Introduction
Bevacizumab and eribulin are novel agents for the
treatment of HER2-negative metastatic breast cancer. Eribulin
improved overall survival in the EMBRACE trials (1). Eribulin has been shown to improve
survival in patients with advanced recurrent breast cancer
previously treated with anthracycline or taxane chemotherapy. It
may be effective in the early treatment of recurrent breast cancer
after surgery, but this has not been reported at this time. On the
other hand, bevacizumab has improved progression-free survival but
not overall survival in several clinical studies (2,3,4).
However, there are many reports that have denied the effectiveness
of bevacizumab for OS, even though it has been suggested to be
effective in improving outcomes in metastatic disease from several
tumor types (5-7).
Recently, real-world data have been reported from ESME in France,
and the efficacy of BEV in improving overall survival was shown
(8). Its excellent tumor reduction
effect is expected to improve symptoms and complications associated
with recurrent tumors, and the lack of a difference in the
incidence of serious adverse events suggests that bevacizumab is a
drug that preserves patients' quality of life and inhibits rapid
tumor progression.
At present, there is no standard for treatment with
these drugs. Making a clinical decision about which treatment
option to choose can be difficult. Clinical data on this question
are lacking. Although there are some reports about an improved
prognosis and fewer adverse events in other cancers by changes in
the sequence of treatment (9,10),
there have been no reports of differences in the effectiveness of
BEV+PTX and eribulin according to their order of
administration.
The purpose of this study was to compare two
treatment strategies, eribulin followed by BEV + PTX versus BEV +
PTX followed by eribulin, to determine whether the order of
administration affects patient outcomes in the real world.
Patients and methods
Patients
The database of the TBCRG, a multicenter study
group, was established to centralize real-world data on metastatic
breast cancer from 14 institutions in Toyama. We used this database
to evaluate the survival benefit in patients who were treated with
bevacizumab + paclitaxel (BEV + PTX) and eribulin. All patients who
started BEV + PTX and eribulin treatment for MBC from August 2011
to June 2018 were selected. Among the 264 patients recorded in the
TBCRG database, 180 patients who started BEV + PTX and eribulin
treatment for HER2-negative MBC were selected. Of these, 84
patients were treated with both BEV + PTX and eribulin sequentially
regardless of the treatment line. The following data were collected
from each institution: age, PS, estrogen receptor (ER) status,
progesterone receptor (PgR) status, adjuvant chemotherapy, most
common metastatic sites, number of metastatic sites, and treatment
line.
We retrospectively reviewed the medical records of
60 cases within the 2nd line and analyzed the following items:
overall survival, time to failure of the strategy, efficacy of
treatment, and adverse events. Computed tomography (CT) was
performed after 2 or 3 months of treatment with eribulin or BEV +
PTX to assess the efficacy. Disease status was assessed according
to the response evaluation criteria in solid tumors (RECIST), and
adverse events were assessed by CTCAE version 4.0. TFS was
calculated as the duration of BEV+PTX and eribulin
administration.
In all institutions, BEV+PTX and eribulin were
continued until progression or unacceptable toxicity. Bevacizumab
(10 mg/kg) was administered biweekly, and paclitaxel (80 mg/m²) was
administered 3 weeks on/1 week off. Eribulin (1.4 mg/m²) was
administered 2 weeks on/1 week off.
To evaluate the influence of the order of treatment,
we compared the efficacy of eribulin followed by BEV + PTX (arm
E-B) with the efficacy of treatment with the reverse treatment
sequence (arm B-E).
Statistical analysis
Arms E-B and B-E were compared with adjustment for
imbalances in patient background factors using a propensity score
matching analysis (PSMA) (11,12).
The PSMA method was used to examine the consistency between the
analysis results, thereby making the clinical findings as robust as
possible. The propensity scores were estimated using a logistic
regression model with treatment line, age, performance status,
number of metastatic sites, recurrence, liver metastasis, and
triple negative status. OS and TFS were analyzed using the
Kaplan-Meier method and compared using the log-rank test. In
addition, univariate Cox regression analysis with the treatment arm
as a covariate was used to estimate the hazard ratio and its
confidence interval. When survival curves crossed over during the
follow-up time, resulting in violation of the proportional hazards
assumption, the two-stage test proposed by Qiu and Sheng (13) was used to compare survival curves
between treatment arms.
Patient background factors were compared between
arms using Pearson's χ2 test or unpaired Student's
t-test, where appropriate. P<0.05 was considered to indicate
statistical significance. Statistical analyses were performed with
JMP software version 14.0 and SAS software version 9.4 (SAS
Institute Inc., Cary, NC, USA).
Results
Baseline patient demographics and
tumor characteristics
The baseline patient characteristics are summarized
in Table I. Thirty-nine patients
were treated with the B-E sequence, and 21 patients were treated
with the E-B sequence; the median ages were 54.4 (range, 30-77) and
54 (range, 34-73) years, respectively. The ER- or PgR-positive
rates were 84.6 and 52.4% for the B-E and E-B groups, respectively.
Five patients in the B-E group and seven patients in the E-B group
had triple-negative breast cancer. Significantly more patients in
the B-E arm than in the E-B arm received it as first line treatment
(32 vs. 9, P=0.0019). Twelve patients in the E-B arm had received
capecitabine or S-1 previously.
 | Table ICharacteristics of the patients. |
Table I
Characteristics of the patients.
| | Number of patients
(%) | |
|---|
| Characteristics | B-E arm (n=39) | E-B arm (n=21) | P-value |
|---|
| Age, median
(range) | 54.4 (30-77) | 54.0 (34-73) | nsa |
| PS | | | nsb |
|
0 | 29 (74.3) | 14 (66.7) | |
|
1 | 9 (23.1) | 5 (23.8) | |
|
2 | 1 (2.6) | 2 (9.5) | |
| De novo metastatic
disease | 34 (87.2) | 19 (90.5) | nsb |
| ER status | | | 0.008b |
|
Positive | 33 (84.6) | 11 (52.4) | |
|
Negative | 6 (15.4) | 10 (47.6) | |
| PgR status | | | nsb |
|
Positive | 26 (66.7) | 9 (42.9) | |
|
Negative | 13 (33.3) | 12 (57.1) | |
|
Triple
negative | 5 (18.5) | 7 (25.0) | nsb |
| Neo/adjuvant
chemotherapy | | | nsb |
|
Taxane | 25 (64.1) | 14 (66.7) | |
|
Anthracycline | 27 (69.2) | 12 (57.1) | |
| Metastatic sites | | | |
|
Bone | 21 (53.9) | 8 (38.1) | nsb |
|
Liver | 18 (46.2) | 6 (28.6) | |
|
Lung | 17 (43.6) | 12 (57.1) | |
|
CNS | 1 (2.6) | 1 (4.8) | |
| Number of metastatic
sites | | | ns b |
|
Within
2 | 23 (59.0) | 13 (61.9) | |
|
3 or
more | 16 (41.0) | 8 (38.1) | |
| Treatment line | | | 0.0019b |
|
1 | 32 (82.0) | 9 (42.9) | |
|
2 | 7 (18.0) | 12 (57.1) | |
Safety
Neutropenia was the most frequent grade ≥3 adverse
event and had a similar incidence in both arms. There was one case
of grade 3/4 hypertension as a result of treatment with BEV + PTX
in each of the arms. There was only one case of grade 3/4
proteinuria in the B-E arm (Table
IIA and B). There were no
differences in adverse events between the two arms, and there were
no deaths.
 | Table IIAdverse events. |
Table II
Adverse events.
| A, Adverse events
of eribulin |
|---|
| | B-E arm (n=39) | E-B arm (n=21) |
|---|
| Toxicity | Grade 1/2 (%) | Grade 3/4 (%) | Grade 1/2 (%) | Grade 3/4 (%) |
|---|
| Leucopenia | 11 (28.2) | 4 (10.3) | 12 (57.1) | 1 (4.8) |
| Neutropenia | 5 (12.8) | 4 (25.6) | 7 (33.3) | 5 (23.8) |
|
Hypertrans-aminasemia | 3 (7.7) | 0 (0) | 6 (28.6) | 0 (0) |
|
Asthenia/fatigue | 17 (43.6) | 1 (2.6) | 8 (38.1) | 0 (0) |
| Peripheral
neuropathy | 21 (53.8) | 0 (0) | 7 (33.3) | 0 (0) |
|
Nausea/vomiting | 6 (15.4) | 0 (0) | 3 (14.3) | 0 (0) |
| Stomatitis | 7 (17.9) | 0 (0) | 3 (14.3) | 0 (0) |
| Dysgeusia | 10 (25.6) | 0 (0) | 6 (28.6) | 0 (0) |
| B, Adverse events
of BEV + PTX |
| | B-E arm (n=39) | E-B arm (n=21) |
| Toxicities | Grade 1/2 (%) | Grade 3/4 (%) | Grade 1/2 (%) | Grade 3/4 (%) |
| Leucopenia | 13 (33.3) | 5 (12.8) | 7 (33.3) | 1 (4.8) |
| Neutropenia | 10 (25.6) | 7 (17.9) | 3 (14.3) | 3 (14.3) |
|
Hypertrans-aminasemia | 6 (15.4) | 0 (0) | 5 (23.8) | 0 (0) |
|
Asthenia/fatigue | 18 (46.2) | 1 (2.6) | 10 (47.6) | 1 (4.8) |
| Peripheral
neuropathy | 30 (76.9) | 1 (2.6) | 9 (42.9) | 0 (0) |
|
Nausea/vomiting | 9 (23.1) | 1 (2.6) | 4 (19.0) | 0 (0) |
| Stomatitis | 12 (30.8) | 0 (0) | 4 (19.0) | 0 (0) |
| Dysgeusia | 11 (28.2) | 0 (0) | 5 (23.8) | 0 (0) |
| Hypertension | 5 (12.8) | 1 (2.6) | 3 (14.3) | 1 (4.8) |
| Proteinuria | 9 (23.1) | 1 (2.6) | 1 (4.8) | 0 (0) |
Efficacy
The overall response rates (ORRs) to eribulin
treatment were 28.9 and 33.3% in the B-E and E-B groups,
respectively, and the difference was not statistically significant
(Table III). In contrast, the
ORRs for BEV + PTX treatment were 66.6 and 23.9% in the B-E and E-B
groups, respectively, and the difference was statistically
significant (P=0.0147) (Table
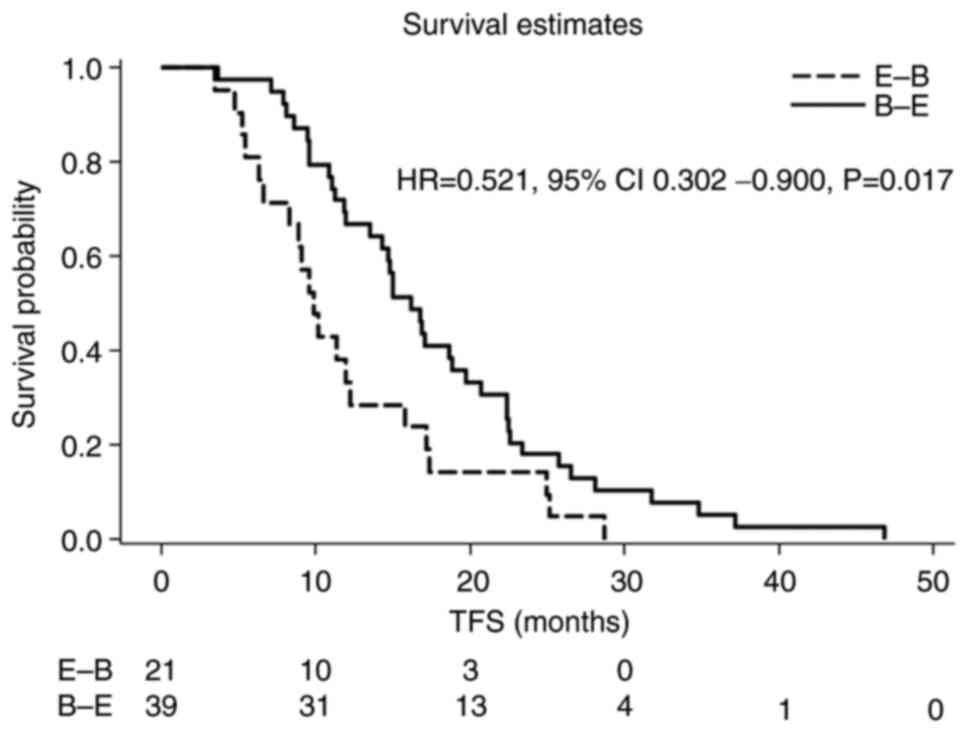
III). In the entire cohort, the median TFS was 16.8 and 9.9
months in the B-E and E-B arms, respectively (HR=0.515, 95% CI
0.298-0.889, P=0.017) (Fig. 1).
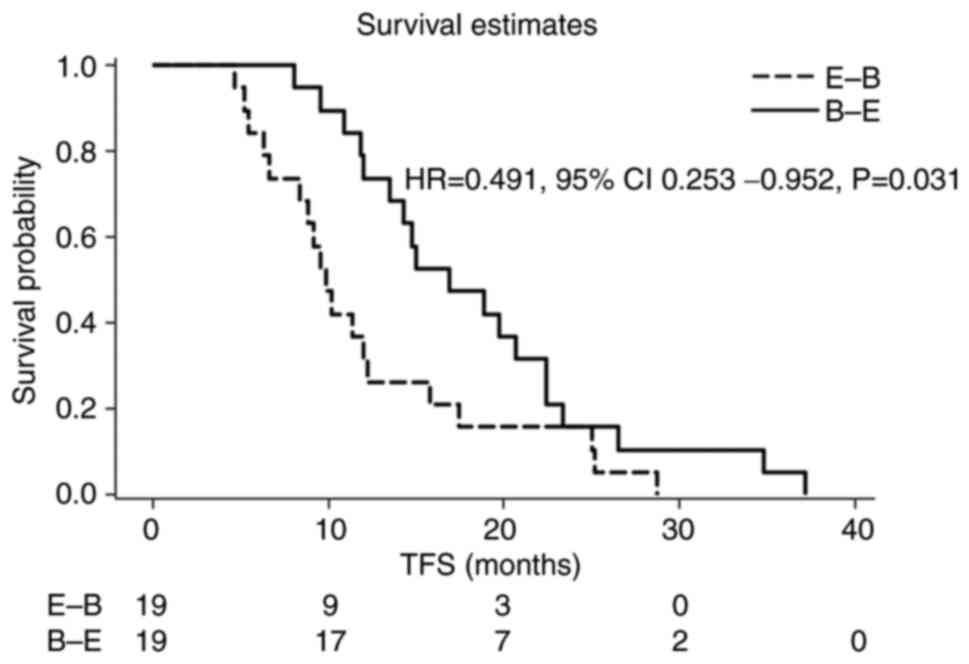
Using PSMA with the caliper of 1.0, 19 pairs (38 patients) were
identified, and their patient characteristics are summarized in
Table IV. TFS was 16.9 and 9.9
months in the B-E and E-B arms, respectively (HR=0.491, 95% CI
0.253-0.952, P=0.031) (Fig. 2).
The results (12 pairs, 24 patients) of PSMA with the caliper of
0.2, which was conducted as a sensitivity analysis (Fig. S1). Despite matching for treatment
line, age, PS, triple-negative status, liver metastasis, and number
of metastatic sites, the E-B and B-E arms showed significant
differences in TFS.
 | Table IIIEfficacy. |
Table III
Efficacy.
| | Number of patients
(%) | |
|---|
| Tumor response | B-E arm (n=39) | E-B arm (n=21) | P-value |
|---|
| Eribulin | | | ns |
|
CR | 1 (2.6) | 0 (0) | |
|
PR | 10 (26.3) | 7 (33.3) | |
|
SD | 11 (29.0) | 6 (28.6) | |
|
PD | 12 (31.6) | 6 (28.6) | |
|
Not
evaluable | 1 (2.6) | 2 (9.5) | |
| BEV+PTX | | | 0.0147 |
|
CR | 2 (5.1) | 1 (4.8) | |
|
PR | 24 (61.5) | 4 (19.1) | |
|
SD | 10 (25.6) | 9 (42.9) | |
|
PD | 3 (7.7) | 6 (28.6) | |
|
Not
evaluable | 0 (0) | 1 (4.8) | |
 | Table IVCharacteristics of the patients after
performing PSMA. |
Table IV
Characteristics of the patients after
performing PSMA.
| | Number of patients
(%) |
|---|
|
Characteristics | B-E arm (n=19) | E-B arm (n=19) |
|---|
| Treatment line | | |
|
1 | 12 (63.2) | 9 (47.4) |
|
2 | 7 (36.8) | 10 (52.6) |
| Age | | |
|
≤50
years | 5 (26.3) | 6 (31.6) |
|
51-60
years | 7 (36.8) | 5 (26.3) |
|
≥61
years | 7 (36.8) | 8 (42.1) |
| PS | | |
|
0 | 14 (73.7) | 13 (68.4) |
|
1 | 4 (21.1) | 4 (21.1) |
|
2 | 1 (5.3) | 2 (10.5) |
| Triple
negative | 5 (26.3) | 8 (42.1) |
| Liver
metastasis | 6 (31.6) | 5 (26.3) |
| Number of
metastatic sites | | |
|
≤2 | 13 (68.4) | 11 (57.9) |
|
≥3 | 6 (31.6) | 8 (42.1) |
Discussion
Patients who received BEV + PTX before eribulin (the
B-E arm) had a significantly longer TFS than patients who received
eribulin before BEV + PTX (the E-B arm) (16.8 vs. 9.0 months).
Overall survival was also longer with the B-E treatment than with
the E-B treatment (28.0 vs. 17.2 months). With regard to
progression-free survival, while our result in the B-E arm was in
line with that reported in E2100, RIBBON-1, AVADO, and JO19901,
which were phase 2 and 3 trials of first-line therapies, our
patients in the E-B arm had a shorter progression-free survival
than obtained in those trials (2,3,4,14).
On the other hand, progression-free survival with eribulin
treatment was 3.7 and 4.1 months in the E-B and B-E arms,
respectively, and the difference was not statistically significant.
Thus, a significant difference appeared during the time to second
progression. With regard to progression-free survival with
eribulin, our results in both the E-B and B-E arms were in line
with those reported in the EMBRACE trial and the 301 trials, which
were phase 3 trials (1,15). When a regimen of BEV + PTX is
administered late, progression-free survival is significantly
decreased compared with initial eribulin treatment. The BEV + PTX
regimen was the second-line treatment in the RIBBON-2 trial
(16), in which the median
progression-free survival was 9.1 months. In the present study,
progression-free survival in the B-E arm was better than in the
RIBBON-2 trial (11.5 months), a phase 3 trial designed to assess
second-line bevacizumab-containing therapy, but it was
significantly decreased in the E-B arm compared to the RIBBON-2
trial (6.2 months). It is supposed that BEV + PTX was administered
after 2 regimens in 57.1% of the E-B arm. Therefore, we performed
PSMA to minimize any imbalances in the background factors between
the two arms. The PSMA analysis yielded results consistent with
those in the overall analysis. Therefore, eribulin treatment is
recommended after BEV + PTX treatment. A similar tendency for TFS
was seen as a result of OS analysis by PSMA. However, a further
follow-up survey is necessary because there were few observation
events.
In regards to the mechanism, there is a difference
in tumor vessel remodelling and reoxygenation between BEV + PTX and
eribulin. Eribulin increases the density of tiny blood vessels and
the supply of oxygenated blood to breast cancer tissue (17). Eribulin may improve the state of
hypoxia relative to bevacizumab treatment. Furthermore, eribulin
stabilizes the microenvironment and may improve the treatment
effect. From these results, it is suggested that the administration
of eribulin after BEV + PTX is most effective.
In the present study, BEV + PTX treatment and
eribulin treatment were generally well tolerated. The incidence of
adverse events with BEV + PTX was similar to that in previous
clinical trials (2-4,14,16).
Eribulin had a manageable profile of adverse events, consistent
with those in previous clinical trials; neutropenia, alopecia,
leukopenia, and peripheral neuropathy were the most common
(1,18-22).
The incidence of grade ≥3 neutropenia was 17.9% in the B-E arm,
which was higher than that in the E-B arm. Therefore, considering
the adverse events of subsequent treatments, we must administer BEV
+ PTX carefully. It is considered that the prognosis can be
effectively improved by reducing adverse events by dose reduction,
withdrawal or maintenance therapies. Indeed, recent data suggest
that maintenance therapy has a positive effect on overall survival
(23,24).
There are more ER+ in the B-E group. If TFS (and OS)
is longer in the ER+ group, then the B-E group would be better for
this reason (if ER were a confounding factor). Therefore, we
examined whether ER is associated with TFS (and OS) using
multivariate Cox regression. We found that ER was not associated
with TFS. For reference, TFS group comparisons were performed
separately for ER+ and ER-, and it was confirmed that TFS was
longer in groups B-E in both subgroups. As we examined the
proportional hazards assumption using the two-stage test proposed
by Qiu and Sheng (13), the
violation of the assumption was not indicated (P =0.337 for OS and
0.766 for TFS) (Figs. S2 and
S3; Table SI). Based on the above discussion,
we believe that the B-E arm can be recommended regardless of ER
status in TFS prolongation.
To our knowledge, this is the first report of the
impact of the sequential treatment of HER2-negative metastatic
breast cancer with BEV + PTX and eribulin. This study has some
limitations. An important limitation is that although it is a
multicenter database, the study design is retrospective and
observational, and the number of cases is small. Although
consistent results were obtained using the PSMA method to reduce
selection bias, it is necessary to confirm the findings in a large,
prospective study.
In conclusion, despite the retrospective nature of
the present analysis and its inherent limitations, the data
presented show that when BEV+PTX and eribulin are administered
sequentially, the prognosis is better if BEV+PTX is administered
first.
Supplementary Material
Kaplan-Meier curves for TFS after PMSA
(12 pairs, 24 patients). PMSA, propensity score matching analysis;
TFS, time to failure of strategy.
Kaplan-Meier curves for TFS after PMSA
(ER+). PMSA, propensity score matching analysis; TFS, time to
failure of strategy.
Kaplan-Meier curves for OS after PMSA
(ER+). PMSA, propensity score matching analysis; OS, overall
survival.
Prognostic analysis of ER-positive
patients.
Acknowledgements
The authors would like to thank Dr Takuya Nagata
(Department of Surgery, Toho University Ohashi Medical Center), Dr
Yasuko Tanada (Department of Surgery, Toyama Nishi General
Hospital), Dr Katsuo Shimada (Department of Surgery, Imizu
Municipal Hospital), Dr Kaoru Kiyohara (Department of Surgery,
Tonami General Hospital), Dr Tetsuro Shimizu (Department of
Surgery, Saiseikai Toyama Hospital), and Dr Keiko Iwata (Department
of Surgery, Kurobe City Hospital) for providing patient data. This
research was presented at The San Antonio Breast Cancer Symposium
in 2020.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KM and TF designed the study concepts and confirm
the authenticity of all the raw data. KM, ME, AY, WF, ZN, KO, KK
and KM collected the data. SM and AN performed statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was conducted according to the guidelines
of The Declaration of Helsinki. However, ethics approval was not
applicable because this was a retrospective study that did not
include procedures outside of common and correct clinical
practice.
Patient consent for publication
Not applicable.
Competing interests
SM received honoraria from Chugai Pharmaceutical
Co., Ltd., and Eisai Co., Ltd., and received research funding
(institution) from Eisai Co., Ltd., outside the submitted work.
References
|
1
|
Cortes J, O'Shaughnessy J, Loesch D, Blum
JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Diéras V,
Delozier T, et al: Eribulin monotherapy versus treatment of
physician's choice in patients with metastatic breast cancer
(EMBRACE): A phase 3 open-label randomised study. Lancet.
377:914–923. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Miller K, Wang M, Gralow J, Dickler M,
Cobleigh M, Perez EA, Shenkier T, Cella D and Davidson NE:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. N Engl J Med. 357:2666–2676. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Robert NJ, Diéras V, Glaspy J, Brufsky AM,
Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SYT, Zhou X,
et al: RIBBON-1: Randomized, double-blind, placebo-controlled,
phase III trial of chemotherapy with or without bevacizumab for
first-line treatment of human epidermal growth factor receptor
2-negative, locally recurrent or metastatic breast cancer. J Clin
Oncol. 29:1252–1260. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Miles DW, Chan A, Dirix LY, Cortés J,
Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F,
et al: Phase III study of bevacizumab plus docetaxel compared with
placebo plus docetaxel for the first-line treatment of human
epidermal growth factor receptor 2-negative metastatic breast
cancer. J Clin Oncol. 28:3239–3247. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sandler A, Grey R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Escudier B, Pluzanska A, Koralewski P,
Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar
B, Bajetta E, et al: AVOREN Trial investigators. Bevacizumab plus
interferon alfa-2a for treatment of metastatic renal cell
carcinoma: A randomised, double-blind phase III trial. Lancet.
370:2103–2111. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Delaloge S, Pérol D, Courtinard C, Brain
E, Asselain B, Bachelot T, Debled M, Dieras V, Campone M, Levy C,
et al: Paclitaxel plus bevacizumab or paclitaxel as first-line
treatment for HER2-negative metastatic breast cancer in a
multicenter national observational study. Ann Oncol. 27:1725–1732.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
LeCaer H, Barlesi F, Corre R, Jullian H,
Bota S, Falchero L, Vergnenegre A, Dujon C, Delhoume JY and Chouaid
C: GFPC 0504 Team. A multicentre phase II randomised trial of
weekly docetaxel/gemcitabine followed by erlotinib on progression,
vs the reverse sequence, in elderly patients with advanced non
small-cell lung cancer selected with a comprehensive geriatric
assessment (the GFPC 0504 study). Br J Cancer. 105:1123–1130.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fiala O, Pesek M, Finek J, Benesova L,
Bortlicek Z and Minarik M: Sequential treatment of advanced-stage
lung adenocarcinoma harboring wild-type EGFR gene: Second-line
pemetrexed followed by third-line erlotinib versus the reverse
sequence. Anticancer Res. 33:3397–3402. 2013.PubMed/NCBI
|
|
11
|
Rosenbaum PR and Rubin DB: The central
role of the propensity score in observational studies for causal
effects. Biometrika. 70:41–55. 1983.
|
|
12
|
Sturmer T, Wyss R, Glynn RJ and Brookhart
MA: Propensity scoresfor confounder adjustment when assessing the
effects of medicalinterventions using nonexperimental study
designs. J Int Med. 275:570–580. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Peihua Q and Jun S: A two-stage procedure
for comparing hazard rate functions. J.R Statist Soc B. 70:191–208.
2008.
|
|
14
|
Aogi K, Masuda N, Ohno S, Oda T, Iwata H,
Kashiwaba M, Fujiwara Y, Kamigaki S, Ito Y, Ueno T and Takashima S:
First-line bevacizumab in combination with weekly paclitaxel for
metastatic breast cancer: Efficacy and safety results from a large,
open-label, single-arm Japanese study. Breast Cancer Res Treat.
129:829–838. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kaufman PA, Awada A, Twelves C, Yelle L,
Perez EA, Velikova G, Olivo MS, He Y, Dutcus CE and Cortes J: Phase
III open-label randomized study of eribulin mesylate versus
capecitabine in patients with locally advanced or metastatic breast
cancer previously treated with an anthracycline and a taxane. J
Clin Oncol. 33:594–601. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brufsky AM, Hurvitz S, Perez E, Swamy R,
Valero V, O'Neill V and Rugo HS: RIBBON-2: A randomized,
double-blind, placebo-controlled, phase III trial evaluating the
efficacy and safety of bevacizumab in combination with chemotherapy
for second-line treatment of human epidermal growth factor receptor
2-negative metastatic breast cancer. J Clin Oncol. 29:4286–4293.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ueda S, Saeki T, Takeuchi H, Shigekawa T,
Yamane T, Kuji I and Osaki A: . In vivo imaging of eribulin-induced
reoxygenation in advanced breast cancer patients: A comparison to
bevacizumab. Br J Cancer. 114:1212–1218. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Goel S, Mita AC, Mita M, Rowinsky EK, Chu
QS, Wong N, Desjardins C, Fang F, Jansen M, Shuster DE, et al: A
phase I study of eribulin mesylate (E7389), a mechanistically novel
inhibitor of microtubule dynamics, in patients with advanced solid
malignancies. Clin Cancer Res. 15:4207–4212. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tan AR, Rubin EH, Walton DC, Shuster DE,
Wong YN, Fang F, Ashworth S and Rosen LS: Phase I study of eribulin
mesylate administered once every 21 days in patients with advanced
solid tumors. Clin Cancer Res. 15:4213–4219. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vahdat LT, Pruitt B, Fabian CJ, Rivera RR,
Smith DA, Tan-Chiu E, Wright J, Tan AR, Dacosta NA, Chuang E, et
al: Phase II study of eribulin mesylate, a halichondrin B analog,
in patients with metastatic breast cancer previously treated with
an anthracycline and a taxane. J Clin Oncol. 27:2954–2961.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cortes J, Vahdat L, Blum JL, Twelves C,
Campone M, Roché H, Bachelot T, Awada A, Paridaens R, Goncalves A,
et al: Phase II study of the halichondrin B analog eribulin
mesylate in patients with locally advanced or metastatic breast
cancer previously treated with an anthracycline, a taxane, and
capecitabine. J Clin Oncol. 28:3922–3928. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Morgan RJ, Synold TW, Longmate JA, Quinn
DI, Gandara D, Lenz HJ, Ruel C, Xi B, Lewis MD, Colevas AD, et al:
Pharmacodynamics (PD) and pharmacokinetics (PK) of E7389 (eribulin,
halichondrin B analog) during a phase I trial in patients with
advanced solid tumors: A California cancer consortium trial. Cancer
Chemother Pharmacol. 76:897–907. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gligorov J, Doval D, Bines J, Alba E,
Cortes P, Pierga JY, Gupta V, Costa R, Srock S, de Ducla S, et al:
Maintenance capecitabine and bevacizumab versus bevacizumab alone
after initial first-line bevacizumab and docetaxel for patients
with HER2-negative metastatic breast cancer (IMELDA): A randomised,
open-label, phase 3 trial. Lancet Oncol. 15:1351–1360.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Park YH, Jung KH, Im SA, Sohn JH, Ro J,
Ahn JH, Kim SB, Nam BH, Oh DY, Han SW, et al: Phase III,
multicenter, randomized trial of maintenance chemotherapy versus
observation in patients with metastatic breast cancer after
achieving disease control with six cycles of gemcitabine plus
paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol.
31:1732–1739. 2013.PubMed/NCBI View Article : Google Scholar
|