Introduction
Primary liver cancer is the third leading cause of
cancer-related mortality worldwide, and hepatocellular carcinoma
(HCC) is the most common type of liver cancer, comprising 75-85% of
cases (1). In Asia, liver cancer
is the fifth most commonly diagnosed type of cancer with a steady
annual increase in incidence and is the second leading cause of
cancer-related mortality (2).
Although resection constitutes one of the optimal methods for the
treatment of patients with HCC, in the majority of Asian centers,
due to a higher volume of cases and limited expertise, the overall
survival (OS) rate of patients remains unsatisfactory and tumor
recurrence is frequent. Therefore, identifying the risk factors for
the OS and relapse-free survival (RFS) of patients with HCC
following a hepatectomy will help to determine other therapeutic
and management strategies. At present, there are prognostic
prediction strategies based on gene expression differences
(3-5)
and mutations (6,7); however, these prediction models
require additional detection methods which are associated with high
costs, and are not suitable for all patients with HCC undergoing
hepatectomy. Thus, an accurate model based on clinicopathological
data is warranted in order to be able to predict the OS and the
probability of tumor recurrence following curative resection.
The present study collected the clinicopathological
and treatment data of 690 patients with HCC and randomly divided
the patients into two cohorts, namely the training and validation
sets (6). Univariate and
multivariate analyses were performed to identify the prognostic
risk events for OS and RFS. In addition, a scoring system was
constructed by counting the cumulative occurrences of
survival-associated risk events. The prognosis prediction scoring
system based on the pathological factors is a low-cost and
easy-to-use tool specifically developed for the prediction of OS
and RFS, and may aid in the risk stratification of patients with
HCC in clinical practice, as well as in clinical trials.
Patients and methods
Patients
The present study included 690 patients with HCC who
underwent surgery at the Sun Yat-Sen Memorial Hospital (Guangzhou,
China) between January, 2013 and December, 2019 (ethics approval
no. SYSEC-KY-KS-2019-039). Curative resection was defined as the
complete removal of the liver tumor tissues with no evidence of
residual microscopic tumors. Serum hepatitis B surface antigen
(HBsAg), hepatitis e antigen (HBeAg) and hepatitis B virus (HBV)
DNA levels were determined using an ELISA kit (Abbott) and
real-time polymerase chain reaction using respective kits (Sansure
Biotech), respectively. Cirrhosis was clinically defined based on
the findings on the ultrasound, computed tomography (CT), upper
gastrointestinal endoscopy and laboratory tests. Patients with a
history of anticancer therapy prior to surgery, those with other
types of malignant tumors, or those who received previous
locoregional therapies such as hepatectomy, radiotherapy,
transarterial chemoembolization, radiofrequency ablation,
percutaneous ethanol injection and patients who were lost to
follow-up following the hepatectomy were excluded from the
study.
Surgery
Overall, the evaluation of the general condition and
liver function reserve of the patients was performed before
surgery. Standard operative techniques for hepatectomy were used.
The tumor was completely removed to ensure that the surgical margin
was free of any residual tumor, while sufficient functional liver
tissue was retained to compensate for liver function, and reduce
operative mortality and post-operative complications. Selective
clamping of the portal vein and hepatic artery was performed when
feasible (8).
Clinicopathological information
The relevant clinicopathological data were extracted
retrospectively from the electronic medical records of the patients
with HCC. HCC was diagnosed according to the Guidelines for
Diagnosis and Treatment of Primary Liver Cancer in China, including
ultrasonography, CT, magnetic resonance imaging (MRI), digital
subtraction angiography, nuclear medical imaging, liver puncture
biopsy, serological molecular markers and pathological diagnosis.
The TNM stage was judged according to the eighth Edition of the
American Joint Committee on Cancer (AJCC) TNM Staging for Liver
Tumors. The cut-off points for age, pre-operative serum
alpha-fetoprotein (AFP), post-operative AFP, albumin (ALB),
alkaline phosphatase (ALP), alanine aminotransferase (ALT),
aspartate aminotransferase (AST), lactic dehydrogenase (LDH), total
bilirubin (TBIL), total cholesterol (TC) and platelet (PLT) levels
before surgery were 50 years, 200 ng/ml, 25 ng/ml, 50 g/l, 100 µ/l,
40 µ/l, 35 µ/l, 252 µ/l, 22.2 µmol/l, 6 mmol/l and
125x109/l, respectively, according to clinical
thresholds.
Follow-up
Post-operative follow-up was scheduled every 3
months with a chest CT scan or an abdominal MRI for the first 2
years and every 6 months thereafter. The primary endpoints of the
study were RFS, which was defined as the time from randomization to
the first documented tumor recurrence, and OS, which was defined as
the time from randomization to death by any causes. Tumor
recurrence was suspected on the detection of new hepatic lesions on
an ultrasound, dynamic CT scan, or MRI. Further investigations
(such as a chest CT scan, full-body bone scan and positron emission
tomography-CT) were performed when there was a clinical suspicion
of extrahepatic metastases.
Statistical analysis
Continuous variables that conformed to the Gaussian
distribution are expressed as the mean ± standard deviation and
compared using the Student's t-test. Otherwise, they are expressed
as the median and interquartile range, and analyzed using the
non-parametric Wilcoxon rank-sum test. Categorical variables are
expressed as percentages and were compared using the χ2
test. The Kaplan-Meier method followed by comparisons with the
log-rank tests was used to calculate the OS and RFS rates.
Clinicopathological and treatment variables found to bear
prognostic significance (P<0.1) in the univariate analysis were
entered into a Cox multivariate proportional hazards model (95%
confidence interval) to determine the independent association with
survival and recurrence (P<0.2 in both cohorts were considered
to be statistically significant) (9,10).
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was carried out using SPSS version
21.0 software (IBM Corp.).
Results
Clinicopathological and treatment
characteristics
In order to illustrate the subsequent statistical
results are repeatable, a total of 690 patients with HCC underwent
curative resection were randomly assigned to the training and
validation groups, each group containing 345 patients (groups A and
B). To validate the randomness of the grouping, the
clinicopathological characteristics and treatments of the two
groups were compared and the significant differences were not found
(P>0.1), which declared the random allocation valid (Table I).
 | Table IClinicopathological characteristics
and treatments of the patients. |
Table I
Clinicopathological characteristics
and treatments of the patients.
| Variable | Group A | Group B | P-value |
|---|
| Clinical
characteristics | | | |
|
Patients
(n) | 345 | 345 | - |
|
Male
(n) | 300 (87.0 %) | 296 (85.8 %) | 0.657 |
|
Age
(years) | 53.0 (42.5-62.0) | 52.0 (44.0-60.0) | 0.638 |
|
Alcohol
consumption (n) | 58 (16.9%) | 65 (19.0%) | 0.475 |
|
HBsAg
(n) | 291 (84.3%) | 294 (85.2%) | 0.751 |
|
HBeAg
(n) | 47 (17.6%) | 35 (13.0%) | 0.140 |
|
HBV DNA (lg,
copies/ml) | 4.4 (3.0-5.6) | 4.1 (3.0-5.5) | 0.277 |
|
ALB
(g/l) | 41.3
(38.2-44.2) | 41.7
(38.2-44.5) | 0.355 |
|
ALT
(U/l) | 39.0
(27.0-64.0) | 37.0
(25.3-55.0) | 0.121 |
|
AST
(U/l) | 45.0
(32.0-70.0) | 43.0
(31.0-65.0) | 0.245 |
|
TBIL
(µmol/l) | 14.7
(11.4-19.9) | 14.6
(11.1-20.4) | 0.980 |
|
ALP
(U/l) | 94.0
(74.0-127.0) | 96.5
(75.0-125.0) | 0.788 |
|
LDH
(U/l) | 224.5
(189-269.8) | 225.0
(185.0-279.5) | 0.959 |
|
TC
(mmol/l) | 4.8 (4.2-5.6) | 5.0 (4.3-5.8) | 0.479 |
|
PLT
(x109/l) | 178.0
(139.0-243.0) | 183.5
(135.0-250.5) | 0.967 |
|
AFP (ng/ml,
BS) | 187.6
(10.2-3493.5) | 177.0
(9.3-3310.0) | 0.933 |
|
AFP (ng/ml,
AS) | 12.2
(4.4-157.5) | 14.3
(4.3-174.7) | 0.470 |
| Pathological
characteristics | | | |
|
Cirrhosis
(n) | 258 (74.8%) | 259 (75.1%) | 0.929 |
|
Ascites
(n) | 57 (16.7%) | 55 (16.1%) | 0.836 |
|
Tumor number
(n) | | | |
|
1 | 259 (75.1%) | 262 (75.9%) | 0.690 |
|
2 | 39 (11.3%) | 45 (13.0%) | |
|
3 | 10 (2.9%) | 9 (2.6%) | |
|
≥4 | 37 (10.7%) | 29 (8.4%) | |
|
Tumor size
(cm) | 5.5 (3.5-10.0) | 6.0 (3.2-10.0) | 0.982 |
|
TNM stage
(n) | | | |
|
I | 130 (37.7%) | 115 (33.3%) | 0.280 |
|
II | 91 (26.4%) | 113 (32.8%) | |
|
III | 106 (30.7%) | 103 (29.9%) | |
|
IV | 18 (5.2%) | 14 (4.1%) | |
|
Differentiation
(n) | | | |
|
Low | 131 (38.3%) | 140 (41.2%) | 0.526 |
|
Median | 149 (43.6%) | 134 (39.3%) | |
|
High | 62 (18.1%) | 67 (19.6%) | |
|
Capsule
invasion (n) | 234 (67.8%) | 226 (65.9%) | 0.590 |
|
Vascular
tumor thrombus (n) | 189 (54.8%) | 189 (55.1%) | 0.933 |
|
Gross tumor
thrombus (n) | 67 (19.4%) | 62 (18.0%) | 0.626 |
|
Vascular
invasion (n) | 205 (59.4%) | 213 (61.7%) | 0.533 |
|
BDG (n) | 14 (4.1%) | 13 (3.8%) | 0.843 |
|
Extrahepatic
metastasis (n) | 29 (8.4%) | 24 (7.0%) | 0.475 |
| Treatment | | | |
|
Pre-operative
antiviral (n) | 121 (41.6%) | 129 (44.0%) | 0.575 |
|
Post-operative
antiviral (n) | 234 (80.4%) | 225 (76.5%) | 0.253 |
|
BTDO
(ml) | 300.0
(150.0-800.0) | 400.0
(150.0-700.0) | 0.696 |
|
TACE
(n) | 170 (53.8%) | 157 (48.6%) | 0.189 |
|
Overall
chemotherapy (n) | 51 (17.0%) | 41 (13.3%) | 0.204 |
|
Portal vein
chemotherapy (n) | 58 (19.5%) | 50 (16.2%) | 0.291 |
Univariate and multivariate analysis
for overall survival
COX regression was conducted to analyze the
associations between the patient variables and OS in groups A and B
(Tables II and III). Univariate regression analysis
revealed that abnormal ALB, AST and ALP levels before surgery,
abnormal AFP levels before and after surgery, ascites, tumor size,
tumor multiplicity, TNM stage, tumor differentiation level, capsule
invasion, vascular tumor thrombus, gross tumor thrombus, vascular
invasion, biliary duct and gallbladder invasion (BDG), extrahepatic
metastasis and blood transfusion during operation (BDTO) were
prognostic factors for OS in the two groups. When factors
associated with outcome in the univariate analyses (P<0.1) were
incorporated into a multivariate model analysis, with P<0.2 set
as the marker for significant differences in both groups, only
ascites, vascular tumor thrombus, extrahepatic metastasis and the
tumor differentiation level were independent risk factors for OS
(Tables II and III).
 | Table IICOX regression analysis of prognostic
variables for overall survival in group A. |
Table II
COX regression analysis of prognostic
variables for overall survival in group A.
| | Univariate | Multivariate |
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Clinical
characteristics | | | | |
|
Sex
(male/female) | 0.851
(0.485-1.491) | 0.572 | | |
|
Age, years
(>50/≤50) | 0.837
(0.574-1.221) | 0.355 | | |
|
Alcohol
consumption (yes/no) | 1.951
(1.233-3.087) | 0.004 | 2.145
(1.039-4.429) | 0.039 |
|
HBsAg
(+/-) | 1.016
(0.597-1.729) | 0.954 | | |
|
HBeAg
(+/-) | 1.274
(0.765-2.121) | 0.352 | | |
|
HBV DNA
(+/-) | 1.192
(0.717-1.979) | 0.498 | | |
|
ALB, g/l
(≥40/<40) | 0.639
(0.433-0.945) | 0.025 | 0.630
(0.350-1.134) | 0.123 |
|
ALT U/l
(≥40/<40) | 1.357
(0.922-1.999) | 0.122 | | |
|
AST, U/l
(≥35/<35) | 1.865
(1.164-2.986) | 0.009 | 1.405
(0.652-3.029) | 0.385 |
|
TBIL, µmol/l
(≥22.2/<22.2) | 1.353
(0.850-2.154) | 0.202 | | |
|
ALP, U/l
(≥100/<100) | 1.877
(1.274-2.764) | 0.001 | 1.307
(0.685-2.494) | 0.416 |
|
LDH, U/l
(≥252/<252) | 1.011
(0.620-1.647) | 0.966 | | |
|
TC, mmol/l
(≥6/<6) | 1.667
(0.993-2.800) | 0.053 | 1.519
(0.770-2.994) | 0.227 |
|
PLT,
(≥125x109/<125x109/l) | 1.220
(0.725-2.053) | 0.453 | | |
|
AFP, ng/ml
(≥200/<200, BS) | 1.677
(1.132-2.484) | 0.010 | 1.167
(0.557-2.445) | 0.683 |
|
AFP, ng/ml
(≥25/<25, AS) | 2.314
(1.542-3.473) | 0.001 | 1.277
(0.589-2.768) | 0.536 |
| Pathological
characteristics | | | | |
|
Cirrhosis
(yes/no) | 1.578
(0.995-2.502) | 0.052 | 2.128
(1.015-4.461) | 0.046 |
|
Ascites
(yes/no) | 1.757
(1.115-2.769) | 0.015 | 1.913
(0.864-4.233) | 0.110 |
|
Tumor size,
cm (>5/≤5) | 2.019
(1.369-2.976) | 0.001 | 0.972
(0.409-2.310) | 0.950 |
|
Tumor number
(multiple/single) | 2.185
(1.476-3.236) | 0.001 | 1.131
(0.558-2.292) | 0.732 |
|
TNM stage
(III + IV/I + II) | 2.326
(1.595-3.391) | 0.001 | 0.437
(0.119-1.600) | 0.211 |
|
Differentiation
(III + IV/I + II) | 1.908
(1.310-2.778) | 0.001 | 1.972
(1.047-3.714) | 0.036 |
|
Capsule
invasion (yes/no) | 2.525
(1.580-4.037) | 0.001 | 2.098
(0.939-4.686) | 0.071 |
|
Vascular
tumor thrombus (yes/no) | 2.922
(1.947-4.450) | 0.001 | 2.389
(1.126-5.068) | 0.023 |
|
Gross tumor
thrombus (yes/no) | 2.774
(1.828-4.210) | 0.001 | 1.325
(0.470-3.739) | 0.595 |
|
Vascular
invasion (yes/no) | 2.334
(1.586-3.436) | 0.001 | 2.099
(0.546-8.073) | 0.281 |
|
BDG
(yes/no) | 4.324
(2.247-8.320) | 0.001 | 2.332
(0.709-7.667) | 0.163 |
|
Extrahepatic
metastasis (yes/no) | 2.677
(1.496-4.791) | 0.001 | 2.979
(1.139-7.791) | 0.026 |
| Treatment | | | | |
|
Pre-operative
antiviral (yes/no) | 0.996
(0.678-1.464) | 0.985 | | |
|
Post-operative
antiviral (yes/no) | 1.160
(0.765-1.759) | 0.486 | | |
|
BTDO, ml
(≥400/<400 ) | 1.544
(1.051-2.269) | 0.027 | 0.437
(0.216-0.886) | 0.022 |
|
TACE
(yes/no) | 0.874
(0.592-1.290) | 0.497 | | |
|
Overall
chemotherapy (yes/no) | 0.530
(0.283-0.993) | 0.048 | 0.340
(0.132-0.875) | 0.025 |
|
Portal vein
chemotherapy (yes/no) | 0.885
(0.546-1.435) | 0.621 | | |
 | Table IIICOX regression of prognostic
variables for overall survival in group B. |
Table III
COX regression of prognostic
variables for overall survival in group B.
| | Univariate | Multivariate |
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Clinical
characteristics | | | | |
|
Sex
(male/female) | 1.046
(0.607-1.803) | 0.870 | | |
|
Age, years
(>50/≤50) | 1.032
(0.708-1.502) | 0.871 | | |
|
Alcohol
consumption (yes/no) | 1.013
(0.649-1.580) | 0.956 | | |
|
HBsAg
(+/-) | 1.254
(0.716-2.197) | 0.428 | | |
|
HBeAg
(+/-) | 1.713
(0.928-3.163) | 0.085 | 1.122
(0.424-2.969) | 0.816 |
|
HBV DNA
(+/-) | 1.602
(0.959-2.676) | 0.072 | 1.726
(0.680-4.383) | 0.251 |
|
ALB, g/l
(≥40/<40) | 0.560
(0.382-0.820) | 0.003 | 0.800
(0.374-1.713) | 0.566 |
|
ALT U/l
(≥40/<40) | 1.247
(0.856-1.816) | 0.250 | | |
|
AST, U/l
(≥35/<35) | 2.708
(1.666-4.399) | 0.001 | 2.324
(0.676-7.992) | 0.181 |
|
TBIL, µmol/l
(≥22.2/<22.2) | 0.910
(0.549-1.509) | 0.715 | | |
|
ALP, U/l
(≥100/<100) | 2.335
(1.589-3.432) | 0.001 | 1.717
(0.836-3.526) | 0.141 |
|
LDH, U/l
(≥252/<252) | 2.373
(1.529-3.683) | 0.001 | 1.042
(0.417-2.607) | 0.929 |
|
TC, mmol/l
(≥6/<6) | 1.210
(0.698-2.096) | 0.497 | | |
|
PLT,
(≥125x109/<125x109/l) | 0.981
(0.634-1.519) | 0.933 | | |
|
AFP, ng/ml
(≥200/<200, BS) | 1.698
(1.140-2.527) | 0.009 | 1.001
(0.392-2.557) | 0.998 |
|
AFP, ng/ml
(≥25/<25, AS) | 2.000
(1.348-2.969) | 0.001 | 1.054
(0.419-2.652) | 0.912 |
| Pathological
characteristics | | | | |
|
Cirrhosis
(yes/no) | 1.109
(0.716-1.718) | 0.643 | | |
|
Ascites
(yes/no) | 2.418
(1.542-3.792) | 0.001 | 3.464
(1.310-9.164) | 0.012 |
|
Tumor size,
cm (>5/≤5) | 2.684
(1.795-4.011) | 0.001 | 0.738
(0.249-2.184) | 0.583 |
|
Tumor number
(multiple/single) | 1.438
(0.959-2.156) | 0.079 | 0.579
(0.212-1.581) | 0.287 |
|
TNM stage
(III+IV/I+II) | 2.638
(1.826-3.813) | 0.001 | 1.502
(0.291-7.744) | 0.627 |
|
Differentiation
(III+IV/I+II) | 2.026
(1.397-2.940) | 0.001 | 2.527
(1.179-5.419) | 0.017 |
|
Capsule
invasion (yes/no) | 1.552
(1.038-2.321) | 0.032 | 0.918
(0.410-2.056) | 0.835 |
|
Vascular
tumor thrombus (yes/no) | 2.423
(1.625-3.611) | 0.001 | 1.904
(0.741-4.892) | 0.181 |
|
Gross tumor
thrombus (yes/no) | 3.126
(2.085-4.687) | 0.001 | 2.701
(0.899-8.119) | 0.077 |
|
Vascular
invasion (yes/no) | 2.410
(1.650-3.520) | 0.001 | 0.521
(0.101-2.682) | 0.436 |
|
BDG
(yes/no) | 3.709
(1.868-7.365) | 0.001 | 1.987
(0.540-7.318) | 0.302 |
|
Extrahepatic
metastasis (yes/no) | 1.923
(0.973-3.801) | 0.060 | 3.706
(1.144-12.002) | 0.029 |
| Treatment | | | | |
|
Pre-operative
antiviral (yes/no) | 1.258
(0.868-1.823) | 0.225 | | |
|
Post-operative
antiviral (yes/no) | 1.544
(1.021-2.335) | 0.039 | 0.860
(0.331-2.233) | 0.757 |
|
BTDO, ml
(≥400/<400) | 2.307
(1.588-3.351) | 0.001 | 1.542
(0.706-3.369) | 0.278 |
|
TACE
(yes/no) | 1.219
(0.830-1.791) | 0.311 | | |
|
Overall
chemotherapy (yes/no) | 1.126
(0.650-1.950) | 0.672 | | |
|
Portal vein
chemotherapy (yes/no) | 0.568
(0.310-1.038) | 0.066 | 0.362
(0.138-0.949) | 0.039 |
Univariate and multivariate analysis
for relapse-free survival
COX regression analysis was also conducted to
determine the associations between variables and RFS in groups A
and B (Tables IV and V). Univariate regression analysis
revealed that the patients' age, abnormal ALB, AST and LDH levels
before surgery, abnormal AFP levels before and after surgery,
ascites, tumor size, tumor number, TNM stage, tumor differentiation
level, capsule invasion, vascular tumor thrombus, gross tumor
thrombus, vascular invasion, BDG, extrahepatic metastasis,
antiviral therapy, BDTO and overall chemotherapy were prognostic
factors for RFS in both groups. When the factors associated with
outcome in univariate analyses (P<0.1) were incorporated into a
multivariate model analysis, with P<0.2 set as the marker for
significant differences in both groups, only the tumor number and
extrahepatic metastasis were independent risk factors for RFS
(Tables IV and V).
 | Table IVCOX regression of prognostic
variables for relapse-free survival in group A. |
Table IV
COX regression of prognostic
variables for relapse-free survival in group A.
| | Univariate | Multivariate |
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Clinical
characteristics | | | | |
|
Sex
(male/female) | 0.978
(0.657-1.457) | 0.913 | | |
|
Age, years
(>50/≤50) | 0.700
(0.534-0.918) | 0.010 | 1.857
(1.126-3.064) | 0.015 |
|
Alcohol
consumption (yes/no) | 1.297
(0.908-1.852) | 0.153 | | |
|
HBsAg
(+/-) | 1.440
(0.955-2.172) | 0.082 | - | - |
|
HBeAg
(+/-) | 0.984
(0.663-1.461) | 0.936 | | |
|
HBV DNA
(+/-) | 1.373
(0.948-1.987) | 0.093 |
0.978(0.555-1.723) | 0.939 |
|
ALB, g/l
(≥40/<40) | 0.767
(0.580-1.013) | 0.062 | 0.647
(0.378-1.108) | 0.113 |
|
ALT U/l
(≥40/<40) | 1.206
(0.915-1.589) | 0.183 | | |
|
AST, U/l
(≥35/<35) | 1.727
(1.248-2.389) | 0.001 | 0.899
(0.478-1.693) | 0.742 |
|
TBIL, µmol/l
(≥22.2/<22.2) | 1.366
(0.972-1.920) | 0.072 | 1.198
(0.646-2.223) | 0.567 |
|
ALP, U/l
(≥100/<100) | 1.724
(1.308-2.272) | 0.001 | 1.027
(0.617-1.710) | 0.918 |
|
LDH, U/l
(≥252/<252) | 1.425
(1.016-2.000) | 0.040 | 1.409
(0.787-2.522) | 0.248 |
|
TC, mmol/l
(≥6/<6) | 1.521
(1.018-2.273) | 0.041 | 2.041
(1.163-3.582) | 0.013 |
|
PLT,
(≥125x109/<125x109/l) | 1.476
(0.995-2.190) | 0.053 | 0.563
(0.286-1.107) | 0.096 |
|
AFP, ng/ml
(≥200/<200, BS) | 1.859
(1.403-2.465) | 0.001 | 1.560
(0.868-2.804) | 0.137 |
|
AFP, ng/ml
(≥25/<25, AS) | 2.076
(1.558-2.767) | 0.001 | 1.166
(0.622-2.186) | 0.631 |
| Pathological
characteristics | | | | |
|
Cirrhosis
(yes/no) | 1.687
(1.199-2.373) | 0.003 | 2.053
(1.092-3.863) | 0.026 |
|
Ascites
(yes/no) | 1.384
(0.973-1.968) | 0.071 | 0.842
(0.392-1.811) | 0.661 |
|
Tumor size,
cm (>5/≤5) | 2.050
(1.551-2.708) | 0.001 | 1.860
(0.906-3.819) | 0.091 |
|
Tumor number
(multiple/single) | 2.088
(1.553-2.808) | 0.001 | 2.815
(1.569-5.049) | 0.001 |
|
TNM stage
(III + IV/I + II) | 2.869
(2.181-3.774) | 0.001 | 0.349
(0.116-0.048) | 0.061 |
|
Differentiation
(III + IV/I + II) | 1.597
(1.214-2.100) | 0.001 | 0.883
(0.522-1.495) | 0.644 |
|
Capsule
invasion (yes/no) | 1.891
(1.388-2.575) | 0.001 | 1.168
(0.654-2.086) | 0.600 |
|
Vascular
tumor thrombus (yes/no) | 2.424
(1.820-3.228) | 0.001 | 1.611
(0.919-2.823) | 0.096 |
|
Gross tumor
thrombus (yes/no) | 3.579
(2.625-4.880) | 0.001 | 4.355
(1.71-10.898) | 0.002 |
|
Vascular
invasion (yes/no) | 2.756
(2.087-3.663) | 0.001 | 1.905
(0.635-5.715) | 0.250 |
|
BDG
(yes/no) | 3.026
(1.685-5.435) | 0.001 | 1.179
(0.409-3.400) | 0.761 |
|
Extrahepatic
metastasis (yes/no) | 2.634
(1.727-4.015) | 0.001 | 2.470
(0.973-6.266) | 0.057 |
| Treatment | | | | |
|
Pre-operative
antiviral (yes/no) | 1.163
(0.883-1.533) | 0.283 | | |
|
Post-operative
antiviral (yes/no) | 1.396
(1.022-1.906) | 0.036 | 0.824
(0.450-1.510) | 0.532 |
|
BTDO, ml
(≥400/<400) | 1.864
(1.416(2.453) | 0.001 | 1.005
(0.587-1.721) | 0.985 |
|
TACE
(yes/no) | 0.877
(0.663-1.159) | 0.356 | | |
|
Overall
chemotherapy (yes/no) | 0.690
(0.470-1.104) | 0.059 | 0.684
(0.374-1.250) | 0.217 |
|
Portal vein
chemotherapy (yes/no) | 0.905
(0.642-1.277) | 0.571 | | |
 | Table VCOX regression of prognostic
variables for relapse-free survival in group B. |
Table V
COX regression of prognostic
variables for relapse-free survival in group B.
| | Univariate | Multivariate |
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Clinical
characteristics | | | | |
|
Sex
(male/female) | 1.016
(0.681-1.514) | 0.939 | | |
|
Age, years
(>50/≤50) | 0.755
(0.574-0.994) | 0.046 | 0.789
(0.481-1.293) | 0.347 |
|
Alcohol
consumption (yes/no) | 0.830
(0.578-1.190) | 0.310 | | |
|
HBsAg
(+/-) | 1.474
(0.963-2.258) | 0.074 | - | - |
|
HBeAg
(+/-) | 2.037
(1.351-3.071) | 0.001 | 2.038
(1.083-3.838) | 0.027 |
|
HBV DNA
(+/-) | 1.135
(0.800-1.609) | 0.478 | | |
|
ALB, g/l
(≥40/<40) | 0.702
(0.527-0.933) | 0.015 | 0.766
(0.473-1.239) | 0.276 |
|
ALT U/l
(≥40/<40) | 1.237
(0.936-1.635) | 0.135 | | |
|
AST, U/l
(≥35/<35) | 1.949
(1.413-2.687) | 0.001 | 1.718
(0.931-3.173) | 0.084 |
|
TBIL, µmol/l
(≥22.2/<22.2) | 1.043
(0.729-1.492) | 0.817 | | |
|
ALP, U/l
(≥100/<100) | 1.210
(0.915-1.600) | 0.181 | | |
|
LDH, U/l
(≥252/<252) | 1.585
(1.136-2.212) | 0.007 | 0.867
(0.477-1.575) | 0.639 |
|
TC, mmol/l
(≥6/<6) | 1.001
(0.667-1.502) | 0.995 | | |
|
PLT,
(≥125x109/<125x109/l) | 1.121
(0.792-1.588) | 0.519 | | |
|
AFP, ng/ml
(≥200/<200, BS) | 1.706
(1.275-2.282) | 0.001 | 1.300
(0.702-2.405) | 0.404 |
|
AFP, ng/ml
(≥25/<25, AS) | 1.800
(1.339-2.421) | 0.001 | 0.988
(0.543-1.795) | 0.967 |
| Pathological
characteristics | | | | |
|
Cirrhosis
(yes/no) | 1.166
(0.847-1.604) | 0.346 | | |
|
Ascites
(yes/no) | 1.995
(1.412-2.819) | 0.001 | 2.003
(1.028-3.902) | 0.041 |
|
Tumor size,
cm (>5/≤5) | 1.881
(1.413-2.503) | 0.001 | 1.426
(0.755-2.694) | 0.274 |
|
Tumor number
(multiple/single) | 1.524
(1.126-2.063) | 0.006 | 1.482
(0.837-2.624) | 0.177 |
|
TNM stage
(III + IV/I + II) | 1.827
(1.379-2.419) | 0.001 | 0.817
(0.288-2.313) | 0.703 |
|
Differentiation
(III + IV/I + II) | 1.678
(1.271-2.216) | 0.001 | 1.464
(0.896-2.392) | 0.128 |
|
Capsule
invasion (yes/no) | 1.462
(1.084-1.973) | 0.013 | 0.943
(0.565-1.573) | 0.821 |
|
Vascular
tumor thrombus (yes/no) | 1.886
(1.417-2.511) | 0.001 | 0.830
(0.486-1.416) | 0.494 |
|
Gross tumor
thrombus (yes/no) | 2.279
(1.642-3.161) | 0.001 | 1.361
(0.638-2.906) | 0.425 |
|
Vascular
invasion (yes/no) | 1.753
(1.304-2.356) | 0.001 | 0.924
(0.344-2.487) | 0.876 |
|
BDG
(yes/no) | 2.671
(1.445-4.940) | 0.002 | 2.199
(0.710-6.809) | 0.172 |
|
Extrahepatic
metastasis (yes/no) | 2.256
(1.387-3.669) | 0.001 | 2.070
(0.748-5.726) | 0.161 |
| Treatment | | | | |
|
Pre-operative
antiviral (yes/no) | 1.095
(0.825-1.454) | 0.530 | | |
|
Post-operative
antiviral (yes/no) | 1.346
(0.996-1.820) | 0.053 | 0.788
(0.433-1.434) | 0.435 |
|
BTDO, ml
(≥400/<400) | 1.626
(1.233-2.143) | 0.001 | 1.141
(0.687-1.895) | 0.612 |
|
TACE
(yes/no) | 1.249
(0.944-1.652) | 0.119 | | |
|
Overall
chemotherapy (yes/no) | 0.949
(0.632-1.426) | 0.802 | | |
|
Portal vein
chemotherapy (yes/no) | 0.580
(0.378-0.891) | 0.013 | 0.397
(0.214-0.737) | 0.003 |
Construction of the prognostic scoring
system
Ascites, vascular tumor thrombus, low tumor
differentiation and extrahepatic metastasis at the time of
hepatectomy were four parameters for the prediction of a poor OS.
Multiple tumors and extrahepatic metastasis were predictive of
tumor recurrence; thus, these variables were weighted to construct
the ascites, vascular tumor thrombus, low tumor differentiation,
extrahepatic metastasis and multiple tumors (AVLEM) score to
predict patient OS or RFS. The OS predictive model was constructed
based on the four aforementioned independent risk factors. The
patients were divided into three subgroups as follows: Grade 0
(G0), no ascites, with highly differentiated tumors, no vascular
tumor thrombus and no extrahepatic metastasis; grade 1 (G1), only
one risk factor was positive; and grade 2 (G2), more than one risk
factor was positive. Similarly, the RFS predictive model was
constructed based on the two of the aforementioned risk factors.
The patients were divided into two subgroups as follows: Grade 0
(G0), single tumor and no extrahepatic metastasis; grade 1 (G1),
multiple tumors or extrahepatic metastasis, multiple tumors
combined with extrahepatic metastasis (Table SI).
Application of the AVLEM score to
predict OS
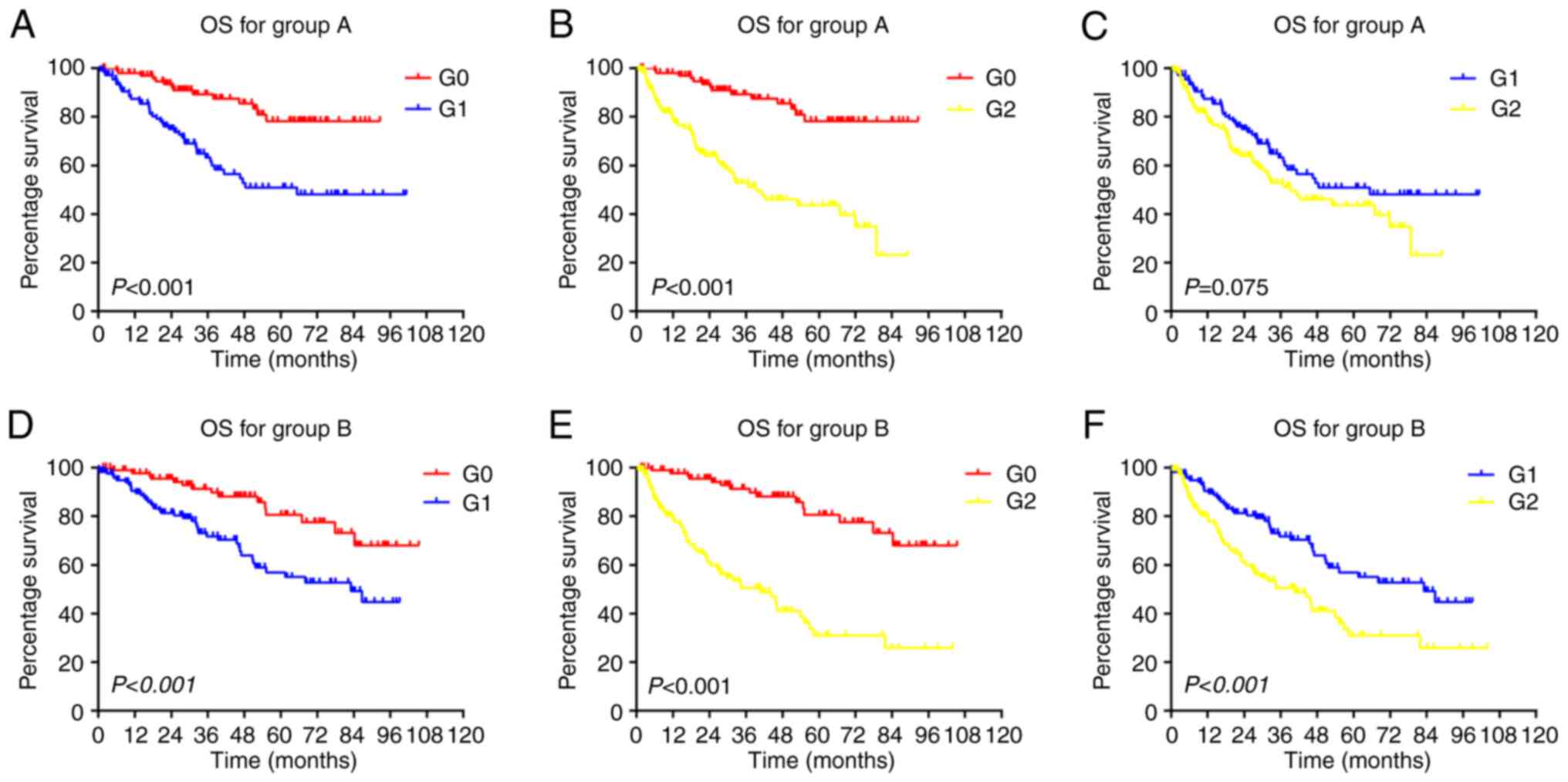
The Kaplan-Meier curves of OS for the patients in
groups A and B are presented in Fig.
1. The average 1-, 3- and 5-year OS rates in the G0 subgroup
were 97.9% (98.0% in group A and 97.8% in group B), 90.4% (89.3% in
group A and 91.4% in group B) and 79.5% (78.3% in group A and 80.7%
in group B), respectively. The average 1-, 3- and 5-year OS rates
in the G1 subgroup were 89.0% (87.5% in group A and 90.5% in group
B), 67.6% (63.3% in group A and 71.8% in group B) and 53.9% (50.9%
in group A and 57.0% in group B), respectively. The average 1-, 3-
and 5-year OS rates in the G2 subgroup were 80.2% (79.89% in group
A and 80.5% in group B), 52.0% (52.9% in group A and 51.1% in group
B) and 38.0% (43.7% in group A and 32.3% in group B), respectively.
Overall, the G0 subgroup had a better OS than the G1 and G2
subgroups (P<0.001). The G1 subgroup also had a better OS than
the G2 subgroup (P<0.001) in the group B cohort; however, the
difference was not significant (P=0.075) in the group A cohort.
Comparisons were also made for each subgroup between groups A and B
(Fig. S1), and it was found that
the OS of the patients did not differ significantly between these
two groups (P>0.05).
Application of the AVLEM score to
predict RFS
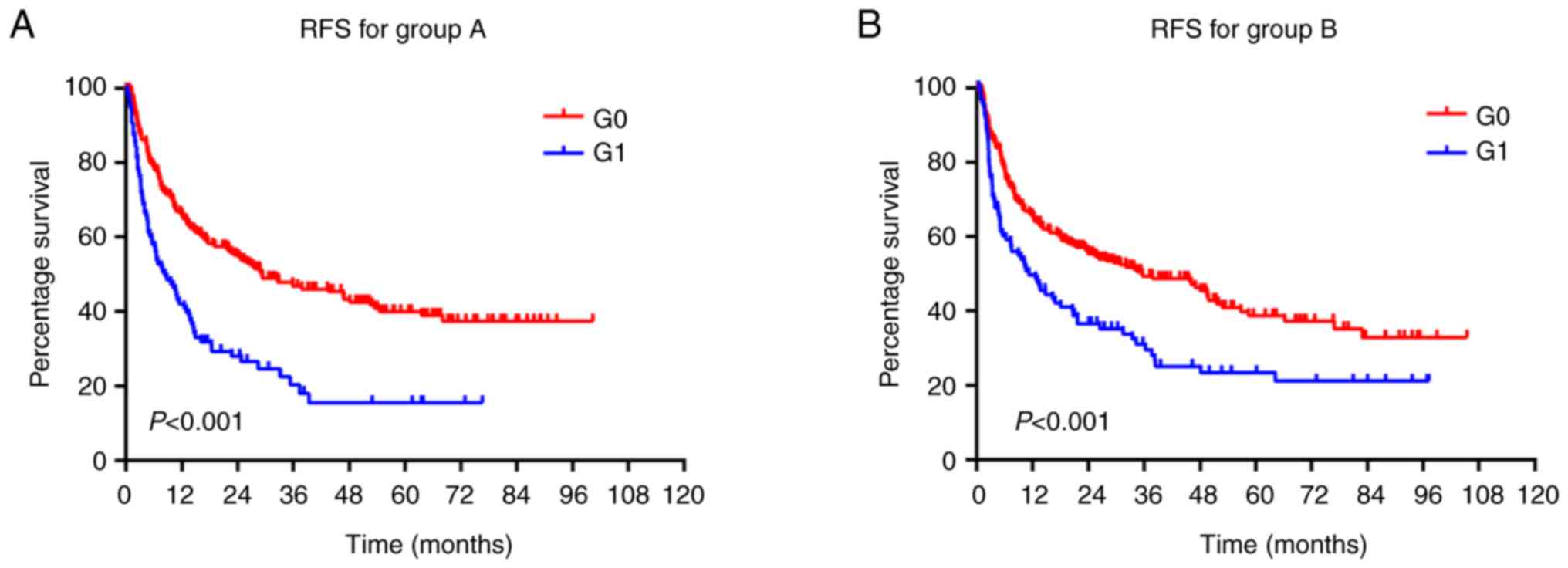
The Kaplan-Meier curves of the RFS of patients in
groups A and B are presented in Fig.
2. The average 1-, 2- and 3-year RFS rates in the G0 subgroup
were 65.7% (65.7% in group A and 65.8% in group B), 55.6% (55.2% in
group A and 56.0% in group B) and 48.2% (46.7% in group A and 49.6%
in group B), respectively. The average 1-, 2- and 3-year RFS rates
in the G1 subgroup were 45.9% (42.0% in group A and 49.7% in group
B), 32.3% (28.0% in group A and 36.5% in group B) and 25.7% (20.3%
in group A and 31.0% in group B), respectively. The G0 subgroup had
a better RFS than the G1 subgroup in both cohorts (P<0.001).
Comparisons were also made for each subgroup between groups A and B
(Fig. S2), and RFS of the
patients was not found to differ significantly between these two
groups (P>0.05).
Discussion
Currently, surgery is considered the best candidate
therapy for patients with solitary HCC, while the prognostic
staging of HCC following curative hepatectomy remains a challenge;
although several staging systems have been proposed, none have been
universally adopted, since HCC is heterogeneous and is influenced
by tumor burden (11), viral
infections (12), liver function
(13), immune response (14) and metabolic abnormalities (15). An ideal prognostic model for risk
stratification needs to be developed with appropriate methods. The
present study collected the information of 690 patients from a
hospital in South China and randomly divided the patients into two
cohorts. First, all the indicators were compared to validate the
randomness of the grouping, and no significant differences were
found for each indicator between the two cohorts. The variables
were then subjected to univariate and multivariate regression
analysis for each cohort. The variables with significant
differences (P<0.1) in the univariate regression analysis were
considered as possible risk factors for further multivariate
regression analysis (9,10). Finally, the risk factors (P<0.2
in multivariate regression analysis in both cohorts) were used to
develop a prognostic scoring system based on the risk factor
cumulative incidence (CuI). These analytical methods illustrate
that the statistical results are repeatable in this population.
Previous studies have focused on the identification
of prognosis-associated biomarkers (16-18),
which normally require additional detections. The AVLEM score used
herein is free of any specific laboratory test and is regarded as
an advantage, since it relies on baseline information, which is
readily available in retrospective analyses. In the patients,
ascites, low tumor differentiation, vascular tumor thrombus and
extrahepatic metastasis were independent predictors for a poor OS.
Based on the CuI for the four aforementioned risk factors, the
patients were divided into the G0 (CuI=0), G1 (CuI=1) and G2 (CuI
≥2) subgroups. The average 1-, 3-, 5-year OS rates were 97.9, 90.4
and 79.5% in the G0 subgroup; 89.0, 67.6 and 53.9% in the G1
subgroup; and 80.2, 52.0 and 38.0% in the G2 subgroup,
respectively. The median OS rate in the G2 subgroup was ~3 years,
the median OS in the other two subgroups, especially G0, was more
than five years. The prognostic strata based on the four
pathological factors can be used for the estimation of patient OS
following hepatectomy. Moreover, the patients' pre-operative
nutritional and immunological status also affects surgical
prognosis. A controlling nutritional status (CONUT), calculated
based on serum ALB, total lymphocyte count, TC, BTDO and other
variables has been reported as an independent predictor of OS. A
higher CONUT score (low ALB, low lymphocyte count and low TC) has
been shown to be significantly associated with a poor OS (19,20).
In the present study, apart from ALB and BTDO, AFP, AST, ALP, tumor
size and capsule invasion were also probable factors for the
prediction of OS when going univariate analysis.
The main reason for HCC being difficult to treat is
its high recurrence rate. The early detection of HCC recurrence and
early intervention can improve patient prognosis following
hepatectomy. However, high-frequency screening for all patients
with HCC is not a cost-effective strategy. Tumor multiplicity and a
large tumor size are two accepted poor prognostic indicators of HCC
recurrence, even though there are other clinicopathological
variables considered to be associated with RFS (21,22).
In the present study, apart from tumor multiplicity, extrahepatic
metastasis was an independent predictor for tumor recurrence; this
is in accordance with published data demonstrating that the
intrahepatic recurrence of HCC is attributable to metastasis
(23). Moreover, patient age,
pre-operative ALB, AST, LDH and AFP levels before and after
surgery, ascites, tumor size and differentiation level, vascular
invasion, treatment and TNM stage were all possible risk factors
related to tumor recurrence in the present study, even though they
were not independent risk factors, these are partially consistent
with published data (24-26);
however, since the variables and the patients included in each
research group varied, the independent prognostic risk factors also
differed. The present study included comprehensive
clinicopathological variables and antitumor and antiviral
treatments, which made the statistical analysis and scoring model
more stringent. The G0 and G1 subgroups were classified according
to risk factor CuI for the further analysis of RFS. Kaplan-Meier
analysis revealed that patients in the G1 subgroup were more likely
to have tumor recurrence compared with those the G0 subgroup.
According to the AVLEM scoring system, high-frequency follow-up and
screening for patients in the G1 subgroup with extrahepatic
metastasis or multiple tumors are more necessary than for patients
in the G0 subgroup without extrahepatic metastasis or with solitary
tumors.
HBV infection remains the leading risk factor for
HCC, with a slight decline in the majority of Asian countries
(2). Patients with HBV infection
suffer from malignancy, as well as chronic hepatitis B infection. A
high HBV-DNA load, intrahepatic metastasis and multicenter
recurrence are independent risk factors for a poor OS (27,28).
HBV reactivation affects the post-operative survival of patients
with HCC with a low pre-operative HBV-DNA level (29), and the loss of HBsAg is associated
with a reduced risk of late recurrence following liver resection in
patients with HBV-related HCC (30); thus, antiviral treatment leads to
an improved OS and a lower HCC recurrence rate following curative
resection in patients with HBV-associated HCC (31,32).
Other researchers have found that including data on the serum
levels of HBsAg or removing data on the level of HBV DNA do not
alter the accuracy of the risk estimation for hepatocellular
carcinoma in chronic hepatitis B (REACH-B) scoring system in
determining the risk of developing HCC in patients with chronic HBV
infection (33). The surgical
outcome of patients with HBsAg-negative HCC has been found to not
differ significantly compared with those with HBsAg-positive HCC
(34). These results suggest that
HBV infection in different HCC patients has diverse effects on
surgical outcomes. In the present study, HBV-associated viral
factors (HBsAg, HBeAg and HBV DNA) and antiviral treatments before
or after hepatectomy were not independent risk factors for
survival. This may be the reason that the viral loads in the
patients in the present study differed, and a high pre-operative
viral load led to a poorer OS and RFS than a low viral load
(28). In addition, the HBV
genotype is associated with tumor recurrence and genotype C results
in greater tumor recurrence rate compared with genotype B (35). Furthermore, different antiviral
drugs also result in significantly different prognoses in patients
with HBV-associated HCC following hepatectomy (36-38);
however, in the present study, patients were not divided into
subgroups for further analysis according to HBV load, viral
genotype and antiviral drugs.
The present study established the AVLEM scoring
system requiring only simple clinicopathological information
(including ascites, vascular tumor thrombus, tumor differentiation
level, extrahepatic metastasis and multiple lesions), which is a
low-cost scoring model to predict recurrence and the OS of patients
with HCC undergoing curative resection; however, this system has
certain limitations. First, the patients used to construct this
scoring system were from a single center, and larger samples from
other centers are required to validate this system. Second, the
present study was a retrospective cross-sectional study, which
limits the amount of data used. Thus, further larger
multi-institutional cohort studies are warranted to validate the
prognostic value of the scoring system used herein, mirroring the
management of HCC in real-life clinical situations. Third, the
effectiveness of the scoring model was only evaluated by analyzing
the OS and RFS of patients in each subgroup. It would be beneficial
to evaluate the receiver operating characteristic curve of this
scoring model by using more HCC cohorts in the future.
Supplementary Material
Kaplan-Meier curves of the OS of
patients in each subgroup in groups A and B. The OS of the two
cohorts was compared in the (A) G0 subgroup (P=0.947), (B) G1
subgroup (P=0.294), and (C) G2 subgroup (P=0.671). The differences
among each cohort were not significant (P>0.05). OS, overall
survival.
Kaplan-Meier curves of the RFS of
patients in each subgroup in groups A and B. The RFS of the two
cohorts was compared in the (A) G0 subgroup (P=0.927), and the (B)
G1 subgroup (P=0.171). The differences among each cohort were not
significant (P>0.05). RFS, relapse-free survival.
The scoring system used in the present
study for predicting OS and RFS based on independent risk
factors.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
National Natural Science Foundation of China (no. 82260603), the
Jiangxi Provincial Natural Science Foundation (no. 20224BAB206005),
and the Education Department Science and Technology Foundation of
Jiangxi (no. GJJ211720).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WD analyzed the data and wrote the manuscript. FC
and YL collected the patient electronic medical records and
scheduled follow-up visits. LX designed the study and revised the
manuscript. WD and FC confirmed the authenticity of all the raw
data. All authors reviewed the results, and have read and approved
the final version of the manuscript.
Ethical approval and consent to
participate
The present study was reviewed and approved by the
Ethics Committee of the Sun Yat-Sen Memorial Hospital, Sun Yat-Sen
University, Guangzhou, China. Written informed consent for this
study provided by all the patients included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang CH, Cheng Y, Zhang S, Fan J and Gao
Q: Changing epidemiology of hepatocellular carcinoma in Asia. Liver
Int. 42:2029–2041. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tang Y, Xu L, Ren Y, Li Y, Yuan F, Cao M,
Zhang Y, Deng M and Yao Z: Identification and validation of a
prognostic model based on three MVI-related genes in hepatocellular
carcinoma. Int J Biol Sci. 18:261–275. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu R, Wang G, Zhang C and Bai DS: A
prognostic model for hepatocellular carcinoma based on
apoptosis-related genes. World J Surg Oncol. 19(70)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Deng FW, Chen D, Wei X, Lu S, Luo X, He J,
Liu J, Meng T, Yang A and Chen H: Development and validation of a
prognostic classifier based on HIF-1 signaling for hepatocellular
carcinoma. Aging (Albany NY). 12:3431–3450. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Huang T, Yan T, Chen G and Zhang C:
Development and validation of a gene mutation-associated nomogram
for hepatocellular carcinoma patients from four countries. Front
Genet. 12(714639)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li Y, Wu J, Li E, Xiao Z, Lei J, Zhou F,
Yin X, Hu D, Mao Y, Wu L and Wenjun L: TP53 mutation detected in
circulating exosomal DNA is associated with prognosis of patients
with hepatocellular carcinoma. Cancer Biol Ther. 23:439–445.
2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou J, Sun HC, Wang Z, Cong WM, Wang JH,
Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, et al: Guidelines for
diagnosis and treatment of primary liver cancer in China (2017
Edition). Liver Cancer. 7:235–260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen ZY, Lusicic A, O'Brien TJ, Velakoulis
D, Adams SJK and Kwan P: Psychotic disorders induced by
antiepileptic drugs in people with epilepsy. Brain. 139:2668–2678.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yin H, Zhou W, Meng J, Zhang D, Wu Z, Wang
T, Wang J, Wang P, Shi X, Wu S, et al: Prognostic factors of
patients with spinal chondrosarcoma: A retrospective analysis of 98
consecutive patients in a single center. Ann Surg Oncol.
21:3572–3578. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Elfadaly AN, Tsilimigras DI, Hyer JM, Paro
A, Bagante F, Ratti F, Marques HP, Soubrane O, Lam V, Poultsides
GA, et al: Impact of tumor burden score on conditional survival
after curative-intent resection for hepatocellular carcinoma: A
multi-institutional analysis. World J Surg. 45:3438–3448.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mgaieth S, Kemp W, Gow P, Fink M, Lubel J,
Nicoll A, Gazzola A, Hong T, Ryan M, Knight V, et al: Impact of
viral hepatitis aetiology on survival outcomes in hepatocellular
carcinoma: A large multicentre cohort study. J Viral Hepat.
24:982–989. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kumada T, Toyoda H, Tada T, Yasuda S and
Tanaka J: Changes in background liver function in patients with
hepatocellular carcinoma over 30 years: Comparison of child-pugh
classification and albumin bilirubin grade. Liver Cancer.
9:518–528. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pham L, Kyritsi K, Zhou T, Ceci L,
Baiocchi L, Kennedy L, Chakraborty S, Glaser S, Francis H, Alpini G
and Sato K: The functional roles of immune cells in primary liver
cancer. Am J Pathol. 192:826–836. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vigano L, Conci S, Cescon M, Fava C,
Capelli P, D'Errico A, Torzilli G, Di Tommaso L, Giuliante F,
Vecchio FM, et al: Liver resection for hepatocellular carcinoma in
patients with metabolic syndrome: A multicenter matched analysis
with HCV-related HCC. J Hepatol. 63:93–101. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zheng Y, Liu Y, Zhao S, Zheng Z, Shen C,
An L and Yuan Y: Large-scale analysis reveals a novel risk score to
predict overall survival in hepatocellular carcinoma. Cancer Manag
Res. 10:6079–6096. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
de Lima LT, Broszczak D, Zhang X, Bridle
K, Crawford D and Punyadeera C: The use of minimally invasive
biomarkers for the diagnosis and prognosis of hepatocellular
carcinoma. Biochim Biophys Acta Rev Cancer.
1874(188451)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang XF, Zhang D, Bu XF, Zhang XH and Cui
L: Identification of a novel miRNA-based recurrence and prognosis
prediction biomarker for hepatocellular carcinoma. BMC
Bioinformatics. 23(479)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Harimoto N, Yoshizumi T, Inokuchi S, Itoh
S, Adachi E, Ikeda Y, Uchiyama H, Utsunomiya T, Kajiyama K, Kimura
K, et al: Prognostic significance of preoperative controlling
nutritional status (CONUT) score in patients undergoing hepatic
resection for hepatocellular carcinoma: A multi-institutional
study. Ann Surg Oncol. 25:3316–3323. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Takagi K, Yagi T, Umeda Y, Shinoura S,
Yoshida R, Nobuoka D, Kuise T, Araki H and Fujiwara T: Preoperative
controlling nutritional status (CONUT) score for assessment of
prognosis following hepatectomy for hepatocellular carcinoma. World
J Surg. 41:2353–2360. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kaibori M, Sakai K, Matsushima H, Kosaka
H, Matsui K, De Velasco MA, Sekimoto M and Nishio K: Patients with
polyclonal hepatocellular carcinoma are at a high risk of early
recurrence and have a poor recurrence-free survival period. Hepatol
Int. 16:135–147. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Erstad DJ and Tanabe KK: Prognostic and
therapeutic implications of microvascular invasion in
hepatocellular carcinoma. Ann Surg Oncol. 26:1474–1493.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Imamura H, Matsuyama Y, Tanaka E, Ohkubo
T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T,
Kawasaki S and Makuuchi M: Risk factors contributing to early and
late phase intrahepatic recurrence of hepatocellular carcinoma
after hepatectomy. J Hepatol. 38:200–207. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sonohara F, Yamada S, Tanaka N, Suenaga M,
Takami H, Hayashi M, Niwa Y, Sugimoto H, Hattori N, Kanda M, et al:
Perioperative and prognostic implication of albumin-bilirubin-TNM
score in Child-Pugh class A hepatocellular carcinoma. Ann
Gastroenterol Surg. 3:65–74. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liang BY, Gu J, Xiong M, Zhang EL, Zhang
ZY, Chen XP and Huang ZY: Tumor size may influence the prognosis of
solitary hepatocellular carcinoma patients with cirrhosis and
without macrovascular invasion after hepatectomy. Sci Rep.
11(16343)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
He HL, Wang Q, Liu L, Luo NB, Su DK and
Jin GQ: Peritumoral edema in preoperative magnetic resonance
imaging is an independent prognostic factor for hepatocellular
carcinoma. Clin Imaging. 75:143–149. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hao S, Fan P, Chen S, Tu C and Wan C:
Distinct recurrence risk factors for intrahepatic metastasis and
multicenter occurrence after surgery in patients with
hepatocellular carcinoma. J Gastrointest Surg. 21:312–320.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang T, Lu JH, Zhai J, Lin C, Yang GS,
Zhao RH, Shen F and Wu MC: High viral load is associated with poor
overall and recurrence-free survival of hepatitis B virus-related
hepatocellular carcinoma after curative resection: A prospective
cohort study. Eur J Surg Oncol. 38:683–691. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Huang G, Lai EC, Lau WY, Zhou WP, Shen F,
Pan ZY, Fu SY and Wu MC: Posthepatectomy HBV reactivation in
hepatitis B-related hepatocellular carcinoma influences
postoperative survival in patients with preoperative low HBV-DNA
levels. Ann Surg. 257:490–505. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yoo S, Kim JY, Lim YS, Han S and Choi J:
Impact of HBsAg seroclearance on late recurrence of hepatitis B
virus-related hepatocellular carcinoma after surgical resection. J
Hepatol. 77:939–946. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang G, Li PP, Lau WY, Pan ZY, Zhao LH,
Wang ZG, Wang MC and Zhou WP: Antiviral therapy reduces
hepatocellular carcinoma recurrence in patients with low HBV-DNA
levels: A randomized controlled trial. Ann Surg. 268:943–954.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chong CC, Wong GL, Wong VW, Ip PC, Cheung
YS, Wong J, Lee KF, Lai PB and Chan HL: Antiviral therapy improves
post-hepatectomy survival in patients with hepatitis B
virus-related hepatocellular carcinoma: A prospective-retrospective
study. Aliment Pharmacol Ther. 41:199–208. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yang HI, Tseng TC, Liu J, Lee MH, Liu CJ,
Su TH, Batrla-Utermann R, Chan HL, Kao JH and Chen CJ:
Incorporating serum level of hepatitis B surface antigen or
omitting level of hepatitis B virus DNA does not affect calculation
of risk for hepatocellular carcinoma in patients without cirrhosis.
Clin Gastroenterol Hepatol. 14:461–468, e462. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yamashita Y, Imai D, Bekki Y, Kimura K,
Matsumoto Y, Nakagawara H, Ikegami T, Yoshizumi T, Shirabe K,
Aishima S and Maehara Y: Surgical outcomes of hepatic resection for
hepatitis b virus surface antigen-negative and hepatitis C virus
antibody-negative hepatocellular carcinoma. Ann Surg Oncol.
22:2279–2285. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen JD, Liu CJ, Lee PH, Chen PJ, Lai MY,
Kao JH and Chen DS: Hepatitis B genotypes correlate with tumor
recurrence after curative resection of hepatocellular carcinoma.
Clin Gastroenterol Hepatol. 2:64–71. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Choi J, Jo C and Lim YS: Tenofovir versus
entecavir on recurrence of hepatitis B virus-related hepatocellular
carcinoma after surgical resection. Hepatology. 73:661–673.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
He L, Xia Z, Shen J, Zhang X, Peng W, Li C
and Wen T: The different effects of adefovir dipivoxil and
telbivudine on the prognosis of hepatitis b virus-related
hepatocellular carcinoma patients after curative resection.
Medicine (Baltimore). 98(e14386)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Qi W, Shen J, Dai J, Wu Y, Zhang Y, Leng
S, Gao F, Ran S, Peng W, Zhang X, et al: Comparison of nucleoside
and nucleotide analogs in the recurrence of hepatitis B
virus-related hepatocellular carcinoma after surgical resection: A
multicenter study. Cancer Med. 10:8421–8431. 2021.PubMed/NCBI View Article : Google Scholar
|