Introduction
Metastasis is the leading cause of morbidity and
mortality in patients with cancer, accounting for ~90% of
cancer-associated deaths. However, the survival rate has recently
increased, owing to earlier detection of cancers and developments
in treatments (1). Particular
cancers tend to metastasize to specific organs, e.g., breast and
prostate cancers prominently develop skeletal metastases (2). Regarding visceral metastasis, the
liver is the most common site for metastasis, followed by the lung
(3).
Early diagnosis, proper staging and an accurate
metastatic workup are critical in oncology. The type and stage of
cancer are both crucial variables in the prognosis. As tumors may
spread to different anatomical locations, a reliable method to
detect distant metastases of malignancies is part of a project for
guiding future staging and appropriate treatment (4). Cancer care is highly dependent on
accurate information on individual tumor spread. For this reason,
early diagnosis and assessment of metastatic workup in individual
patients require several imaging modalities. However, this method
is time-consuming, costy and unpleasant for the patient (5). In high-risk patients, occult
metastases are evaluated during staging using chest X-rays,
abdominal ultrasounds and bone scintigraphy, while computed
tomography (CT), positron emission tomography with CT (PET/CT) and
magnetic resonance imaging (MRI) are increasingly being used as
they are more advanced and comprehensive in the detection of both
primary and metastatic lesions (6). Previous clinical studies have
indicated that 18F-fluorodeoxyglucose (18FDG)
PET/CT has significantly higher sensitivity and specificity in the
diagnosis and staging of certain cancers than CT alone, even though
it is more expensive and uses radioactive ions, i.e.
18FDG (6). Whole-body
MRI (WB-MRI) has been proposed as another effective whole-body
approach for assessing both local invasiveness and distant
metastases in patients with newly diagnosed cancers in recent years
(7,8). WB-MRI provides several advantages,
including the absence of ionizing radiation, high soft-tissue
contrast, low cost, the absence of radioactive substances, improved
availability and its safe use in patients with renal impairment
(6,9). WB-MRI primarily provides structural
information (revealing a detailed image of the pathology or lesion)
on tumor spread; however, the absence of functional datasets has
been resolved by incorporating WB-DWI into medical practice
(10). WB-DWI shortens examination
interpretation times by directing the radiologist's attention to
abnormalities, which may then be investigated on anatomic sequences
(11,12). In addition, WB-DWI with background
body signal suppression (DWIBS) enables volumetric capture of DWIs
of the entire body. This idea differs from traditional DWI, which
has been demonstrated to be effective during free breathing and has
a crucial role in WB imaging in oncology patients (13). Initial studies on TNM staging of
lung cancer using WB-MRI vs. PET/CT reveal that both modalities
give adequate accuracy and effectiveness. Whole-body MRI tends to
be more effective in identifying brain and liver metastases,
whereas PET/CT appears to be more effective in detecting lymph node
(LN) and soft tissue metastases (14).
The present study aimed to determine the efficacy of
WB-MRI in assessing metastasis in patients with newly diagnosed
cancers using histopathologic data as the reference method.
Patients and methods
Study design
This prospective study was conducted at a single
center (Shahid Hemn Teaching Hospital; Sulaimani, Iraq) from April
2018 to July 2020. It was performed on patients newly diagnosed
with cancers who have had a whole-body MRI scan.
Inclusion and exclusion criteria
Only patients with malignancies proven by histology
and patients with metastatic lesions proven by histopathology or
cytology were included. Patients whose MRI sequences were
incomplete, low-quality and/or had no histopathological evidence of
metastatic lesions were eliminated from the study. Low-quality or
incomplete MRI included MRI exams with incomplete sequence(s),
which is particularly common in elderly patients who are unable to
tolerate the scan, or uncooperative patients who do not obey
breathing instructions and motion artifacts that may impair images
and lead to lower accuracy.
Measurements
Sensitivity is the proportion of true-positive tests
out of all patients with a condition. It was calculated as follows:
Sensitivity=(True Positives)/(True Positives + False
Negatives).
Specificity is the percentage of true negatives out
of all subjects who do not have a disease or condition.
Calculation: Specificity=(True Negatives)/(True Negatives + False
Positives).
Accuracy measures how correct a diagnostic test
identifies and excludes a given condition. Calculation:
Accuracy=(True Negatives + True Positives)/(True Negatives + True
Positives + False Negatives + False Positives).
Radiological evaluation
In the current study, five sequences were used,
including anatomical and functional data between three orthogonal
planes (coronal, axial and sagittal). A surface body coil (Philips
ACHIEVA; 1.5 Tesla; Philips Medical Systems); was used to acquire
the WB-MRI images. The same MR platform was used to scan all
patients without giving contrast material. The evaluation lasted
for ~1 h. Table I lists the MRI
parameters in detail. For improved lesion detection, the DWIBS
images were inverted black-and-white grayscale. Conventional
sequences were employed to confirm positive DWIBS results or
artifacts. On a workstation, two board-certified radiologists with
10 years of expertise in WB-MRI evaluated the findings. Imaging
data were categorized based on nodal and distant metastasis and
stored in an Excel sheet. The histopathology sample was ordered by
a multidisciplinary team or the treating physician for clinical
purposes. If histological examination of the samples provided an
outcome, no further follow-up was performed. In the case of
involvement of just one organ, a sample was taken from the
suspicious lesion. In multi-organ metastases, only one lesion was
confirmed and all other lesions were considered positive.
 | Table IWhole-body MRI sequences and
parameters for oncologic patient evaluation. |
Table I
Whole-body MRI sequences and
parameters for oncologic patient evaluation.
| Sequence | T1WI | T1WI | T2WI | T2 STIR | WB-DWIBS |
|---|
| Plane | Coronal | Sagittal | Axial | Coronal | Coronal |
| Involved
regions | Vertex to toes | Whole spine | Vertex to
mid-thigh | Vertex to toes | Vertex to
mid-thigh |
| Slices, n | 34 | 20 | 152 | 34 | 230 |
| Gap, mm | 1 | 0.4 | 0.6 | 1 | 0 |
| Thickness, mm | 6 | 4 | 6 | 6 | 2 |
| TR, msec | 412 | 500 | 1,000 | 570 | 1,400 |
| TE, msec | 4 | 16 | 80 | 80 | 70 |
| b-value,
sec/mm2 | - | - | - | - | 0-800 |
| Phase encoding | Right/left | Feet-head |
Anterior-posterior | Right/left |
Anterior-posterior |
| Respiratory
motion | Breath-hold | Free-breathing | Breath-hold | Breath-hold | Free-breathing |
| Total scan time
(min) | 15-20 | 3-6 | 9-10 | 5-10 | 20-25 |
To simplify the process, the WB-MRI was divided into
three categories: Nodal, skeletal and visceral metastases.
Patient-based analysis was used for visceral metastases, whereas
region-based analysis was used for skeletal systems and LN
groups.
Statistical analysis
To strengthen the identification of the collected
datasets, statistical analysis using two softwares, SPSS (version
25; IBM Corporation) and Excel 2016 (Microsoft Corporation), was
performed. The presence or absence of metastasis was used to
classify the findings. Using 2x2 cross-tabulation data, the
sensitivity, specificity and accuracy of WB-MRI were estimated.
Results
Patient characteristic
A WB-MRI scan was performed on 153 patients
diagnosed with primary cancers. Only 43 individuals with distant
metastatic lesions, confirmed by histology, were included. WB-MRI
was used to evaluate a total of 43 consecutive patients (24 males
and 19 females) who had just been diagnosed with cancer, with a
mean age of 56±15.2 (range, 18-83) years. Histopathological
confirmation was used as the gold standard. The present study
included a variety of primary tumors. Breast cancer was the most
prevalent primary tumor, accounting for 16.2%. Breast cancer was
the most common primary tumor in seven cases followed by prostate
cancer in six cases. Table II
provides an account of the locations/types of primary tumors within
the cohort.
 | Table IITypes/locations of primary tumors in
the patients (n=43). |
Table II
Types/locations of primary tumors in
the patients (n=43).
| Primary
malignancy | N |
|---|
| Melanoma | 4 |
| Sarcoma | 4 |
| Bronchogenic
cancer | 2 |
| Prostate
cancer | 6 |
| Breast cancer | 7 |
| Endometrial
cancer | 3 |
| Ovarian cancer | 2 |
| Colorectal
cancer | 2 |
| Thyroid
carcinoma | 3 |
| Carcinoma of
unknown primary origin | 6 |
| Neuroendocrine
tumor | 1 |
| Mesothelioma | 1 |
| Testicular
tumor | 2 |
| Total | 43 |
Efficacy
According to the patient-based method, there was a
concordance between the WB-MRI and histological confirmation in 41
of 43 cases (95.3%). The skeletal system was the most prevalent
location of metastasis. There was a concordance between WB-MRI and
histological confirmation of visceral metastases. There were no
false-positive or false-negative outcomes recorded. On WB-MRI, 12
patients had hepatic metastasis, 10 had pulmonary metastasis, 4 had
peritoneal metastasis and only one had brain metastasis.
For the LNs, a region-based analysis was performed.
A total of 258 areas were evaluated on 43 individuals.
Lymphadenopathy was defined as any LN measuring >10 mm in the
short axis diameter or any abnormal LN of any size that had newly
appeared. WB-MRI verified 38 of the 258 LNs as positive, whereas
histology confirmed 36. Two LN areas were first considered to be
negative on imaging but turned out to be positive on histology. The
presence or absence of metastases was determined in all skeletal
areas. WB-MRI was used to evaluate 301 regions of the 43 patients,
with 66 sites considered positive. The accuracy for detecting bone
metastases was 100%. There were no false-positive or false-negative
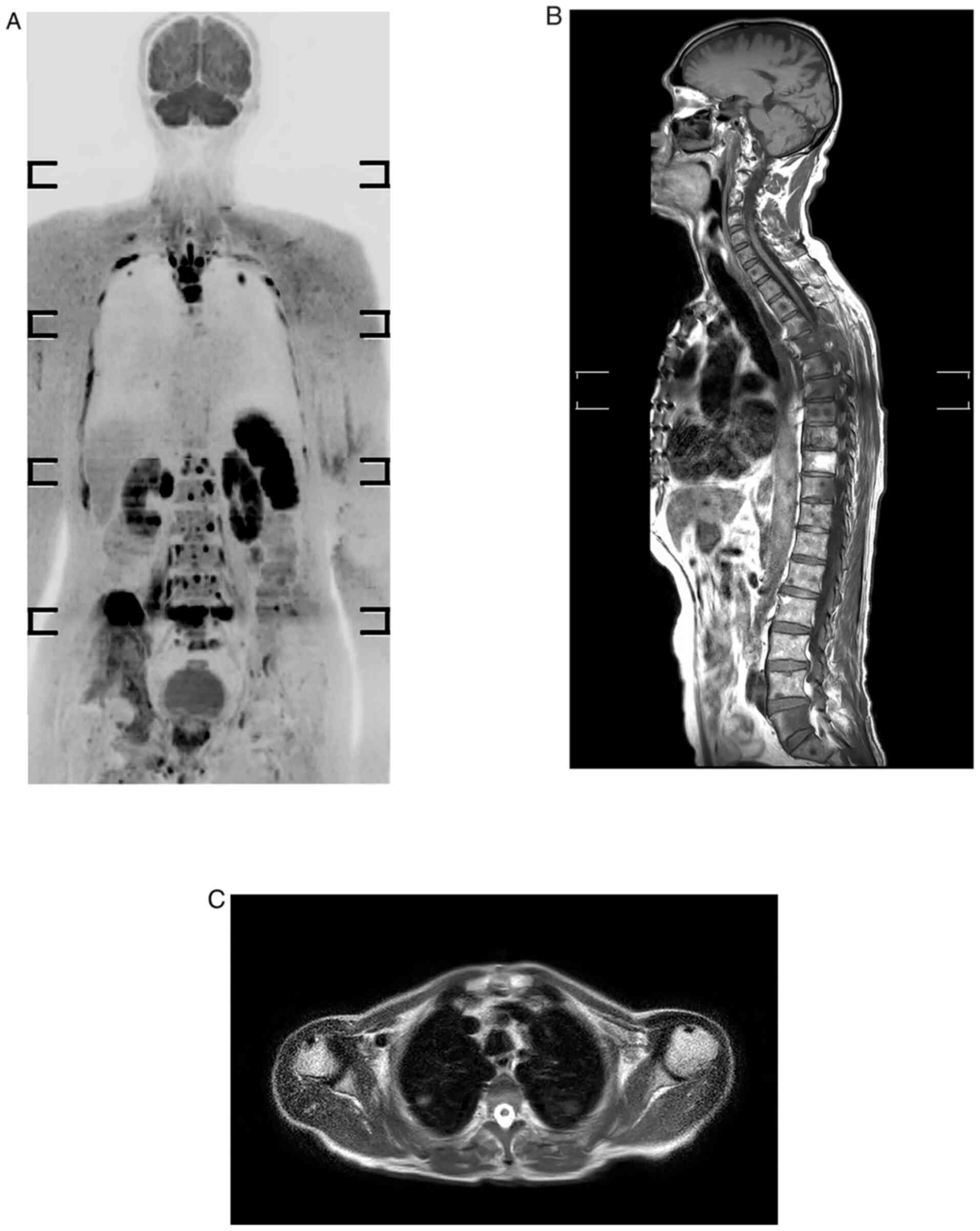
results for skeletal and visceral metastases. The sagittal plane is
strongly recommended in oncology because spine curvatures may be
responsible for partial volume effects that conceal certain lesion
on coronal plane. In the current study, the accuracy for bone
metastasis was 100%; this high result is explained by the efficacy
of DWBIS and the provision of images in three orthogonal planes
(Fig. 1).
The study found that WB-MRI was 100% accurate in
diagnosing skeletal and visceral metastases but only 98.45%
accurate in diagnosing LN metastases. The sensitivity, specificity
and accuracy of visceral, skeletal and nodal metastases are all
provided in Table III.
 | Table IIISensitivity, specificity and accuracy
of visceral, skeletal and nodal metastases based on histological
findings. |
Table III
Sensitivity, specificity and accuracy
of visceral, skeletal and nodal metastases based on histological
findings.
| MRI finding | Visceral
metastasis | Bone
metastasis | Nodal
metastasis |
|---|
| Sensitivity, % | 100 | 100 | 94.74 |
| Specificity, % | 100 | 100 | 99.09 |
| Accuracy, % | 100 | 100 | 98.45 |
Discussion
A proper staging work-up and monitoring are required
for assessing prognosis and management strategies in patients with
cancer (15). Bone scans and PET
scans are commonly utilized in the initial work-up in patients with
cancer to evaluate metastases. These techniques expose the
cancer-affected patient to potentially hazardous radiation
(16). A previous study has been
performed to demonstrate the utility of WB-MRI in resolving this
dilemma, as MRI is generally more readily available and is a
radiation-free technique. Adding to that, MRI may detect metastatic
lesions in bone before the occurrence of changes in bone
metabolism, making them visible on bone scans (16). WB-MRI has been used for both
primary staging and monitoring of different malignancies,
particularly cancers that commonly metastasize to the bone, brain
and abdominal organs, such as breast cancer and colorectal cancer
(17). WB-MRI has also been used
as a diagnostic tool for imaging of various hematologic
malignancies and bone marrow pathologies, such as lymphoma and
multiple myeloma (17).
False-positive results, particularly in enlarged LNs
due to inflammatory processes or normal-sized LNs carrying
micro-metastases, may decrease MRI or CT sensitivity and
specificity. Comparison of WB-MRI to other imaging modalities, such
as PET-CT, bone scintigraphy and conventional cross-sectional
imaging, has been performed in numerous studies (18-20).
Barchetti et al (21)
compared WB-MRI to PET-CT and discovered that it had a sensitivity,
specificity and accuracy of 99, 98 and 98%, respectively. Regarding
visceral metastasis, a recent meta-analysis reported that DWI had a
sensitivity of 82.8% and specificity of 80.1% for detecting lung
nodules (22). MRI sensitivity and
specificity are reduced when pulmonary nodules are <10 mm in
size. Regier et al (23)observed a sensitivity of 97% for
nodules with a diameter of >10 mm, while it dropped to 86% for
nodules 6-9 mm in size and 43.8% for lesions 5 mm or less. Goda
et al (24) discovered a
sensitivity, specificity and accuracy of 64, 88 and 76%,
respectively, and an accuracy of 100% for hepatic lesions. DWIBS
was used in the present study instead of DWI, which is more
sensitive than DWI, as demonstrated in a recent study published by
Eissawy et al (25). In the
current study, WB-MRI demonstrated 100% accuracy for visceral
metastases. The high percentages obtained in the current study,
notably in detecting lung lesions, may be attributed to the use of
DWIBS, which allows for free-breathing scanning of moving visceral
organs and lesions. In metastatic cancer, there is a limited
indication that the lesions detected by WB-MRI contain
pathologically viable tumor cells (26). However, in the present study,
histology confirmed the radiological findings.
The DWIBS sequence increases the detection of
pulmonary lesions. Usuda et al (27) performed a study on 55 patients with
lung cancer, concluding that the DWIBS sequence may identify
multiple metastatic lesions across the body and differentiate
malignancy from benignity in only one examination. Although DWIBS
alone may be somewhat sensitive, characterization of lesions is not
always achievable due to impeded diffusion in both malignant and
nonmalignant processes. To avoid false-positive and false-negative
outcomes, correlation with morphologic imaging data is required.
Another factor that improves diagnostic accuracy in the present
study is the use of a surface coil rather than a main magnet coil,
which considerably improves the pulmonary spatial resolution, as
indicated by Paruthikunnan et al (28).
Primary malignancies frequently metastasize to LNs,
and LN involvements have an impact on patient management (29). WB-DWI offers a functional imaging
component that may enhance LN characterization by providing
information on tissue characteristics over a wide field at
appropriate acquisition times, making it a practical staging and
screening method (30). WB-DWI was
suggested as an alternative to conventional 18FDG PET/CT
for lymphoma staging (31). DWI
reveals microstructural and cellular changes in malignant vs.
normal LNs. Goda et al (24) determined that the sensitivity,
specificity and accuracy for LN detection were 77, 85 and 83%,
respectively. Changing the size parameters to bigger or smaller
cut-offs may affect sensitivity and specificity, as a low cut-off
value would increase sensitivity but reduce specificity (24). This may explain why the sensitivity
of WB-DWI-MRI varies from 60 to 90% in different trials (32,33).
Schmidt et al (17)
reported that WB-MRI detected 92% of LNs with diameters >12 mm.
However, the detection accuracy decreased to 67% for LNs sized 6-12
mm. The findings of Sigovan et al (34)indicated that DWI had high
sensitivity, specificity and accuracy (91, 83 and 85%,
respectively) in distinguishing benign from malignant enlarged
mediastinal LNs. The present study found that WB-MRI has 98.45%
accuracy in diagnosing LN metastases. Porta-hepatis LNs had the
maximum precision. The lowest precision was recorded in mediastinal
LNs, where image quality may be compromised by pulsation artifacts
(24). Compared with WB-MRI,
PET/CT appears to have an increased sensitivity for neoplastic
axillary and mediastinal LNs (18).
The most prevalent malignant bone lesion is bone
metastasis. Skeletal involvement occurs in 30-70% of all patients
with cancer (35). Currently,
99mTc-phosphonate-based scintigraphy is a well-established approach
for screening for skeletal metastases in the body. However, in the
absence of an osteoblastic response, lesions may be undetectable in
the early stage of the disease. In addition, misperception of
tracer uptake in healing fractures or degenerative illness may
result in false-positive results. The diagnostic performance of MRI
for skeletal metastases has been compared to bone scintigraphy in
several studies with greater specificity and sensitivity in the
early detection of skeletal metastases (36). Recent meta-analyses have indicated
that WB-MRI has higher diagnostic accuracy than bone scan and CT in
identifying primary and metastatic lesions in patients with
prostate cancer (37,38). The sensitivity and specificity of
WB-MRI in detecting bone metastases were reported to be 95%,
whereas the values obtained for bone scan were only 78 and 85%, and
for CT, only 77 and 83%, respectively (35,37,38).
Goda et al (24)
demonstrated that WB-DWI detected bone lesions with a sensitivity,
specificity and accuracy of 88, 94 and 92%, respectively. According
to a meta-analysis conducted by Yang et al (36), the sensitivity rates for PET/CT,
CT, MRI and bone scintigraphy were 89.7, 72.9, 90.6 and 86.0%,
respectively, while the specificity rates were 96.8, 94.8, 95.4 and
81.4%, respectively. This increased precision may even help in
determining a more suitable prognosis for each patient. The
acquisition of the whole spine sequence enhances the image
accuracy, since pathological fractures may be obscured on the
coronal plane. The sagittal plane is strongly suggested in oncology
because spine curvatures may be responsible for partial volume
effects that conceal certain coronal plane lesions. In the current
study, the accuracy for bone metastasis was 100%; this high result
is explained by the efficacy of DWBIS and the provision of images
in three orthogonal planes.
Each imaging modality has limitations that may lead
to bias in the efficacy of WB-MRI. The long scanning duration in
the present study is a limitation, particularly for uncooperative
and elderly patients. The only criterion for LN evaluation is the
size; as the LN is a highly cellular tissue, it exhibited high
signal intensity on the DWIBS sequence, regardless of the size.
Further limitations of the present study are the relatively small
sample size and the exclusion of pulmonary lesions >10 mm in
size. The current study's main strength is that histopathology data
are employed as the gold standard for included patients. To the
best of our knowledge, no previous study has compared WB-MRI to
histology.
In conclusion, WB-MRI in three orthogonal planes,
including the DWIBS sequence, may be utilized efficiently and
accurately to examine patients with malignancies for metastasis.
Further studies in the area of this issue are required.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
RJR was a major contributor to the conception of the
study. FHK and MNH were involved in the literature review and the
writing of the manuscript. RQS and AMS contributed to the
conception and the design of the study. SFA, SHA and SN analysed
and interpreted the data. RQS and SHK were involved in the
literature review, the design of the study, the revision of the
manuscript and in the processing of the figures. KKM, SMM and SHM
approved the final manuscript version to be published. SHT and SSO
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The Arab Board Scientific Committee (Sulaimani
branch, Iraq) and the local ethical committee of the University of
Sulaimani/College of Medicine (Sulaimani, Iraq) approved this study
(no. 2018-02). Written informed consent to participate in the study
was obtained from the patients.
Patient consent for publication
All patients provided written informed consent
regarding the publication of their data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guan X: Cancer metastases: Challenges and
opportunities. Acta Pharm Sin B. 5:402–418. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Heindel W, Gübitz R, Vieth V, Weckesser M,
Schober O and Schäfers M: The diagnostic imaging of bone
metastases. Dtsch Arztebl Int. 111:741–747. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Imam K and Bluemke DA: MR imaging in the
evaluation of hepatic metastases. Magn Reson Imaging Clin N Am.
8:741–756. 2000.PubMed/NCBI
|
|
4
|
Guimarães MD, Noschang J, Teixeira SR,
Santos MK, Lederman HM, Tostes V, Kundra V, Oliveira AD, Hochhegger
B and Marchiori E: Whole-body MRI in pediatric patients with
cancer. Cancer Imaging. 17(6)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schlemmer HP, Schäfer J, Pfannenberg C,
Radny P, Korchidi S, Müller-Horvat C, Nägele T, Tomaschko K,
Fenchel M and Claussen CD: Fast whole-body assessment of metastatic
disease using a novel magnetic resonance imaging system: Initial
experiences. Invest Radiol. 40:64–71. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Godinho MV, Lopes FPPL and Costa FM:
Whole-body magnetic resonance imaging for the assessment of
metastatic breast cancer. Cancer Manag Res. 10:6743–6756.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lauenstein TC and Semelka RC: Emerging
techniques: Whole-body screening and staging with MRI. J Magn Reson
Imaging. 24:489–498. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schmidt GP, Haug AR, Schoenberg SO and
Reiser MF: Whole-body MRI and PET-CT in the management of cancer
patients. Eur Radiol. 16:1216–1225. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bisschop C, de Heer EC, Brouwers AH,
Hospers GAP and Jalving M: Rational use of 18F-FDG
PET/CT in patients with advanced cutaneous melanoma: A systematic
review. Crit Rev Oncol Hematol. 153(103044)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li B, Li Q, Nie W and Liu S: Diagnostic
value of whole-body diffusion-weighted magnetic resonance imaging
for detection of primary and metastatic malignancies: A
meta-analysis. Eur J Radiol. 83:338–344. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nievelstein RA and Littooij AS: Whole-Body
MRI in Pediatric Oncology. Img Ped Oncol. 107–135. 2019.
|
|
12
|
Pasoglou V, Michoux N, Tombal B and
Lecouvet F: Optimising TNM staging of patients with prostate cancer
using WB-MRI. J Belg Soc Radiol. 100(101)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kwee TC, Takahara T, Ochiai R, Nievelstein
RA and Luijten PR: Diffusion-weighted whole-body imaging with
background body signal suppression (DWIBS): Features and potential
applications in oncology. Eur Radiol. 18:1937–1952. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Puls R, Kühn JP, Ewert R and Hosten N:
Whole-body magnetic resonance imaging for staging of lung cancer.
Front Radiat Ther Oncol. 42:46–54. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Akay S, Kocaoglu M, Emer O, Battal B and
Arslan N: Diagnostic accuracy of whole-body diffusion-weighted
magnetic resonance imaging with 3.0 T in detection of primary and
metastatic neoplasms. J Med Imaging Radiat Oncol. 57:274–282.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kachewar SG: Using DWIBS MRI technique as
an alternative to bone scan or PET scan for whole-body imaging in
oncology patients. Acta Radiol. 52(788)2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schmidt GP, Reiser MF and Baur-Melnyk A:
Whole-body MRI for the staging and follow-up of patients with
metastasis. Eur J Radiol. 70:393–400. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schmidt GP, Baur-Melnyk A, Haug A,
Heinemann V, Bauerfeind I, Reiser MF and Schoenberg SO:
Comprehensive imaging of tumor recurrence in breast cancer patients
using whole-body MRI at 1.5 and 3 T compared to FDG-PET-CT. Eur J
Radiol. 65:47–58. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sohaib SA, Koh DM, Barbachano Y, Parikh J,
Husband JE, Dearnaley DP, Horwich A and Huddart R: Prospective
assessment of MRI for imaging retroperitoneal metastases from
testicular germ cell tumours. Clin Radiol. 64:362–367.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ciliberto M, Maggi F, Treglia G, Padovano
F, Calandriello L, Giordano A and Bonomo L: Comparison between
whole-body MRI and Fluorine-18-Fluorodeoxyglucose PET or PET/CT in
oncology: A systematic review. Radiol Oncol. 47:206–218.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Barchetti F, Stagnitti A, Megna V, Al
Ansari N, Marini A, Musio D, Monti ML, Barchetti G, Tombolini V,
Catalano C and Panebianco V: Unenhanced whole-body MRI versus
PET-CT for the detection of prostate cancer metastases after
primary treatment. Eur Rev Med Pharmacol Sci. 20:3770–3776.
2016.PubMed/NCBI
|
|
22
|
Li B, Li Q, Chen C, Guan Y and Liu S: A
systematic review and meta-analysis of the accuracy of
diffusion-weighted MRI in the detection of malignant pulmonary
nodules and masses. Acad Radiol. 21:21–29. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Regier M, Schwarz D, Henes FO, Groth M,
Kooijman H, Begemann PG and Adam G: Diffusion-weighted MR-imaging
for the detection of pulmonary nodules at 1.5 Tesla:
Intraindividual comparison with multidetector computed tomography.
J Med Imaging Radiat Oncol. 55:266–274. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Goda HH, Abd Elkareem HA, Ahmed EA,
Megally HI, Khalaf MI, Taha AM and Mohamed HEG: Whole body
diffusion-weighted MRI in detection of metastasis and lymphoma: A
prospective longitudinal clinical study. Egypt J Radiol Nucl Med.
51:1–2. 2020.
|
|
25
|
Eissawy MG, Saadawy AMI, Farag K, Akl T
and Kamr WH: Accuracy and diagnostic value of diffusion-weighted
whole body imaging with background body signal suppression (DWIBS)
in metastatic breast cancer. Egypt J Radiol Nucl Med.
52(74)2021.
|
|
26
|
Iwamura H, Kaiho Y, Ito J, Anan G, Satani
N, Matsuura T, Tamura R, Murakami K, Koyama K and Sato M:
Evaluation of tumor viability for primary and bone metastases in
metastatic castration-resistant prostate cancer using whole-body
magnetic resonance imaging. Case Rep Urol.
2018(4074378)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Usuda K, Iwai S, Yamagata A, Iijima Y,
Motono N, Matoba M, Doai M, Yamada S, Ueda Y, Hirata K, et al:
Diffusion-weighted whole-body imaging with background suppression
(DWIBS) is effective and economical for detection of metastasis or
recurrence of lung cancer. Thorac Cancer. 12:676–684.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Paruthikunnan SM, Kadavigere R and
Karegowda LH: Accuracy of whole-body DWI for metastases screening
in a diverse group of malignancies: Comparison with conventional
cross-sectional imaging and nuclear scintigraphy. AJR Am J
Roentgenol. 209:477–490. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Torabi M, Aquino SL and Harisinghani MG:
Current concepts in lymph node imaging. J Nucl Med. 45:1509–1518.
2004.PubMed/NCBI
|
|
30
|
Tunariu N, Blackledge M, Messiou C,
Petralia G, Padhani A, Curcean S, Curcean A and Koh DM: What's new
for clinical whole-body MRI (WB-MRI) in the 21st century. Brit J
Radiol. 93(20200562)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kharuzhyk S, Zhavrid E, Dziuban A,
Sukolinskaja E and Kalenik O: Comparison of whole-body MRI with
diffusion-weighted imaging and PET/CT in lymphoma staging. Eur
Radiol. 30:3915–3923. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Balbo-Mussetto A, Cirillo S, Bruna R,
Gueli A, Saviolo C, Petracchini M, Fornari A, Lario CV, Gottardi D,
De Crescenzo A and Tarella C: Whole-body MRI with
diffusion-weighted imaging: A valuable alternative to
contrast-enhanced CT for initial staging of aggressive lymphoma.
Clin Radiol. 71:271–279. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Albano D, Patti C, La Grutta L, Agnello F,
Grassedonio E, Mulè A, Cannizzaro G, Ficola U, Lagalla R, Midiri M
and Galia M: Comparison between whole-body MRI with
diffusion-weighted imaging and PET/CT in staging newly diagnosed
FDG-avid lymphomas. Eur J Radiol. 85:313–318. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sigovan M, Akl P, Mesmann C, Tronc F,
Si-Mohamed S, Douek P and Boussel L: Benign and malignant enlarged
chest nodes staging by diffusion-weighted MRI: An alternative to
mediastinoscopy? Brit J Radiol. 91(20160919)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang HL, Liu T, Wang XM, Xu Y and Deng SM:
Diagnosis of bone metastases: A meta-analysis comparing 18FDG PET,
CT, MRI and bone scintigraphy. Eur Radiol. 21:2604–2617.
2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Engelhard K, Hollenbach HP, Wohlfart K,
von Imhoff E and Fellner FA: Comparison of whole-body MRI with
automatic moving table technique and bone scintigraphy for
screening for bone metastases in patients with breast cancer. Eur
Radiol. 14:99–105. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shen G, Deng H, Hu S and Jia Z: Comparison
of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the
diagnosis of bone metastases in patients with prostate cancer: A
meta-analysis. Skelet Radiol. 43:1503–1513. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu LP, Cui LB, Zhang XX, Cao J, Chang N,
Tang X, Qi S, Zhang XL, Yin H and Zhang J: Diagnostic performance
of diffusion-weighted magnetic resonance imaging in bone
malignancy: Evidence from a meta-analysis. Medicine (Baltimore).
94(e1998)2015.PubMed/NCBI View Article : Google Scholar
|