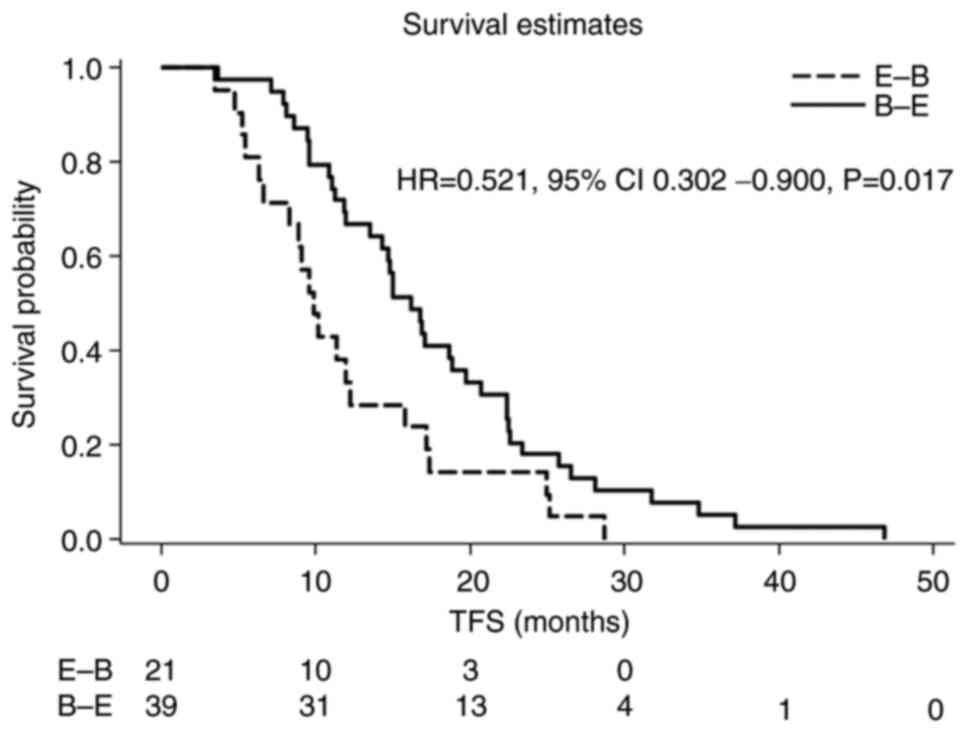
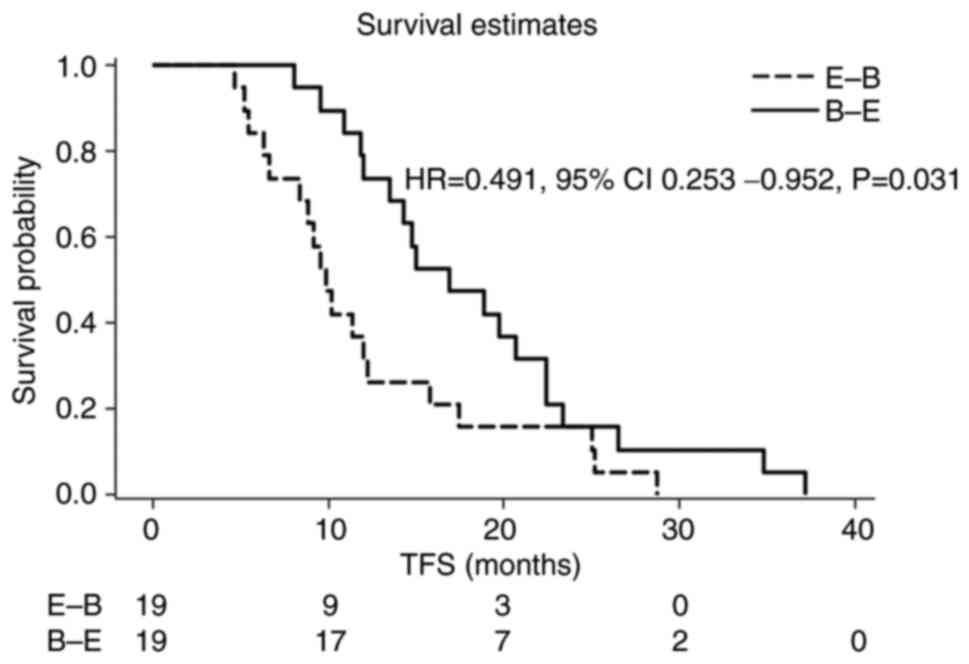
|
1
|
Cortes J, O'Shaughnessy J, Loesch D, Blum
JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V,
Delozier T, et al: Eribulin monotherapy versus treatment of
physician's choice in patients with metastatic breast cancer
(EMBRACE): A phase 3 open-label randomised study. Lancet.
377:914–923. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Miller K, Wang M, Gralow J, Dickler M,
Cobleigh M, Perez EA, Shenkier T, Cella D and Davidson NE:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. N Engl J Med. 357:2666–2676. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Robert NJ, Diéras V, Glaspy J, Brufsky AM,
Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SYT, Zhou X,
et al: RIBBON-1: Randomized, double-blind, placebo-controlled,
phase III trial of chemotherapy with or without bevacizumab for
first-line treatment of human epidermal growth factor receptor
2-negative, locally recurrent or metastatic breast cancer. J Clin
Oncol. 29:1252–1260. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Miles DW, Chan A, Dirix LY, Cortés J,
Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F,
et al: Phase III study of bevacizumab plus docetaxel compared with
placebo plus docetaxel for the first-line treatment of human
epidermal growth factor receptor 2-negative metastatic breast
cancer. J Clin Oncol. 28:3239–3247. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sandler A, Grey R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Escudier B, Pluzanska A, Koralewski P,
Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar
B, Bajetta E, et al: AVOREN Trial investigators. Bevacizumab plus
interferon alfa-2a for treatment of metastatic renal cell
carcinoma: A randomised, double-blind phase III trial. Lancet.
370:2103–2111. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Delaloge S, Pérol D, Courtinard C, Brain
E, Asselain B, Bachelot T, Debled M, Dieras V, Campone M, Levy C,
et al: Paclitaxel plus bevacizumab or paclitaxel as first-line
treatment for HER2-negative metastatic breast cancer in a
multicenter national observational study. Ann Oncol. 27:1725–1732.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
LeCaer H, Barlesi F, Corre R, Jullian H,
Bota S, Falchero L, Vergnenegre A, Dujon C, Delhoume JY and Chouaid
C: GFPC 0504 Team. A multicentre phase II randomised trial of
weekly docetaxel/gemcitabine followed by erlotinib on progression,
vs the reverse sequence, in elderly patients with advanced non
small-cell lung cancer selected with a comprehensive geriatric
assessment (the GFPC 0504 study). Br J Cancer. 105:1123–1130.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fiala O, Pesek M, Finek J, Benesova L,
Bortlicek Z and Minarik M: Sequential treatment of advanced-stage
lung adenocarcinoma harboring wild-type EGFR gene: Second-line
pemetrexed followed by third-line erlotinib versus the reverse
sequence. Anticancer Res. 33:3397–3402. 2013.PubMed/NCBI
|
|
11
|
Rosenbaum PR and Rubin DB: The central
role of the propensity score in observational studies for causal
effects. Biometrika. 70:41–55. 1983.
|
|
12
|
Sturmer T, Wyss R, Glynn RJ and Brookhart
MA: Propensity scoresfor confounder adjustment when assessing the
effects of medicalinterventions using nonexperimental study
designs. J Int Med. 275:570–580. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Peihua Q and Jun S: A two-stage procedure
for comparing hazard rate functions. J.R Statist Soc B. 70:191–208.
2008.
|
|
14
|
Aogi K, Masuda N, Ohno S, Oda T, Iwata H,
Kashiwaba M, Fujiwara Y, Kamigaki S, Ito Y, Ueno T and Takashima S:
First-line bevacizumab in combination with weekly paclitaxel for
metastatic breast cancer: Efficacy and safety results from a large,
open-label, single-arm Japanese study. Breast Cancer Res Treat.
129:829–838. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kaufman PA, Awada A, Twelves C, Yelle L,
Perez EA, Velikova G, Olivo MS, He Y, Dutcus CE and Cortes J: Phase
III open-label randomized study of eribulin mesylate versus
capecitabine in patients with locally advanced or metastatic breast
cancer previously treated with an anthracycline and a taxane. J
Clin Oncol. 33:594–601. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brufsky AM, Hurvitz S, Perez E, Swamy R,
Valero V, O'Neill V and Rugo HS: RIBBON-2: A randomized,
double-blind, placebo-controlled, phase III trial evaluating the
efficacy and safety of bevacizumab in combination with chemotherapy
for second-line treatment of human epidermal growth factor receptor
2-negative metastatic breast cancer. J Clin Oncol. 29:4286–4293.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ueda S, Saeki T, Takeuchi H, Shigekawa T,
Yamane T, Kuji I and Osaki A: . In vivo imaging of eribulin-induced
reoxygenation in advanced breast cancer patients: A comparison to
bevacizumab. Br J Cancer. 114:1212–1218. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Goel S, Mita AC, Mita M, Rowinsky EK, Chu
QS, Wong N, Desjardins C, Fang F, Jansen M, Shuster DE, et al: A
phase I study of eribulin mesylate (E7389), a mechanistically novel
inhibitor of microtubule dynamics, in patients with advanced solid
malignancies. Clin Cancer Res. 15:4207–4212. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tan AR, Rubin EH, Walton DC, Shuster DE,
Wong YN, Fang F, Ashworth S and Rosen LS: Phase I study of eribulin
mesylate administered once every 21 days in patients with advanced
solid tumors. Clin Cancer Res. 15:4213–4219. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vahdat LT, Pruitt B, Fabian CJ, Rivera RR,
Smith DA, Tan-Chiu E, Wright J, Tan AR, Dacosta NA, Chuang E, et
al: Phase II study of eribulin mesylate, a halichondrin B analog,
in patients with metastatic breast cancer previously treated with
an anthracycline and a taxane. J Clin Oncol. 27:2954–2961.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cortes J, Vahdat L, Blum JL, Twelves C,
Campone M, Roché H, Bachelot T, Awada A, Paridaens R, Goncalves A,
et al: Phase II study of the halichondrin B analog eribulin
mesylate in patients with locally advanced or metastatic breast
cancer previously treated with an anthracycline, a taxane, and
capecitabine. J Clin Oncol. 28:3922–3928. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Morgan RJ, Synold TW, Longmate JA, Quinn
DI, Gandara D, Lenz HJ, Ruel C, Xi B, Lewis MD, Colevas AD, et al:
Pharmacodynamics (PD) and pharmacokinetics (PK) of E7389 (eribulin,
halichondrin B analog) during a phase I trial in patients with
advanced solid tumors: A California cancer consortium trial. Cancer
Chemother Pharmacol. 76:897–907. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gligorov J, Doval D, Bines J, Alba E,
Cortes P, Pierga JY, Gupta V, Costa R, Srock S, de Ducla S, et al:
Maintenance capecitabine and bevacizumab versus bevacizumab alone
after initial first-line bevacizumab and docetaxel for patients
with HER2-negative metastatic breast cancer (IMELDA): A randomised,
open-label, phase 3 trial. Lancet Oncol. 15:1351–1360.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Park YH, Jung KH, Im SA, Sohn JH, Ro J,
Ahn JH, Kim SB, Nam BH, Oh DY, Han SW, et al: Phase III,
multicenter, randomized trial of maintenance chemotherapy versus
observation in patients with metastatic breast cancer after
achieving disease control with six cycles of gemcitabine plus
paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol.
31:1732–1739. 2013.PubMed/NCBI View Article : Google Scholar
|