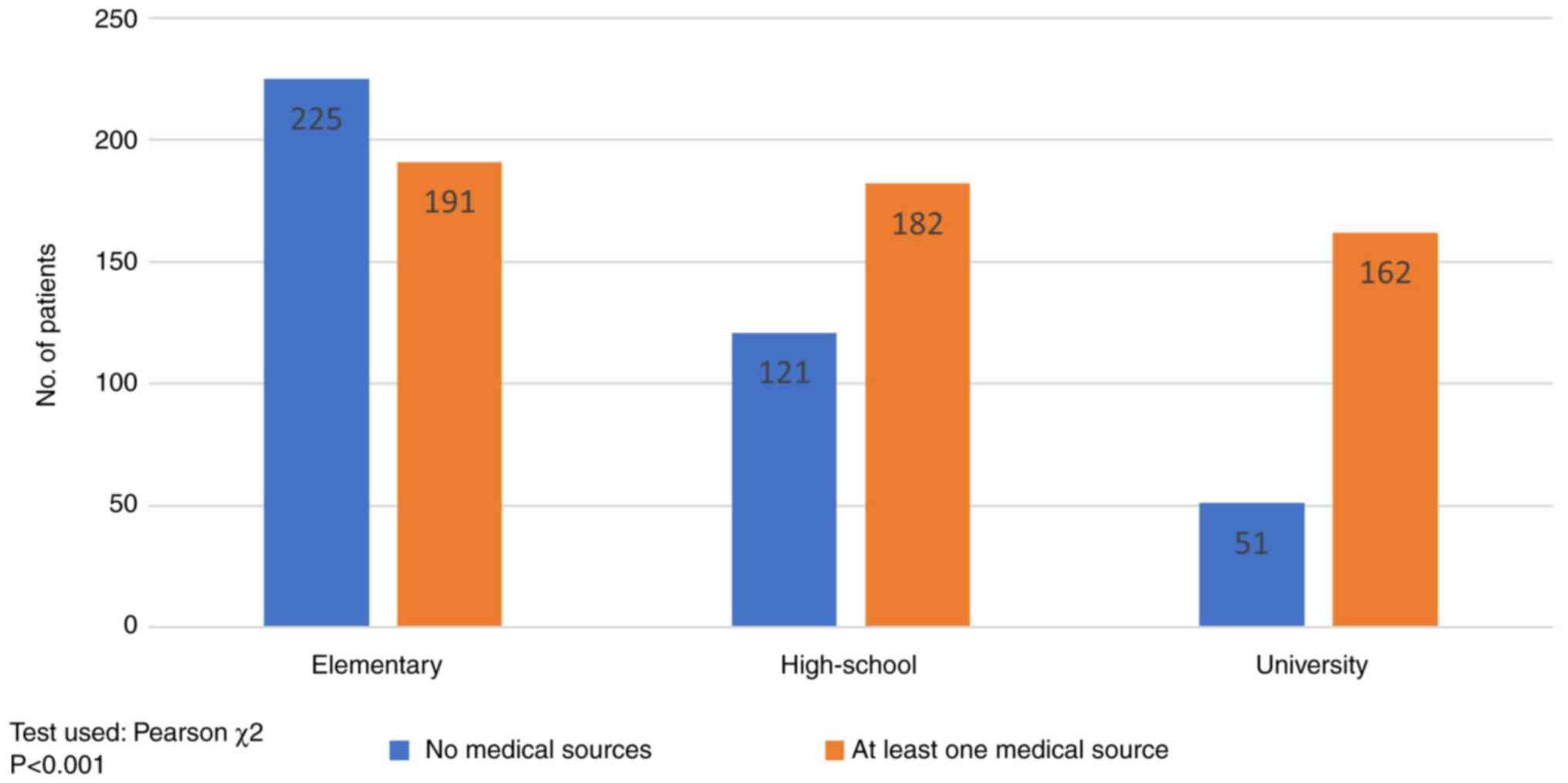
according to the level of education. Fig. 6 shows that high-school (60.06%,
182/303) or university (76.05%, 162/213) graduates relied on a
medical source of information (oncologist, general practitioner
recommendation), while primary education patients (54.08%, 225/416)
were influenced by non-medical sources in particular (mass-media,
social platforms or friends'/family advice) (P<0.001).
The main source of information in non-vaccinated
patients was mass-media (TV, radio) at a rate of 72.38% as compared
to 29.67% among vaccinated patients. In vaccinated patients the
primary source of information was the recommendation by the
oncologist (59.70%) and the general practitioner (56.76%).
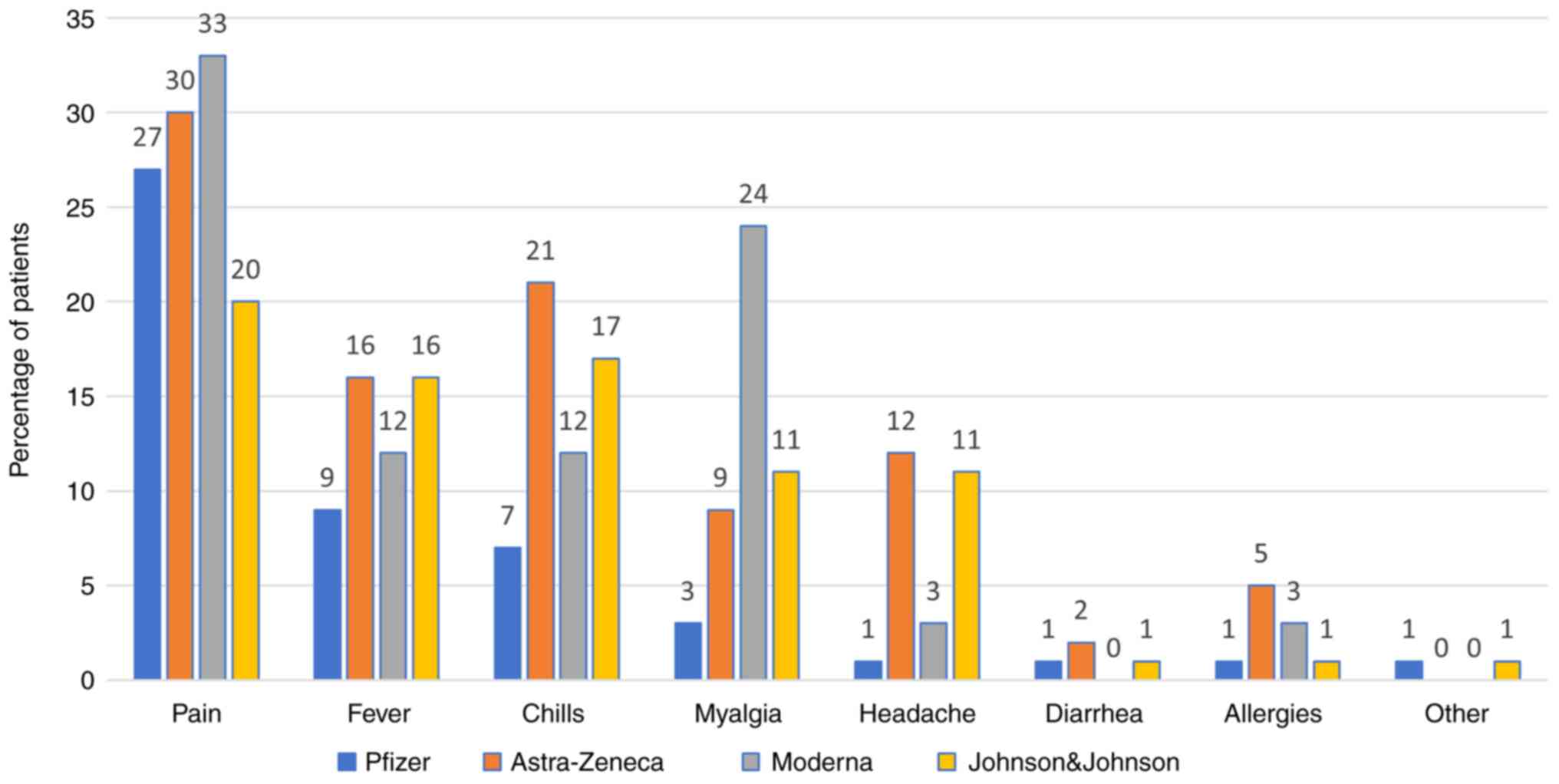
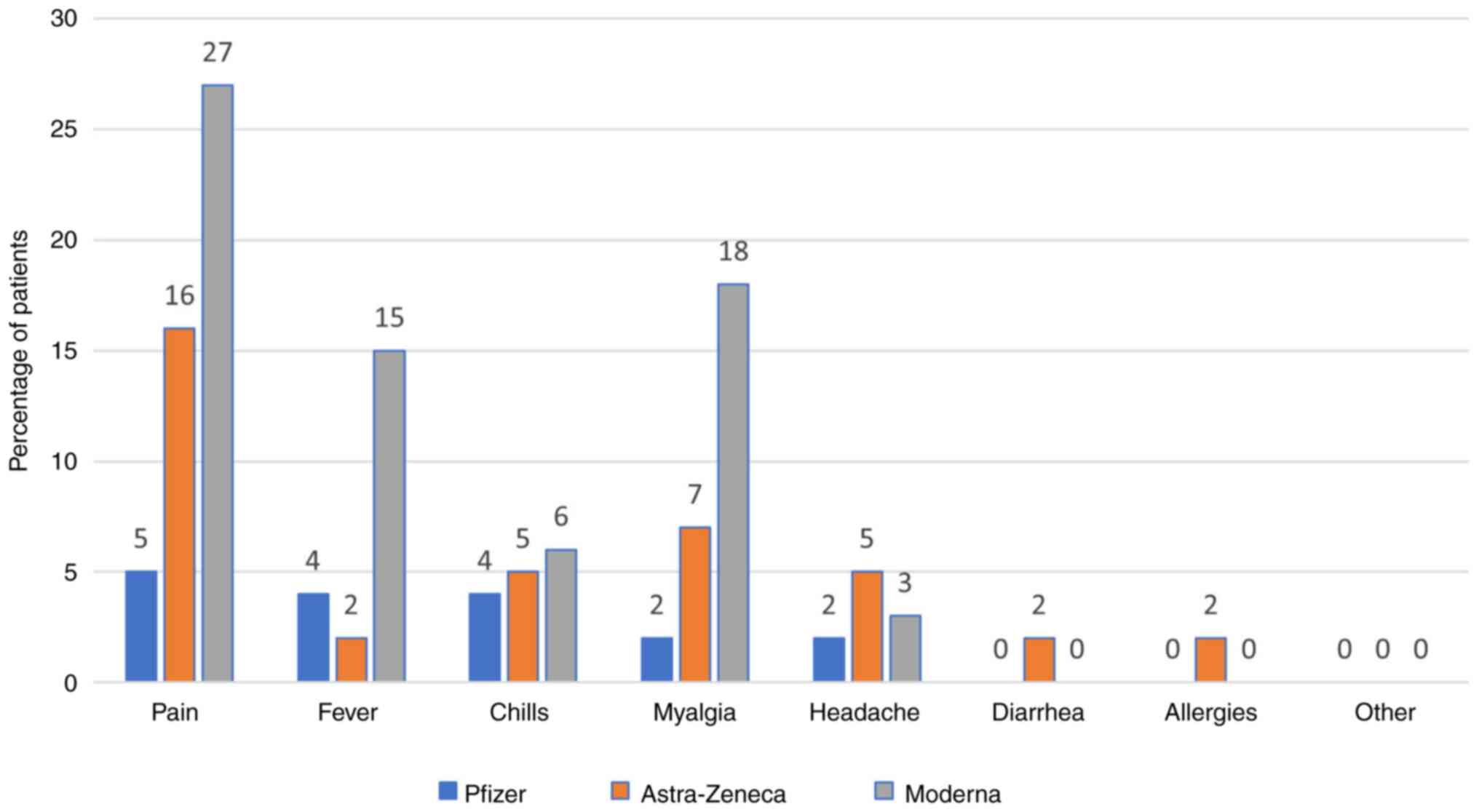
Side effects
The most commonly reported side effect was pain at
the injection site after the first (Pfizer vaccine, 27.04%) and the
second dose (Pfizer vaccine, 4.85%). All the side effects are shown
in Figs. 7 and 8.
Discussion
COVID-19 vaccination hesitancy rates vary in the
general population worldwide (13). This attitude towards COVID-19
vaccines among cancer patients was reported in other papers as well
(14,15,16).
According to the findings of this study, the Romanian cancer
patients also need more comprehensible information regarding this
issue.
In Romania, the first dose of COVID-19 vaccine was
administered on December 27, 2020 and all eligible individuals were
encouraged to get vaccinated, cancer patients in particular. The
European Society for Medical Oncology (ESMO) issued a statement
recommending vaccination in cancer patients, monitoring of side
effects and specific patient education. The European Medicines
Agency (EMA)-approved RNA vaccines are safe and have no
contraindications in patients with active cancer or in
immunocompromised patients (17).
The study we conducted to investigate the attitudes
of cancer patients regarding the COVID-19 vaccination, one year
from the start of the vaccination campaign, was based on 932
analyzed patients: 58.05% were vaccinated and 41.95% not
vaccinated. These results are comparable to data from the general
population of Romania, where the rate of vaccination amounts to
only 41.44% of the total population (18). Of the total number of patients
enrolled in the study, 31.54% had a history of COVID-19 infection
and 55.44% of these patients wanted to receive the vaccine. This
finding is comparable to the data from a study published in
Vaccines in 2022 regarding the general population of
Romania, where 33% of participants had a history of SARS-CoV-2
infection with different degrees of severity (9).
As far as the influenza vaccination is concerned,
41.20% of the total number of patients analyzed in our study
received such a vaccine, a higher rate as compared to the findings
of another study conducted in the Romanian cancer population
published in ESMO Open in 2021, before the start of SARS-CoV-2
vaccination campaign, where only 15.9% of patients had been
vaccinated against influenza the previous year (19). Higher rates of influenza
vaccination may be a useful by-product of the COVID-19 vaccination
campaigns.
In Romania, 4 types of COVID-19 vaccine were
available, all approved for use in cancer patients as well.
Vaccinated cancer patients preferred Pfizer-BioNTech (72%),
followed by Johnson & Johnson (14%). This finding is consistent
with the general population, with a significantly higher number of
Pfizer vaccines administered daily as compared to other vaccines
(18). The drivers for this
preference for Pfizer may be that it is EMA approved and shown to
be 95% safe and effective in people 16 years of age and older
(20).
To get a better understanding of attitudes towards
vaccination in our study, the vaccinated patients were asked about
how they made their decision while non-vaccinated patients about
their arguments against vaccination. The reasons in vaccinated
patients were the following: 63.96% observed the physicians'
recommendations, 56.56% reported fear of the side effects caused by
the SARS-CoV-2 infection, 53.05% wanted to protect their loved ones
and 46.58% wanted their life to return to normal as soon as
possible. These rates mirror the findings from the study published
in Vaccines in 2022, conducted on the general population of
Romania which assessed how much Romanians trusted physicians and
the Romanian medical system and showed that 65% had confidence in
the recommendations made by Romanian physicians, but only 26%
trusted the Romanian health system (7). This low percentage of confidence
results from problems encountered by the health system in recent
years such as lack of medicines, medical equipment and modern
facilities, as well as deadly hospital fires (21,22).
We compared the reasons provided by non-vaccinated
patients and 67.52% reported fear of vaccine-induced side effects,
28.13% did not consider the vaccine necessary and 20.46% did not
trust the current scientific data about it. We asked them if they
intended to get vaccinated and 50.38% promptly answered they were
unwilling to get vaccinated, 20.97% considered getting vaccinated
on completion of their oncological treatment, 18.93% expected more
evidence of vaccine efficacy and 9.46% were willing to get
vaccinated as soon as possible. This is how our results compare to
the findings in French [53.7% reported the desire to get
vaccinated, 29.7% considered they were not ready and 16.6% refused
vaccination (14)], Portuguese
[84% intended to get vaccinated, 16% had not yet decided or were
reluctant (23)] and Serbian
cancer patients, respectively [10% certainly rejected vaccination
(24)].
The main reason the population continues to reject
vaccination is the fear of side effects. Recent studies have begun
to systematically examine the frequency and types of side effects
(25,26). The authors highlight that the
adverse events reported (cerebral thrombosis, Guillain-Barré
syndrome, myocarditis, pericarditis) are not COVID-19
vaccination-specific side effects as compared with their background
rate in the general population.
In our study, the most common side effect was pain
at the injection site after the first and the second dose (for the
Pfizer vaccine, 27.04 and 4.85%, respectively). Other side effects
include fever, chills, headache, diarrhea, allergies, however all
with significantly lower rates (around 1.28-2.81% for Pfizer). For
other side effects, patients mentioned chest pain, dizziness,
finger numbness, but rates were low (0.51%). We have comparative
data from the study conducted in cancer patients in London by Monin
et al reporting a lower rate of side effects in cancer
patients compared to vaccinated healthy patients (46% vs. 62% and
29% vs. 69%) after the first and the second dose, respectively
(27). A study published in The
Breast which included breast and gynecologic cancer patients
receiving active treatment in Germany found that the COVID-19
vaccines were well tolerated while undergoing systemic cancer
therapy with no additional side effects reported as compared to the
general population (28). The data
provide solid evidence of the effectiveness of vaccinating patients
while receiving active treatment.
In our study, we found a statistically significant
association between the vaccination rate and both the place of
residence and the level of education, as independent factors. These
findings are comparable to those of the study published in
Supportive Care in Cancer regarding patients in Portugal
which showed that lower levels of education and rural residence are
predictive factors of COVID-19 vaccination hesitancy (23).
Multiple sources of information regarding the
COVID-19 infection and vaccines are used. As shown in Figs. 5 and 6, patients opted for different sources of
information, depending on their level of education and area of
residence. It is a known fact that mass-media often promote
misinformation, channeling the opinions of different experts
instead of official recommendations, which leads to increased
uncertainty, anxiety and confusion in the general population and
especially among cancer patients (29). The effects of ‘fake news’ were
evident in the study conducted on the general population of
Romania, where 47% of participants believed that a secret
organization that controls the world wants to decrease the global
population by means of vaccination (7,9). In
February 2022 in Romania, only 44.62% of the total population of
19.29 million had received a COVID-19 vaccine (30).
Our study suggests that the information on vaccines
needs to be tailored based on the patients' educational level.
Also, public information campaigns designed on different levels of
complexity may prove more efficient in Romania. Future research
including a larger population with various subpopulations would
provide further validation to our study. Also, this pilot study
which highlights a correlation between the formal education level
and the rate of vaccine acceptance gives a cue that public
awareness-raising campaigns worldwide should be tailored to the
patients' level of understanding.
The large number of cancer patients and the number
of oncology centers included is the main strength of our study. The
fact that it is the first study of this kind in Romania is the
second. The first limitation of our study is the heterogeneity of
participants and the lack of information on their medical
background which made it impossible to demonstrate if the surveyed
population is representative for the cancer patient population as a
whole. Also, a better stratification of patients as well as an
analysis of comorbidities would have helped to further clarify the
patients' attitudes. Another limitation of our study is that the
survey included non-standardized questions and that it was a
multicenter study, leading to differences in terms of the health
care providers-patient communication. However, to the best of our
knowledge, there are no globally validated questionnaires to
appropriately reflect our patients' characteristics and serve the
objective of our study.
This study demonstrates that Romanian cancer
patients are reluctant to receive the COVID-19 vaccines. In
summary, their hesitancy is mainly influenced by the fear of side
effects; mass-media (TV, radio) and social platforms play the main
role as source of information among non-vaccinated patients, while
for vaccinated patients the primary source of information is the
recommendation by the oncologists or the general practitioners.
Although scientific evidence to support the efficacy
and safety of vaccines exists, patients are prone to
misinformation. The residence in rural areas and the lower level of
education were identified as predictors of vaccination
hesitancy.
Also, there is a need for specific
vaccination-related education of the general population, especially
in the rural areas, to improve the success of preventive measures
and information campaigns implemented by health care
authorities.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RV was involved in conceptualization and
supervision. LMB, GM, LD, TTV, IC, DS, AI, LAT, SH, RC, AG, AH, AU,
DP and LOU contributed to data collection. DV performed the
statistical analysis, CMO, RL and RV were involved in data analysis
and confirmed the authenticity of all the raw data. RL was involved
in writing and preparing the original draft. CMO was involved in
writing, reviewing and editing. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of The Declaration of Helsinki and was approved by the
Institutional Ethics Committee of the ‘Prof. Dr. Octavian Fodor’
Regional Institute of Gastroenterology and Hepatology (Cluj-Napoca,
Romania; approval no. 1677/09.12.2021). All patients provided a
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Glatter KA and Finkelman P: History of the
Plague: An ancient pandemic for the age of COVID-19. Am J Med.
134(176)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zaorsky NG, Churilla TM, Egleston BL,
Fisher SG, Ridge JA, Horwitz EM and Meyer JE: Causes of death among
cancer patients. Ann Oncol. 28:400–407. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Palmieri C, Turtle L, Docherty A, Harrison
E, Drake T, Greenhalf B, Openshaw PJ, Baillie JK and Semple MG:
1670O prospective data of first 1,797 hospitalized patients with
cancer and COVID-19 derived from the COVID-19 clinical information
network and international severe acute respiratory and emerging
infections consortium, WHO coronavirus clinical characterization
consortium. Ann Oncol. 31(S992)2020.
|
|
4
|
Rüthrich MM, Giessen-Jung C, Borgmann S,
Classen AY, Dolff S, Grüner B, Hanses F, Isberner N, Köhler P,
Lanznaster J, et al: COVID-19 in cancer patients: Clinical
characteristics and outcome-an analysis of the LEOSS registry. Ann
Hematol. 100:383–393. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Anderson EJ, Rouphael NG, Widge AT,
Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens
LJ, Pruijssers AJ, et al: Safety and immunogenicity of SARS-CoV-2
mRNA-1273 vaccine in older adults. N Engl J Med. 383:2427–2438.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lee LYW, Cazier JB, Starkey T, Briggs SEW,
Arnold R, Bisht V, Booth S, Campton NA, Cheng VWT, Collins G, et
al: COVID-19 prevalence and mortality in patients with cancer and
the effect of primary tumour subtype and patient demographics: A
prospective cohort study. Lancet Oncol. 21:1309–1316.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Smith LE, Amlôt R, Weinman J, Yiend J and
Rubin GJ: A systematic review of factors affecting vaccine uptake
in young children. Vaccine. 35:6059–6069. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jacobson RM, St Sauver JL and Finney
Rutten LJ: Vaccine Hesitancy. Mayo Clin Proc. 90:1562–1568.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mărcău FC, Purec S and Niculescu G: Study
on the refusal of vaccination against COVID-19 in Romania. Vaccines
(Basel). 10(261)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kelkar AH, Blake JA, Cherabuddi K, Cornett
H, McKee BL and Cogle CR: Vaccine enthusiasm and hesitancy in
cancer patients and the impact of a webinar. Healthcare (Basel).
9(351)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dror AA, Eisenbach N, Taiber S, Morozov
NG, Mizrachi M, Zigron A, Srouji S and Sela E: Vaccine hesitancy:
The next challenge in the fight against COVID-19. Eur J Epidemiol.
35:775–779. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Joshi A, Kaur M, Kaur R, Grover A, Nash D
and El-Mohandes A: Predictors of COVID-19 vaccine acceptance,
intention, and hesitancy: A scoping review. Front Public Health.
9(698111)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Barrière J, Gal J, Hoch B, Cassuto O,
Leysalle A, Chamorey E and Borchiellini D: Acceptance of SARS-CoV-2
vaccination among French patients with cancer: A cross-sectional
survey. Ann Oncol. 32:673–674. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Villarreal-Garza C, Vaca-Cartagena BF,
Becerril-Gaitan A, Ferrigno AS, Mesa-Chavez F and Platas A and
Platas A: Attitudes and factors associated with COVID-19 vaccine
hesitancy among patients with breast cancer. JAMA Oncol.
7:1242–1244. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chan WL, Ho YT, Wong CK, Choi HC, Lam KO,
Yuen KK, Kwong D and Hung I: Acceptance of COVID-19 vaccination in
cancer patients in Hong Kong: Approaches to improve the vaccination
rate. Vaccines (Basel). 9(792)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Garassino MC, Vyas M, de Vries EGE,
Kanesvaran R, Giuliani R and Peters S: European Society for Medical
Oncology. The ESMO call to action on COVID-19 vaccinations and
patients with cancer: Vaccinate. Monitor. Educate. Ann Oncol.
32:579–581. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
COVID 19 Up To Date https://datelazi.ro/ (accessed March 7, 2022).
|
|
19
|
Gheorghe AS, Negru ŞM, Nițipir C, Mazilu
L, Marinca M, Gafton B, Ciuleanu TE, Schenker M, Dragomir RD,
Gheorghe AD, et al: Knowledge, attitudes and practices related to
the COVID-19 outbreak among Romanian adults with cancer: A
cross-sectional national survey. ESMO Open.
6(100027)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Polack FP, Thomas SJ, Kitchin N, Absalon
J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED,
Zerbini C, et al: Safety and efficacy of the BNT162b2 mRNA Covid-19
vaccine. N Engl J Med. 383:2603–2615. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Radio Free Europe/Radio Liberty: Inside
Romania's Hospitals As COVID Crisis Intensifies. https://www.rferl.org/a/romania-hospitals-covid-crisis/31520509.html.
Accessed March 10, 2022.
|
|
22
|
The New York Times: Romania's underfunded
health system creaks under the pressure of the pandemic. https://www.nytimes.com/2021/10/01/world/romanias-underfunded-health-system-creaks-under-the-pressure-of-the-pandemic.html.
Accessed March 10, 2022.
|
|
23
|
de Sousa MJ, Caramujo C, Júlio N,
Magalhães JC, Basto R, Fraga T, Gomes IF, Monteiro AR, Pazos I and
Sousa G: Acceptance of SARS-CoV-2 vaccination among cancer patients
in Portugal: attitudes and associated factors. Support Care Cancer.
30:4565–4570. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Matovina Brko G, Popovic M, Jovic M, Radic
J, Bodlovic Kladar M, Nikolic I, Vidovic V, Kolarov Bjelobrk I,
Kukic B, Salma S, et al: COVID-19 vaccines and cancer patients:
acceptance, attitudes and safety. J BUON. 26:2183–2190.
2021.PubMed/NCBI
|
|
25
|
Gallo K, Goede A, Mura C, Abel R, Moahamed
B, Preissner S, Nahles S, Heiland M, Bourne PE, Preissner R and
Mallach M: A comparative analysis of COVID-19 vaccines based on
over 580,000 cases from the vaccination adverse event reporting
system. Vaccines (Basel). 10(408)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hertel M, Heiland M, Nahles S, von Laffert
M, Mura C, Bourne PE, Preissner R and Preissner S: Real-world
evidence from over one million COVID-19 vaccinations is consistent
with reactivation of the varicella-zoster virus. J Eur Acad
Dermatol Venereol. 36:1342–1348. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Monin L, Laing AG, Muñoz-Ruiz M, McKenzie
DR, Del Molino Del Barrio I, Alaguthurai T, Domingo-Vila C, Hayday
TS, Graham C, Seow J, et al: Safety and immunogenicity of one
versus two doses of the COVID-19 vaccine BNT162b2 for patients with
cancer: Interim analysis of a prospective observational study.
Lancet Oncol. 22:765–778. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Forster M, Wuerstlein R, Koenig A, Amann
N, Beyer S, Kaltofen T, Degenhardt T, Burges A, Trillsch F, Mahner
S, et al: COVID-19 vaccination in patients with breast cancer and
gynecological malignancies: A German perspective. Breast.
60:214–222. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stoeklé HC, Sekkate S, Angellier E, Hervé
C and Beuzeboc P: Refusal of anti-coronavirus disease 2019
vaccination in cancer patients: Is there a difference between the
sexes? Eur J Cancer. 155:54–55. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vaccination situation in Romania | Covid
vaccination (Internet). (cited Jun 30, 2022). https://vaccinare-covid.gov.ro/situatia-vaccinarii-in-romania.
|