Introduction
The number of breast cancer cases is increasing
annually throughout the world and it has more than doubled from
~800,000 in 1990 to 1.68 million in 2016(1). The 10-year survival rate for stage I
primary breast cancer is >95% with good prognosis; however, that
for metastatic/recurrent breast cancer is only ~5% (2). Proper definitive treatment is
important to prevent metastasis and recurrence. Surgical treatment
is known to have an important role as the initial treatment. The
standard surgery for breast cancer is generally mastectomy or
partial mastectomy; i.e., breast-conserving surgery (BCS), but it
is important to obtain a negative surgical margin, particularly in
BCS. A meta-analysis by the Early Breast Cancer Trialists'
Collaborative Group reported that positive surgical margins in BCS
increased breast cancer-related mortality (3). Accurate diagnosis of the spread of
breast cancer is necessary to obtain negative surgical margins. The
gold standard imaging examination for investigating the spread of
breast cancer is ultrasound (US) (4). However, for diagnoses made by US, the
rate of positive surgical margins is 24-27% (5,6),
which is clearly insufficient and requires to be improved. As an
alternative technique, magnetic resonance imaging (MRI) with the
patient in the prone position has recently been used for this
purpose. The prone position has the advantages of minimal
deterioration of image quality due to respiratory movements and of
improved ability to examine intraductal components of the mammary
glands without distortion, as they are stretched when the breast is
‘hanging’ in this position (7,8). At
present, US and MRI in the prone position are in widespread use as
standard methods despite a lack of significant results from
randomized controlled trials (RCTs) (6). An RCT conducted in 2001 compared the
reoperation rate for BCS between a group with MRI in the prone
position (n=816) and a triple assessment group of mammography (MG),
US and core needle biopsy (non-MRI group, n=807). There was no
significant reduction in the reoperation rate in the MRI group
(153/816, 19%) compared with the non-MRI group (156/807, 19%; odds
ratio: 0.96, 95% CI: 0.75-1.24, P=0.77) (9). A disadvantage of MRI in the prone
position is that it differs from the operating position (10,11).
Even if spread of the lesion can be correctly diagnosed, it is
considered that a certain shift occurs due to the change in posture
between imaging and surgery. The present study was performed based
on the notion that supine MRI has an advantage compared with prone
MRI because the images are acquired in the same position as the
surgical position. In addition, MRI image rendering technology has
improved to the point that previous problems associated with supine
MRI have been overcome. The aim of the present study was therefore
to examine the rate of positive surgical margins for supine
MRI.
Patients and methods
Patients
Enrolled in this multi-center retrospective study
were 1,150 consecutive patients with a diagnosis of breast cancer
who underwent BCS between January 2012 and December 2013 at Sapporo
Medical University Hospital, Sapporo Breast Surgical Clinic,
Sapporo-Kotoni Breast Clinic, Shin-Sapporo Breast Clinic or Higashi
Sapporo Hospital (Sapporo, Japan). Inclusion criteria were as
follows: Patients with BCS who underwent preoperative MRI performed
in the supine position at the above-mentioned hospitals between
January 2012 and December 2013. Patients with bilateral and stage Ⅳ
breast cancer were excluded. For all patients, surgeries were
performed by certified surgeons and BCS was performed in all cases
based on the decision of each surgeon. Surgical margin-negative was
defined as no microscopically observable tumor at the margin. MRI
was performed using the GE SIGNA Excite 1.5T, GE SIGNA HGX 1.5T
(Cytiva) or PHILIPS Ingenia 1.5T Ver.4.3 (Philips Medical Systems,
Inc.). There was no standardized technique for partial resection at
any of the institutions and the operative method was selected based
on curative ability and adaptability. All patients underwent MG, US
and clinical best examination. At Sapporo Medical University
Hospital (Sapporo, Japan), but no other institution, rapid
intraoperative pathological examination was performed in four
directions and additional resection was performed if the result was
cancer-positive. The final pathological diagnoses were obtained by
an individual pathologist at the respective institution or by a
dedicated pathologist at an affiliated pathology laboratory. If
either non-invasive or invasive cancer was identified, the patient
was considered cancer-positive. Furthermore, the margin was
compared between patients with different HER2 status.
Statistical analysis
The clinicopathological parameters were compared
between the margin-positive and -negative groups using the
χ2 test. P<0.05 was considered to indicate
statistical significance. JMP11 software (SAS Institute, Inc.) was
used for statistical analysis.
Results
Patients' background
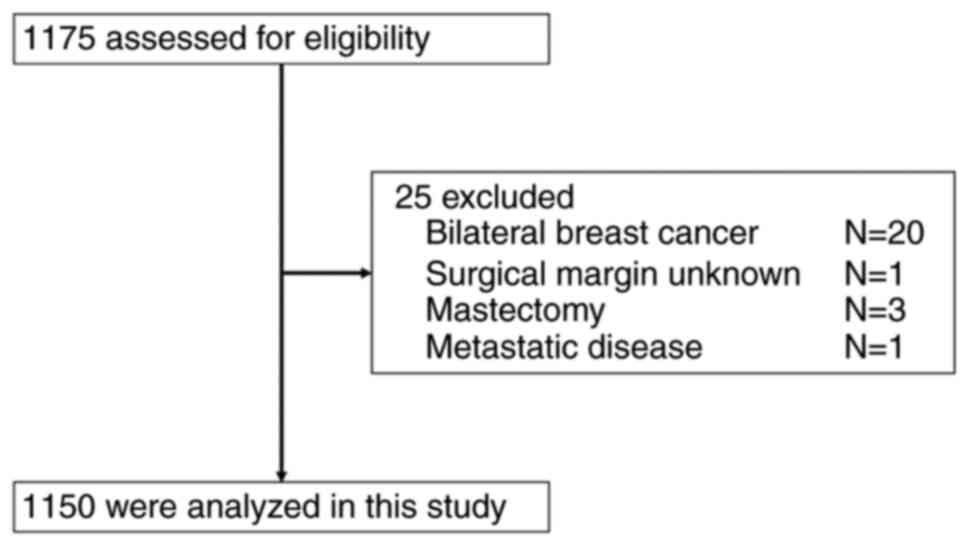
After excluding 25 cases of bilateral breast cancer
and stage IV cancer, 1,150 of the 1,175 patients were enrolled in
the study (Fig. 1). All of these
women (median age, 55 years; range, 29-97 years) underwent MRI in
the supine position prior to BCS for breast cancer.
Patients' characteristics
The patient characteristics, rate of positive
surgical margins and clinicopathological factors are listed in
Table I. Regarding the rate of
positive surgical margins determined by supine MRI (primary
endpoint), 215/1,150 patients (18.8%) had positive and 930/1,150
(81.2%) had negative margins.
 | Table ICharacteristics of the patients of the
present study (n=1,150). |
Table I
Characteristics of the patients of the
present study (n=1,150).
| Parameter | Value |
|---|
| Age, years | 55 (29-97) |
| cT | |
|
0 | 181 (15.8) |
|
1 | 738 (64.6) |
|
2 | 213 (18.6) |
|
3 | 6 (0.5) |
|
4 | 5 (0.4) |
| pN | |
|
0 | 888 (81.8) |
|
1-3 | 197 (18.2) |
| ER | |
|
Positive | 975 (85.3) |
|
Negative | 168 (14.7) |
| PgR | |
|
Positive | 825 (73.2) |
|
Negative | 302 (26.8) |
| HER2 | |
|
Positive | 121 (11.4) |
|
Negative | 944 (88.6) |
| ER/HER2 status | |
|
ER- and
HER2+ | 41 (3.6) |
|
Any | 1,104 (96.4) |
| NG | |
|
1 | 592 (67.8) |
|
2 | 80 (9.2) |
|
3 | 201 (23.0) |
| Ly | |
|
Positive | 423 (40.5) |
|
Negative | 622 (59.5) |
| Margin | |
|
Positive | 215 (18.8) |
|
Negative | 930 (81.2) |
Positive margin rate
The rate of positive surgical margins was
substantially higher in patients of the HER2-positive type than in
those with HER2-negative type breast cancer (6.5 and 2.9%,
respectively; χ2 P=0.0103; Table II).
 | Table IIComparison of clinicopathological
factors between margin-positive and margin-negative cases. |
Table II
Comparison of clinicopathological
factors between margin-positive and margin-negative cases.
| Parameter | Margin positive
(n=218) | Margin negative
(n=932) | P-value |
|---|
| Age, years | 53 (29-83) | 55 (30-97) | 0.5109 |
| cT | | | 0.6903 |
|
0 | 33 (15.4) | 148 (16.0) | |
|
1 | 142 (66.0) | 596 (64.2) | |
|
2 | 38 (17.7) | 175 (18.7) | |
|
3 | 2 (0.9) | 4 (0.4) | |
|
4 | 0 (0) | 5 (0.5) | |
| pN | | | 0.5561 |
|
0 | 174 (83.3) | 714 (81.5) | |
|
1-3 | 35 (16.7) | 162 (18.5) | |
| ER | | | 0.7555 |
|
Positive | 184 (86.0) | 791 (85.2) | |
|
Negative | 30 (14.0) | 138 (14.8) | |
| PgR | | | 0.5662 |
|
Positive | 160 (74.8) | 665 (72.8) | |
|
Negative | 54 (25.2) | 248 (27.2) | |
| HER2 | | | 0.1624 |
|
Positive | 28 (14.2) | 93 (10.7) | |
|
Negative | 169 (85.8) | 775 (89.3) | |
| ER/HER2 status | | | 0.0103 |
|
ER- and
HER2+ | 14 (6.5) | 27 (2.9) | |
|
Any | 201 (93.5) | 903 (97.1) | |
| NG | | | 0.5513 |
|
1 | 112 (68.3) | 480 (67.7) | |
|
2 | 18 (11.0) | 62 (8.7) | |
|
3 | 34 (20.7) | 167 (23.6) | |
| Ly | | | 0.0857 |
|
Positive | 71 (35.2) | 352 (41.8) | |
|
Negative | 131 (64.8) | 491 (58.2) | |
Discussion
The rate of positive surgical margins for MRI in the
supine position in the present study was 18.8%, which is similar to
19.7-20.0%, the rates reported for MRI in the prone position
(6,12-14).
US may also be used for preoperative imaging and has the advantages
of being simple and inexpensive. It may be performed in the
operating position; however, it has the disadvantage that only part
of the breast may be visualized at a time. For this reason, MRI
imaging in the prone position has been employed for preoperative
assessment (15). The present
results indicated comparable rates of positive margins for supine-
and prone-position MRI. As the present data were obtained between
2012 and 2013, it is possible that the current positive margin rate
is further reduced when examined by MRI using the most advanced
technology. In the present subgroup analysis of tumors with a
diameter of 2 cm or less, the positive rate was almost comparable
between supine and prone MRI, similar to the results of previous
studies (16,17). However, only the tumor size cannot
be deduced from the positive rate. The present study indicated an
increased rate of positive margins for prone MRI in the case of
tumors >2 cm, but this was not the case for supine MRI in the
current study. It may be difficult to determine the extent of
resection in larger tumors imaged with prone-position MRI, as the
relationship of the resection line to the complex tumor differs
considerably between the prone imaging and supine surgical
positions. By contrast, spatial variation is minimized with supine
MRI due to its similarity with the supine surgical position. A
total of three RCTs (6,9,12) of
perioperative procedures using supine MRI reported percentages of
positive margins, although the positive margin rates were different
for ductal carcinoma in situ and invasive ductal carcinoma.
The combined positive margin rate of 19.7-20.0% is comparable to
the 18.8% reported in the present study.
Higher positive margin rates have been reported with
both supine- and prone-position MRI for the HER2-positive type
compared with non-HER2 type breast cancer (18). In the latter study, there are
reports of a higher rate of positive margins in HER-positive breast
cancer, while this does not appear to be the case for ER-positive
patients. A previous RCT (6) has
reported that positive margins are more likely to occur in cases of
in situ HER2-positive breast cancer because ductal spread is
poorly detected even by MRI. Expected improvements in MRI
image-rendering technology should increase iability to detect
ductal carcinoma in situ (16,19).
Prospective cohort studies will then be required to evaluate the
efficacy of the new technology. Previous studies have reported a
difference in tumor size between positive and negative surgical
margin rates (12-14),
but in the present study, no such difference was found.
There are certain limitations to the present study.
As its retrospective design may have introduced bias, the results
should be interpreted with caution. In the present study, there was
no prone position group included, so there was no direct comparison
between prone- and supine-position patients. Furthermore, as it
appears that there were differences in procedures among the
different hospitals included, this may have introduced
heterogeneity. However, it may be assumed that the data are
meaningful because of the relatively large size of the study
cohort. The correlation between
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
gene mutation and positive resection margins or between
PI3K/AKT/mTOR pathway-related mutations and positive resection
margins is important in the analysis of clinicopathological
factors, but lack of preparation, including informed consent, made
it difficult to examine this in the present study.
In conclusion, the rate of positive surgical margins
in patients who underwent preoperative imaging with supine MRI
prior to BCS was 18.8%, which is similar to the 20% reported
previously (6). Supine MRI may
provide useful information for determining the extent of
resection.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GK, HS, DK, TMiz and IT conceived and planned the
present study in detail. FS, AW, YK, MO, AO, HM, TMik, YY, TMat, TO
and HK extracted the entirety of patient data, performed the data
collation and put the data into a form in which it could be entered
into statistical software. AW, YK and DK performed analysis and
interpretation of the patient data. HM, TMiz, HK and TO critically
revised the manuscript for important intellectual content. GK
drafted the manuscript. IT provided overall supervision and gave
final approval for the version to be published. All authors read
and approved the final manuscript. GK and HS confirmed the
authenticity of all the raw data.
Ethics approval and consent to
participate
This study adhered to ethical tenets of The
Declaration of Helsinki and Ethical Principles for Medical Research
Involving Human Subjects, and was approved by the Clinical Trial
Center of Sapporo Medical University, Japan (342-179).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sharma R: Breast cancer incidence,
mortality and mortality-to-incidence ratio (MIR) are associated
with human development, 1990-2016: Evidence from global burden of
disease study 2016. Breast Cancer. 26:428–445. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Greenberg PA, Hortobagyi GN, Smith TL,
Ziegler LD, Frye DK and Buzdar AU: Long-term follow-up of patients
with complete remission following combination chemotherapy for
metastatic breast cancer. J Clin Oncol. 14:2197–2205.
1996.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eggemann H, Ignatov T, Beni A, Costa SD
and Ignatov A: Ultrasonography-guided breast-conserving surgery is
superior to palpation-guided surgery for palpable breast cancer.
Clin Breast Cancer. 14:40–45. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Koh J, Park AY, Ko KH and Jung HK: Can
enhancement types on preoperative MRI reflect prognostic factors
and surgical outcomes in invasive breast cancer? Eur Radiol.
29:7000–7008. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Balleyguier C, Dunant A, Ceugnart L,
Kandel M, Chauvet MP, Chérel P, Mazouni C, Henrot P, Rauch P,
Chopier J, et al: Preoperative breast magnetic resonance imaging in
women with local ductal carcinoma in situ to optimize surgical
outcomes: Results from the randomized phase III trial IRCIS. J Clin
Oncol. 37:885–892. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Houssami N, Turner RM and Morrow M:
Meta-analysis of pre-operative magnetic resonance imaging (MRI) and
surgical treatment for breast cancer. Breast Cancer Res Treat.
165:273–283. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Houssami N, Turner R and Morrow M:
Preoperative magnetic resonance imaging in breast cancer:
Meta-analysis of surgical outcomes. Ann Surg. 257:249–255.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Turnbull L, Brown S, Harvey I, Olivier C,
Drew P, Napp V, Hanby A and Brown J: Comparative effectiveness of
MRI in breast cancer (COMICE) trial: A randomised controlled trial.
Lancet. 375:563–571. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yamashiro N, Tozaki M, Ogawa T, Kawano N,
Suzuki T, Ozaki S, Sakamoto N, Abe S and Fukuma E: Preoperative MRI
marking technique for the planning of breast-conserving surgery.
Breast Cancer. 16:223–228. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sakakibara M, Nagashima T, Sangai T,
Nakamura R, Fujimoto H, Arai M, Kazama T, Hashimoto H, Nakatani Y
and Miyazaki M: Breast-conserving surgery using projection and
reproduction techniques of surgical-position breast MRI in patients
with ductal carcinoma in situ of the breast. J Am Coll Surg.
207:62–68. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen K, Zhu L, Chen L, Li Q, Li S, Qiu N,
Yang Y, Su F and Song E: Circumferential shaving of the cavity in
breast-conserving surgery: A randomized controlled trial. Ann Surg
Oncol. 26:4256–4263. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Langhans L, Tvedskov TF, Klausen TL,
Jensen MB, Talman ML, Vejborg I, Benian C, Roslind A, Hermansen J,
Oturai PS, et al: Radioactive seed localization or wire-guided
localization of nonpalpable invasive and in situ breast cancer: A
randomized, multicenter, open-label trial. Ann Surg. 266:29–35.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
McGhan LJ, Wasif N, Gray RJ, Giurescu ME,
Pizzitola VJ, Lorans R, Ocal IT, Stucky CC and Pockaj BA: Use of
preoperative magnetic resonance imaging for invasive lobular
cancer: Good, better, but maybe not the best? Ann Surg Oncol. 17
(Suppl 3):S255–S262. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Peters NH, van Esser S, van den Bosch MA,
Storm RK, Plaisier PW, van Dalen T, Diepstraten SC, Weits T,
Westenend PJ, Stapper G, et al: Preoperative MRI and surgical
management in patients with nonpalpable breast cancer: The
MONET-randomised controlled trial. Eur J Cancer. 47:879–886.
2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lai HW, Huang RH, Wu YT, Chen CJ, Chen ST,
Lin YJ, Chen DR, Lee CW, Wu HK, Lin HY and Kuo SJ:
Clinicopathologic factors related to surgical margin involvement,
reoperation, and residual cancer in primary operable breast
cancer-an analysis of 2050 patients. Eur J Surg Oncol.
44:1725–1735. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chandwani S, George PA, Azu M, Bandera EV,
Ambrosone CB, Rhoads GG and Demissie K: Role of preoperative
magnetic resonance imaging in the surgical management of
early-stage breast cancer. Ann Surg Oncol. 21:3473–3480.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bae SJ, Ahn SG, Yoon CI, Yang BS, Lee HW,
Son EJ and Jeong J: Measuring tumor extent based on subtypes using
magnetic resonance imaging: Radiologic-pathologic discordance and
high positive margin rates in breast cancer. J Breast Cancer.
22:453–463. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Petrillo A, Fusco R, Petrillo M, Triunfo
F, Filice S, Vallone P, Setola SV, Rubulotta M, Di Bonito M,
Rinaldo M, et al: Added value of breast MRI for preoperative
diagnosis of ductal carcinoma in situ: Diagnostic performance on
362 patients. Clin Breast Cancer. 17:e127–e134. 2017.PubMed/NCBI View Article : Google Scholar
|