Introduction
Recently, novel immunotherapies directed against
CTLA-4 (Cytotoxic T Lymphocyte Antigen-4) and PD-1 (Programmed
Death antigen-1) afford the opportunity to effectively treat
loco-regional and metastatic melanoma, ensuring significant
reduction of risk for relapse, with relatively durable responses
and definitely with a considerable improvement of survival
(1,2).
Monotherapy with anti-PD-1 drugs, i.e. Nivolumab or
Pembrolizumab, represents the standard adjuvant treatment in BRAF
wild type melanoma patients (3,4).
The combination of Nivolumab and anti-CTLA4,
Ipilimumab, in BRAF wild type and negative PD-L1 tumors, or
anti-PD1 alone in melanoma with PD-L1 expression, are instead the
most recent two options for metastatic disease (1,5).
Nevertheless, in this new therapeutic scenario, over
half patients with melanoma, especially melanotic one, during or
immediately after adjuvant anti-PD1 treatment recurs, or does not
respond to modern immunotherapy or, moreover, relapses after a
temporary response (1,3-5).
For patients with primary resistance or progressing
to anti-PD-1 or even to the combination the prognosis is extremely
unfavorable, with a second/following line including Ipilimumab,
alkylating chemotherapy, or agents in experimental trials.
Among them, there are many patients that can develop
peculiar refractory superficial metastases.
In-transit metastases represent cutaneous
localization of melanoma that appear between a primary tumor and
its regional lymph node basin as part of the natural lymphatic
dissemination process. In presence of lymph node involvement their
incidence is 20% (6).
The prognosis of patients with in-transit melanoma
metastases is unfavorable with 5-year survival ranging from 12 to
37% (7).
The surgical excision of isolated lesions represents
the elective treatment.
For metastases, which primarily show or develop
resistance to standard therapies, electroporation associated with
chemotherapy (electrochemotherapy-ECT) seems to be a new potential
therapeutic option in particular in combination with immunotherapy,
since it elicits a systemic immune response other than directly
treat deep-seated tumors. The biological rational is represented by
the ECT induction of immunogenic cancer cells death. In fact, this
procedure can determine a more efficient antigen presentation of
tumor-derived antigens by APCs (antigen-presenting cells),
particularly CD8+ antigen-specific T cells. Anti-PD-1 drugs revert
T-cell exhaustion induced by PD-1/PD-L1 (programmed death-ligand 1)
and PD-L2 (programmed death-ligand 2) engagement on CD8+ T cells.
Moreover, ECT may also be beneficial in the priming phase of the
antitumor immune responses, provoking long-lived tumor
antigen-specific CD8+ T-cell effectors (8).
However no data from trials exist to evaluate the
potential role of the combination or the correct sequence of these
two therapeutic options-loco-regional and systemic treatment-but
only anecdotic/retrospective cases in papers. Moreover, nowadays it
is unknown the appropriate timing and sequence in terms of
restoring efficacy of immunotherapy in resistance events (9-13).
Our experience on a patient with initial in-transit
metastases on the scalp and then loco regional lymph node and bone
metastases showed the crucial beneficial role of a peculiar
sequence of electrochemotherapy and immunotherapies.
Case report
A 73-year old male patient with no significant
medical history, except for rheumatic polymyalgia regressed after
steroid therapy, was subjected to excision of cutaneous lesion on
the scalp in November 2020. The histological exam revealed a
lentigo-maligna melanoma in vertical growth phase, with the
following features: Clark level IV invasion, 1.7 mm Breslow index,
10 mitoses/mm2, TIL non brisk, absence of ulceration,
regression, microsatellitosis and lymphovascular invasion, presence
of perineural invasion and of melanin pigmentation.
In December 2020, the patient underwent
intra-parotid sentinel lymph nodes biopsy (SLNB) and radicalization
of previous exeresis. The histological skin-related report was
positive for the presence of multiple melanoma foci accountable as
satellite nodules of lentigo-maligna melanoma infiltrating the
dermis for a maximum thickness of 1.1 mm (greater nodule). The
parenchyma of salivary gland resulted free from pathological
lesions. The histological examination of SLNB in the intra and
periglandular tissue showed: one of these, in its subcapsular
location, was positive for rare cells ascribable to metastases from
melanoma. Total body CT-scan resulted in plausible no evidence of
loco-regional and distant metastases (some unspecific micro-nodular
to pulmonary parenchyma bilaterally, mainly to the upper lung lobes
and angiomatous lesion of ~68 mm localized in the fourth hepatic
segment, known in patient's history).
Furthermore, no BRAF gene mutation was identified.
It ended for an onset stage IIIC (AJCC 2017) of the melanoma
disease (pT2apN2cM0).
In January 2021 the patient started anti-PD1
Pembrolizumab 200 mg flat dose therapy with adjuvant purpose.
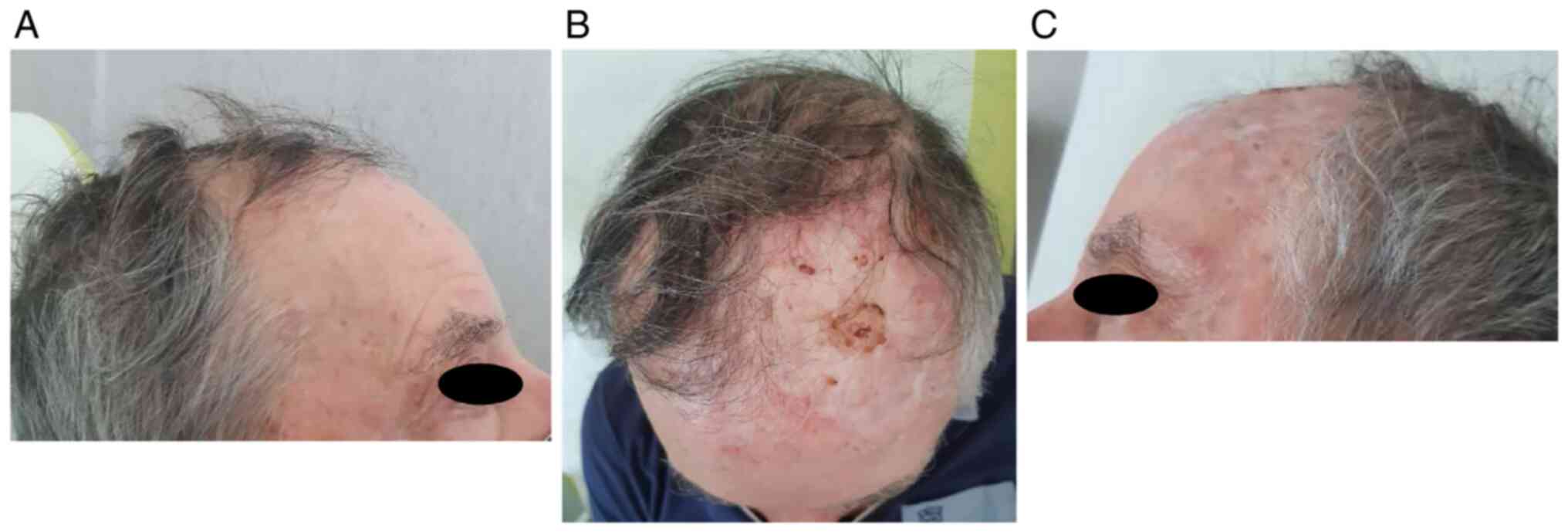
For the appearance of a suspected pigmented
peri-scar skin lesion on the scalp (frontal region), the patient
underwent surgery in May 2021. Histological examination confirmed
the clinical suspicion of in-transit metastases.
A total body CT-scan of June 2021 showed unchanged
findings and the patient continued Pembrolizumab without
interruptions.
Subsequently for the rapidly progressive disease on
the scalp in May-June 2021, the patient, after discussion in the
multidisciplinary context and signing the informed consent to the
treatment, was subjected to ECT to treat the scalp and its
superficial lymphatic drainage pathways. Considering the
oligo-progression of the disease (no visceral and brain melanoma
localizations), the characteristics resistance of cutaneous lesions
to elective treatments and the potential synergy between ECT and
immunotherapy, the patient followed the same oncological treatment
as first line therapy.
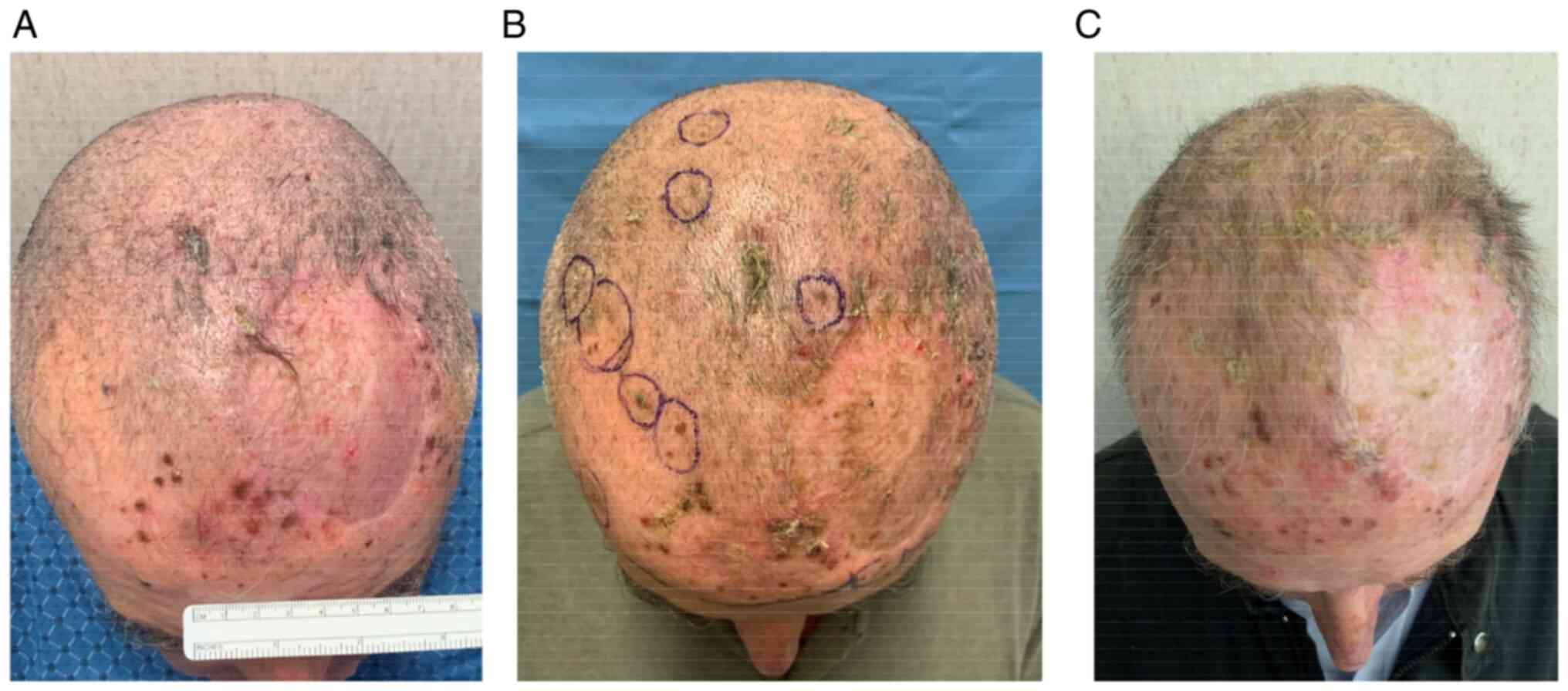
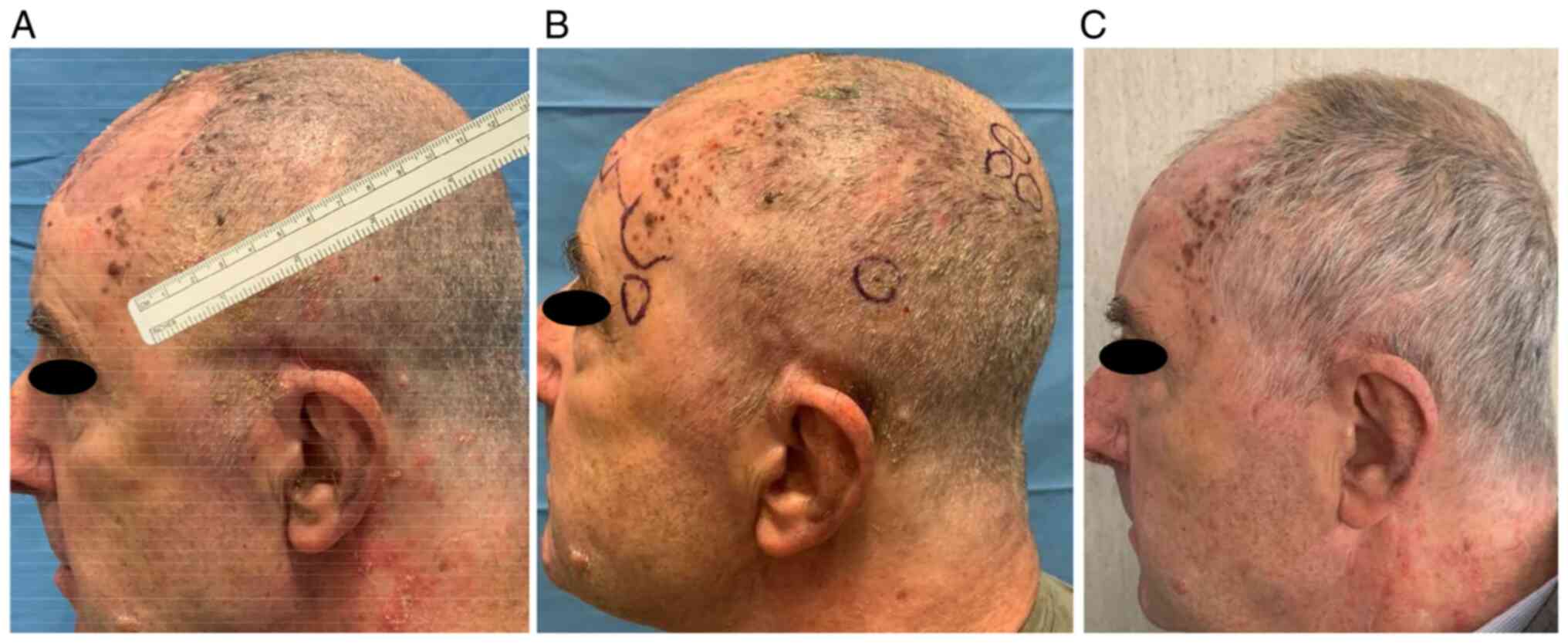
In particular, he underwent two ECT administrations
with intravenous injection of bleomycin 30,000 UI through linear
electrode and with a 6 weeks interval (June 2021-131 pulses- and
August 2021-273 pulses-). He showed a partial response (persistence
of disease on the left frontal-temporal region) and no significant
adverse events [only pruritus G2 according to common toxicities
criteria adverse events (CTCAE) version 5.0 was referred] Fig. 1, Fig.
2 and Fig. 3.
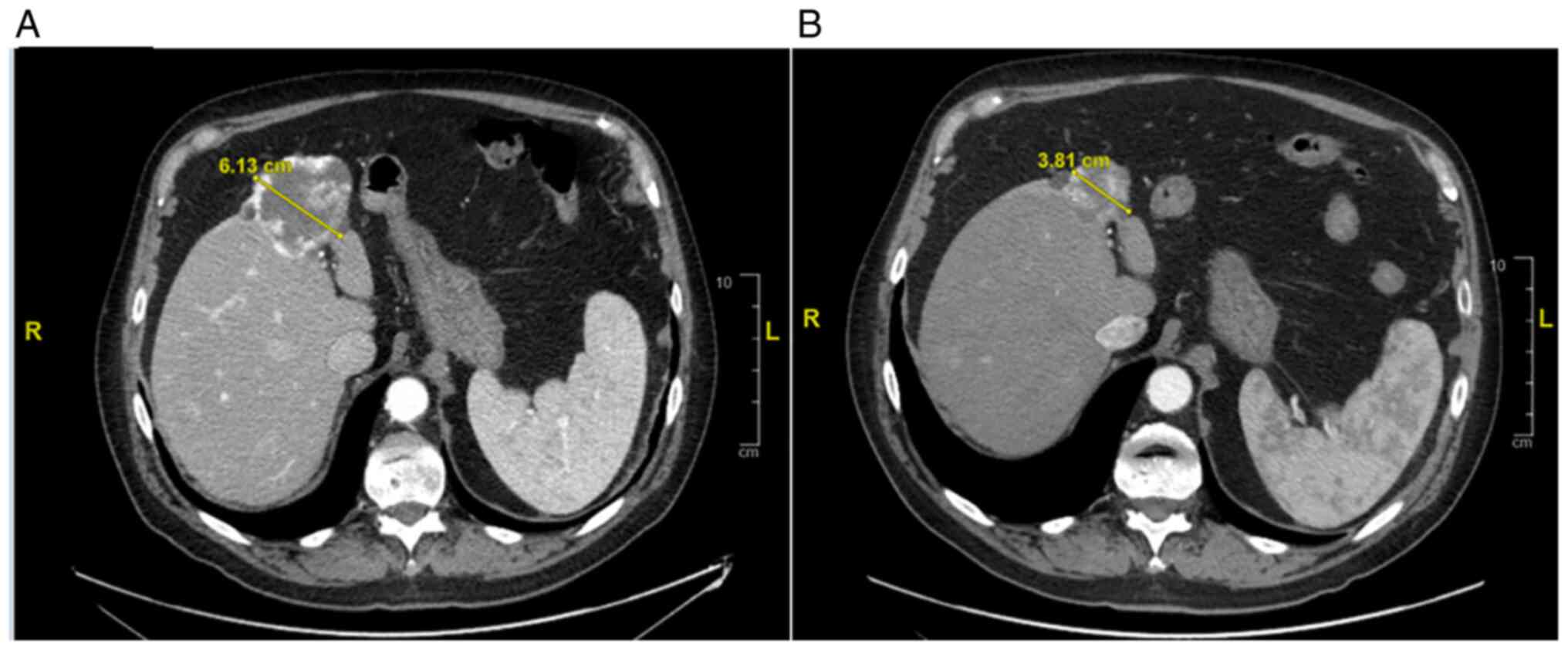
In September 2021, the patient repeated a total body
CT-scan with the evidence of a strange behavior of the known liver
lesion (dimensional reduction from 68 to 40 mm) Fig. 4.
To better define the subsequent therapeutic process,
the multidisciplinary team decided for a diagnostic investigation
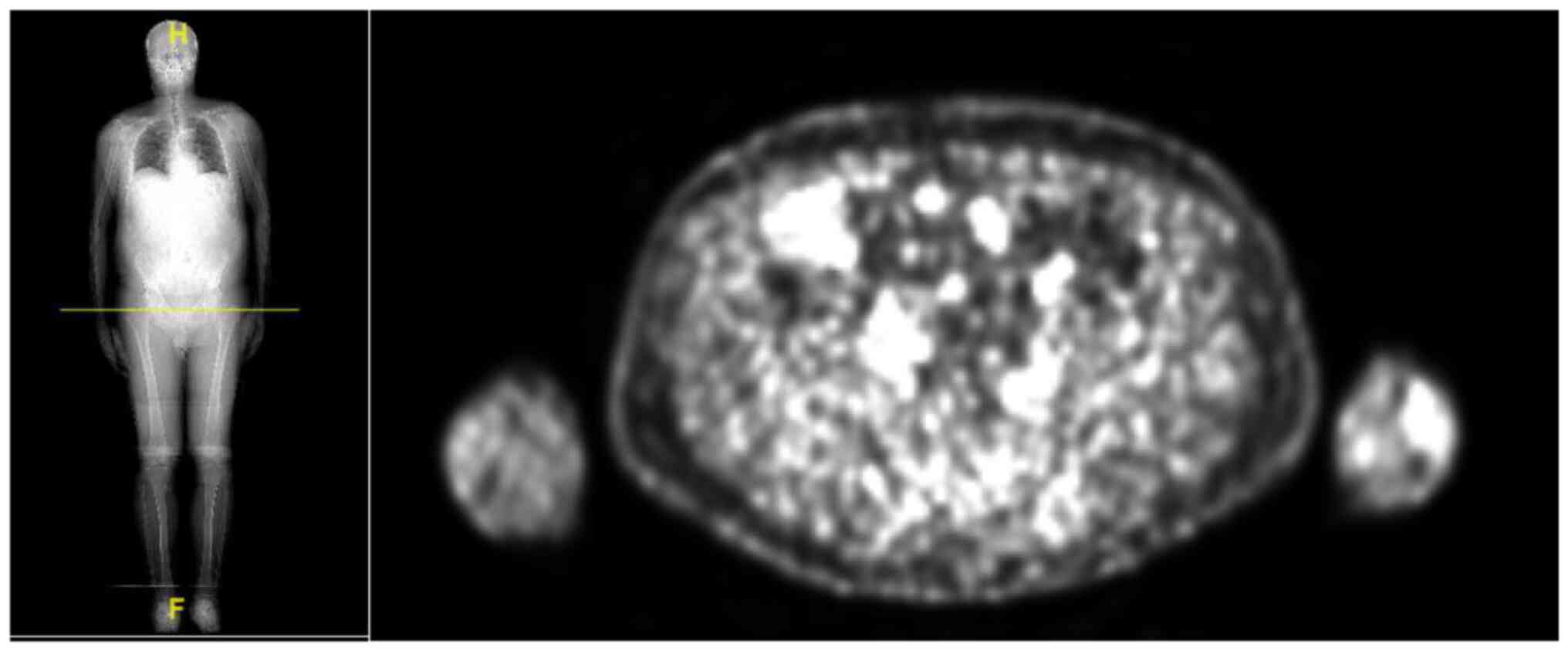
using 18-F-FDG PET-CT scan.
PET-CT scan carried out in October 2021 showed
pathological uptake in the right iliac bone, in the right
intra-parotid and left preauricular lymph nodes, and in the
in-transit metastases especially at the left frontal-parietal
region. There was no liver uptake. Thus, the case was discussed
with radiologists: the focal lesion of liver seemed to be a
fibrotic involution of the known angioma, but the appearance of
lymph nodes in the neck could indicate a progression of disease.
Instead, for the complex identification of intramedullary
localization by CT scan, the time of onset of bone metastasis
remained uncertain Fig. 5.
No clinical trials were active for our patient, thus
he started a second line therapy with anti-CTLA4 Ipilimumab. After
the first administration the patient had immediately benefit with
the resolution of feeling of encumbrance of lymph node in parotid
region and with the remission of the known nodules of the scalp.
Nevertheless after four canonical doses of Ipilimumab ended in
February 2022, the patient developed acute hypophysitis (grade 4-G4
according to CTCAE version 5.0). This peculiar immune-related
adverse event was characterized by asthenia, hyporexia with
consequent dehydration and hospital admission. He performed RMN of
the sella turcica with reduction of physiological contrast uptake
in the neurohypophysis. Thus a replacement steroid therapy was
started after the load dose because of the corticotropic axis
failure, with immediate advantage.
Then, he was hospitalized again for evidence of
significant asymptomatic hyperglycemia (G4 CTCAE 5.0). Thus,
diabetes mellitus type I was diagnosed and the patient undertook
insulin therapy.
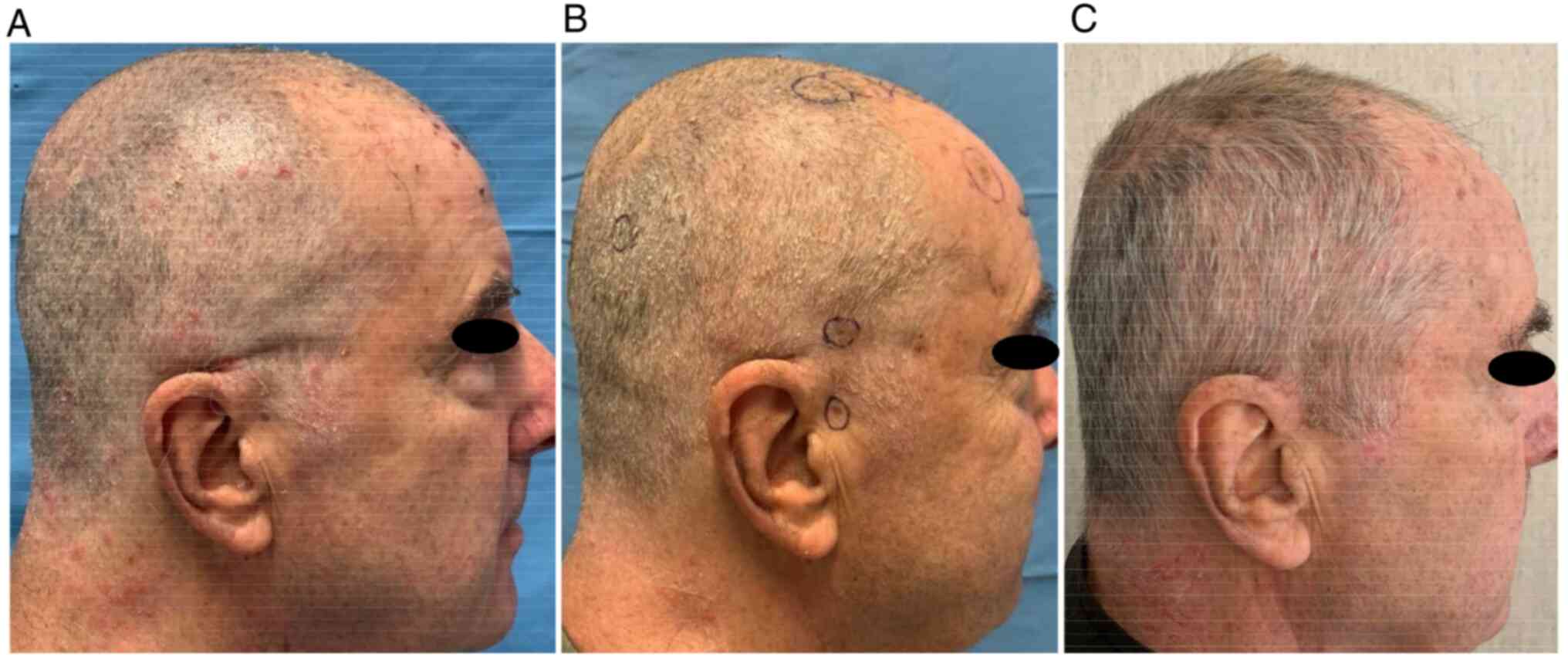
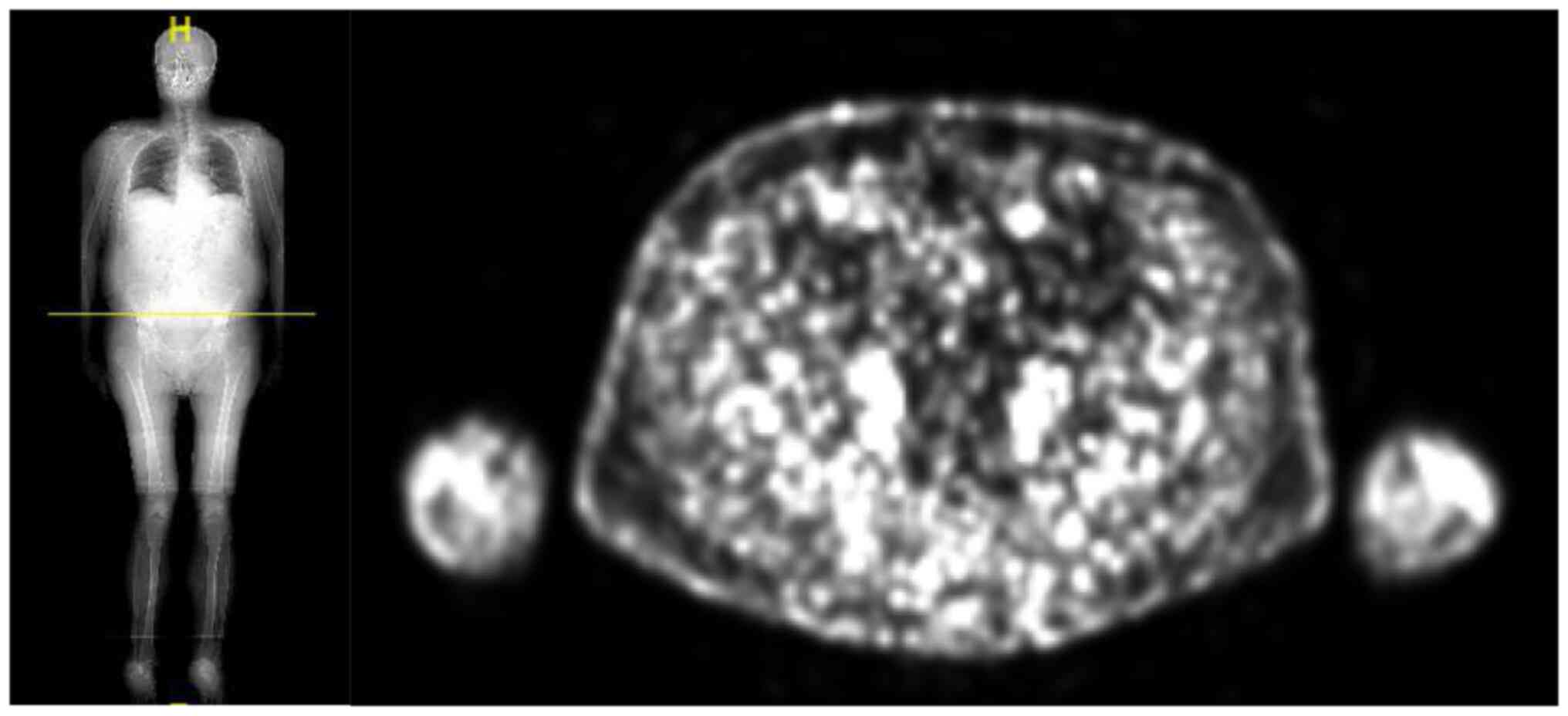
PET-CT scan performed in March 2022 showed a
complete response of disease Fig.
6.
During Pembrolizumab treatment we observed the onset
of lymphopenia; instead eosinophilia, progressive lymphocyte
increase to normal values and lastly neutropenia appeared firstly
during the ECT and then with Ipilimumab.
Although these adverse effects, the patient had a
very fast and considerable improvement on its quality of life
especially for the remission of strongly pronounced skin-lesions on
the scalp. Until now, after 12 months from the first ECT and 6
months from the starting of anti-CTLA4 immunotherapy, the patient
is in excellent clinical condition and complete response of disease
still maintains Fig. 7.
Discussion
In this manuscript we reported a case report of
metastatic melanotic melanoma patient receiving Pembrolizumab,
electrochemotherapy and then Ipilimumab for cutaneous and finally
loco-regional lymph nodes and distant bone metastases with
experience of dramatic clinical-radiological benefit.
Specifically our patient progressed during adjuvant
treatment with in-transit melanotic metastases on the scalp. It is
reported that melanin pigment and melanogenesis have a crucial
function in the progression of melanotic melanoma determining
resistance to immunotherapy, probably by glycolysis and
hypoxia-inducible factor 1-alpha (HIF-1a) activation and their
immunosuppressive effects. In fact a negative correlation between
tumor pigmentation and diseases outcome was shown (14).
Therefore, after the discussion in the Skin Cancer
Multidisciplinary Group of our University Hospital, to overcome the
melanotic metastasis' resistance to Pembrolizumab, the patient
underwent electrochemotherapy obtaining partial and then nearly
complete response with the Ipilimumab treatment.
The electrochemotherapy was safe; the patient only
reported pruritus in the scalp, treated with antihistamine
medications. During the treatment with Ipilimumab the patient
developed hypophysitis with corticotropic axis failure and type 1
diabetes mellitus: thus an insulin therapy and cortisone acetate
was started.
ECT combines the inducted electroporation of cancer
cells with the concurrent infusion of bleomycin as cytotoxic
chemotherapy, more often than cisplatin. A pulsed electrical
current enhances the cell permeability expounding the event of
revocable electroporation. ECT also determines anti-vascular effect
resulting in increased tumor cell hypoxia. Therefore, locally
bleomycin is more active with negligible systemic adverse events
(15,16).
In addition to determine cell death, ECT can
generate a local and systemic immune reaction as a result of
releasing tumor associated antigens (TAA) from electroporated
cancer cells.
TAA are neoantigens able to evoke an immune response
primarily mediated by cytotoxic-T lymphocytes (CTL). Furthermore,
TAA seem to be captured by local dendritic cells and then presented
to tumor-specific CTL in draining lymph nodes (17-20).
Some reports showed the efficacy of ECT in the
treatment of in-transit or subcutaneous metastases from cutaneous
melanoma. An objective response rate from 60 to 90% was achieved in
the palliative management of unresectable recurrent cutaneous
disease. This response is usually long-lasting; moreover ECT is an
easy, rapid and effective procedure which can be repeated (21-23).
However, a long-term complete response of these peculiar
localizations of metastasis seems to be difficult to obtain due to
spread of cancer cells into lymphatic vessels (24-27).
In a similar way as ECT, a systemic and unexpected
response to a local treatment derives from the radiotherapy of
lesions, due to the release of TAA, named abscopal effect. It has
been also demonstrated in patients affected by metastatic melanoma:
distant lesions have showed a clinical-radiological response.
Thus, it has been hypothesized that the association
of ECT with anti-PD1/anti-CTLA4 could represent an effective
strategy to induce an immunological durable and synergic response
against the cancer (11,28-32).
The role of hypoxia in this combination treatment
has virtually been examined, particularly in melanotic melanoma.
The response to radiation/ECT joint with immune checkpoint
inhibition seems to be dependent on the hypoxia level (33) that could be measured to prescribe
appropriate dose of radiotherapy and the number of ECT
administrations.
Trials utilizing concomitant stereotactic body
radiotherapy/and immunotherapy are still ongoing or recently
completed without results about their synergistic impact and the
best schedule for the two treatments (NCT02659540 with preliminary
results¸ NCT04581382, NCT03850691, NCT03297463, NCT02406183,
NCT04017897). Only one trial-NCT03448666-, still ongoing, aims to
evaluate the activity of ECT and Pembrolizumab in patients with
superficial or superficial and visceral metastases. ECT is
administrated after the first cycle of immunotherapy. Starting with
ECT could determine intralesional necrosis reducing loco-regional
immunotherapy efficacy; instead, ECT administration after the first
dispensation demonstrated a synergistic effect in retrospective
series (11), probably due to the
aforementioned mechanism of neo-antigens release. However,
immunotherapy alone could induce an optimal local and systemic
performance without the use of ECT. Thus, the immunotherapy-ECT
sequence can be considered in oligo-progressing disease, for
instance.
Considering that Ipilimumab shows uncommon, not
immediate but long lasting action: usually the complete response
was achieved after some months from the start (34). According to our experience, ECT
leads to a local response and probably to an acceleration of the
systemic one. Thus, we hypothesize that ECT in two administrations
could increase and accelerate the efficacy of Ipilimumab.
Finally, randomized clinical trials and
translational researches are needed to shed the light on the
improved combination/sequence/number of administrations of ECT and
systemic immunotherapy in order to offer the most appropriate
therapies, especially for patients with superficial (and also
visceral) metastases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FM and FDF performed the patient treatments
(immunotherapy and electrochemotherapy respectively) and they were
major contributors in writing the paper and contributed to design
and conception. They verified efficacy, monitored patient and
collected images and medical data. RB and PB formulated and
supervised treatment plans. FM and FDF confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The patient consented to the collection of data and
images for the aim of research and for publication in written
form.
Patient consent for publication
Written consent was obtained from the patient. He
authorized us to publish his disease history and his images.
Competing interests
FM was a consultant/advisory board member for BMS
and MSD. The rest of the authors declare that they have no
competing interests.
References
|
1
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J,
Dummer R, et al: Five-year survival with combined nivolumab and
ipilimumab in advanced melanoma. N Engl J Med. 381:1535–1546.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Weber J, Mandala M, Del Vecchio M, Gogas
HJ, Arance AM, Cowey CL, Dalle S, Schenker M, Chiarion-Sileni V,
Marquez-Rodas I, et al: Adjuvant nivolumab versus ipilimumab in
resected stage III or IV melanoma. N Engl J Med. 377:1824–1835.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eggermont AMM, Blank CU, Mandalà M, Long
GV, Atkinson VG, Dalle S, Haydon AM, Meshcheryakov A, Khattak A,
Carlino MS, et al: Adjuvant pembrolizumab versus placebo in
resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant
metastasis-free survival results from a double-blind, randomised,
controlled, phase 3 trial. Lancet Oncol. 22:643–654.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Robert C, Ribas A, Schachter J, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, et al:
Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006):
Post-hoc 5-year results from an open-label, multicentre,
randomised, controlled, phase 3 study. Lancet Oncol. 20:1239–1251.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kretschmer L, Beckmann I, Thoms KM,
Mitteldorf C, Bertsch HP and Neumann C: Factors predicting the risk
of in-transit recurrence after sentinel lymphonodectomy in patients
with cutaneous malignant melanoma. Ann Surg Oncol. 13:1105–1112.
2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pawlik TM, Ross MI, Thompson JF, Eggermont
AM and Gershenwald JE: The risk of in-transit melanoma metastasis
depends on tumor biology and not the surgical approach to regional
lymph nodes. J Clin Oncol. 23:4588–4590. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Campana LG, Testori A, Mozzillo N and
Rossi CR: Treatment of metastatic melanoma with
electrochemotherapy. J Surg Oncol. 109:301–307. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Quaresmini D, Di Lauro A, Fucci L,
Strippoli S, De Risi I, Sciacovelli AM, Albano A, Achille G,
Montepara M, Russo S, et al: Electrochemotherapy as a trigger to
overcome primary resistance to Anti-PD-1 treatment: A case report
of melanoma of the scalp. Front Oncol. 11(742666)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brizio M, Fava P, Astrua C, Cavaliere G
and Savoia P: Complete regression of melanoma skin metastases after
electrochemotherapy plus ipilimumab treatment: An unusual clinical
presentation. Eur J Dermatol. 25:271–272. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mozzillo N, Simeone E, Benedetto L,
Curvietto M, Giannarelli D, Gentilcore G, Camerlingo R, Capone M,
Madonna G, Festino L, et al: Assessing a novel
immuno-oncology-based combination therapy: Ipilimumab plus
electrochemotherapy. Oncoimmunology. 4(e1008842)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Heppt MV, Eigentler TK, Kähler KC, Herbst
RA, Göppner D, Gambichler T, Ulrich J, Dippel E, Loquai C, Schell
B, et al: Immune checkpoint blockade with concurrent
electrochemotherapy in advanced melanoma: A retrospective
multicenter analysis. Cancer Immunol Immunother. 65:951–959.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Karaca B, Yayla G, Erdem M and Gürler T:
Electrochemotherapy with anti-PD-1 treatment induced durable
complete response in heavily pretreated metastatic melanoma
patient. Anticancer Drugs. 29:190–196. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Slominski RM, Sarna T, Płonka PM, Raman C,
Brożyna AA and Slominski AT: Melanoma, melanin, and melanogenesis:
The Yin and Yang relationship. Front Oncol.
12(842496)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sersa G, Miklavcic D, Cemazar M, Rudolf Z,
Pucihar G and Snoj M: Electrochemotherapy in treatment of tumours.
Eur J Surg Oncol. 34:232–240. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kranjc S, Kranjc M, Scancar J, Jelenc J,
Sersa G and Miklavcic D: Electrochemotherapy by pulsed
electromagnetic field treatment (PEMF) in mouse melanoma B16F10 in
vivo. Radiol Oncol. 50:39–48. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Roux S, Bernat C, Al-Sakere B,
Ghiringhelli F, Opolon P, Carpentier AF, Zitvogel L, Mir LM and
Robert C: Tumor destruction using electrochemotherapy followed by
CpG oligodeoxynucleotide injection induces distant tumor responses.
Cancer Immunol Immunother. 57:1291–1300. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Di Gennaro P, Gerlini G, Urso C, Sestini
S, Brandani P, Pimpinelli N and Borgognoni L:
CD4+FOXP3+ T regulatory cells decrease and
CD3+CD8+ T cells recruitment in TILs from
melanoma metastases after electrochemotherapy. Clin Exp Metastasis.
33:787–798. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gerlini G, Sestini S, Di Gennaro P, Urso
C, Pimpinelli N and Borgognoni L: Dendritic cells recruitment in
melanoma metastasis treated by electrochemotherapy. Clin Exp
Metastasis. 30:37–45. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu CC, Yang H, Zhang R, Zhao JJ and Hao
DJ: Tumour-associated antigens and their anti-cancer applications.
Eur J Cancer Care. 26(e12446)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sersa G, Stabuc B, Cemazar M, Miklavcic D
and Rudolf Z: Electrochemotherapy with cisplatin: The systemic
antitumour effectiveness of cisplatin can be potentiated locally by
the application of electric pulses in the treatment of malignant
melanoma skin metastases. Melanoma Res. 10:381–385. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kaehler KC, Egberts F and Hauschild A:
Electrochemotherapy in symptomatic melanoma skin metastases:
Intraindividual comparison with conventional surgery. Dermatol
Surg. 36:1200–1202. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mozzillo N, Caracò C, Mori S, Di Monta G,
Botti G, Ascierto PA, Caracò C and Aloj L: Use of neoadjuvant
electrochemotherapy to treat a large metastatic lesion of the cheek
in a patient with melanoma. J Transl Med. 10(131)2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Möller MG, Salwa S, Soden DM and
O'Sullivan GC: Electrochemotherapy as an adjunct or alternative to
other treatments for unresectable or in-transit melanoma. Expert
Rev Anticancer Ther. 9:1611–1630. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kis E, Oláh J, Ócsai H, Baltas E, Gyulai
R, Kemény L and Horvath AR: Electrochemotherapy of cutaneous
metastases of melanoma-a case series study and systematic review of
the evidence. Dermatol Surg. 37:816–824. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Colombo GL, Di Matteo S and Mir LM:
Cost-effectiveness analysis of electrochemotherapy with the
Cliniporator™ vs other methods for the control and
treatments of cutaneous and subcutaneous tumors. Therap Clin Risk
Manag. 4:541–548. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Campana LG, Mocellin S, Basso M, Puccetti
O, De Salvo GL, Chiarion-Sileni V, Vecchiato A, Corti L, Rossi CR
and Nitti D: Bleomycin-based electrochemotherapy: Clinical outcome
from a single institution's experience with 52 patients. Ann Surg
Oncol. 16:191–199. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Snoj M, Paulin-Kosir Z, Cemazar S and
Sersa G: Long lasting complete response in melanoma treated by
electrochemotherapy. Eur J Cancer. 4 (Suppl)(S2)2006.
|
|
29
|
Queirolo P, Marincola F and Spagnolo F:
Electrochemotherapy for the management of melanoma skin metastasis:
A review of the literature and possible combinations with
immunotherapy. Arch Dermatol Res. 306:521–526. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Goggins CA and Khachemoune A: The use of
electrochemotherapy in combination with immunotherapy in the
treatment of metastatic melanoma: A focused review. Int J Dermatol.
58:865–870. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Longo F, Perri F, Caponigro F, Della
Vittoria Scarpati G, Guida A, Pavone E, Aversa C, Muto P, Giuliano
M, Ionna F and Solla R: Boosting the immune response with the
combination of electrochemotherapy and immunotherapy: A new weapon
for squamous cell carcinoma of the head and neck? Cancers (Basel).
12(2781)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Maglietti F, Tellado M, De Robertis M,
Michinski S, Fernández J, Signori E and Marshall G: Electroporation
as the immunotherapy strategy for cancer in veterinary medicine:
State of the art in Latin America. Vaccines (Basel).
8(537)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hompland T, Fjeldbo CS and Lyng H: Tumor
hypoxia as a barrier in cancer therapy: Why levels matter. Cancers
(Basel). 13(499)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Saenger YM and Wolchok JD: The
heterogeneity of the kinetics of response to ipilimumab in
metastatic melanoma: Patient cases. Cancer Immun.
8(1)2008.PubMed/NCBI
|