Introduction
Prostate cancer (PCa) was the second-most common
cause of cancer-related fatalities in humans in 2020 and the most
common cancer in men (1). The most
recognized biomarker for the early identification of PCa is serum
prostate-specific antigen (PSA). PSA is highly specific for PCa.
The widespread use of PSA testing has increased the detection rate
of asymptomatic PCa, defined as highly differentiated PCa (2). Although there are more alternatives
for the early diagnosis of PCa thanks to the development of new
biomarkers including SelectMDx, ConfirmMDx, Pca3, MIPS, ExoDX and
mpMRI, PSA testing remains the most widely used screening tool due
to its favorable affordability and applicability (3). Most recently, the United States
Preventive Services Task Force recently updated their guidelines,
which upgraded the PSA recommendation level from a D as a
screening-based level to a C as an advocate for personal screening
(4,5). However, several studies have
demonstrated that PSA concentrations may be influenced by
additional factors that may help to cause bias in identifying PCa
(6-8).
Overdiagnosis or under-diagnosis affected by numerous factors, may
result in inappropriate and unnecessary therapy (9). Therefore, screening PCa based on PSA
concentration still has certain problems to be solved (10).
Inflammation is one of the most significant and
well-known variables influencing cancer development (11). Hematological indicators that can
indicate the state of the immune-inflammatory response in patients
with cancer have recently received increasing attention (12,13).
Systemic immune inflammatory index (SII), C-reactive protein (CRP)
levels, neutrophil-to-lymphocyte ratio (NLR) and
platelet-to-lymphocyte ratio (PLR) are some of these measures.
Because NLR and PLR are readily available and inexpensive, they
have been extensively examined in several malignancies (14-16).
PLR is a systemic parameter based on inflammation. Previous
research has explored the diagnostic function of PLR in patients
with PCa; however, findings remain inconclusive. Yuksel et
al (17) found that PLR may
distinguish between benign prostatic hyperplasia and PCa,
ultimately serving as a diagnostic tool for PCa. Conversely, Lee
et al (18) determined that
pre-biopsy PLR is not predictive of clinically significant PCa
(CSPCa), and thus, does not provide diagnostic value for PLR.
Indeed, there may be some correlation between PLR and PSA
metabolism, which may lead to detection bias in PCa diagnosis.
Furthermore, to the best of the authors' knowledge, it was found
that this phenomenon has never been reported before.
Consequently, a secondary data analysis was
performed on the National Health and Nutrition Examination Survey
(NHANES) data. After controlling for a large number of influencing
factors, it was sought to clarify the relationship between PLR and
PSA concentration in men without PCa in the USA.
Materials and methods
Data availability
Since 1960, the NHANES, which is designed to
estimate the health and nutritional status of adults and children
in the US, has been conducted by the National Center for Disease
Control (CDC) and the Prevention National Center for Health
Statistics. Demographic and methodological details can be found on
the NHANES website (www.cdc.gov/nchs/nhanes, accessed on October 7, 2022).
The National Center approved the NHANES protocols for the Health
Statistics Research Ethics Review Board.
Study population
The NHANES uses a stratified, multi-stage random
sampling design and is a nationally representative nutrition survey
of the general USA population. Five cycles of NHANES data from 2001
to 2010 were integrated into the present study. The data used for
the second analysis included PSA concentrations, socio-demographic
data and laboratory data. Participants were excluded from the
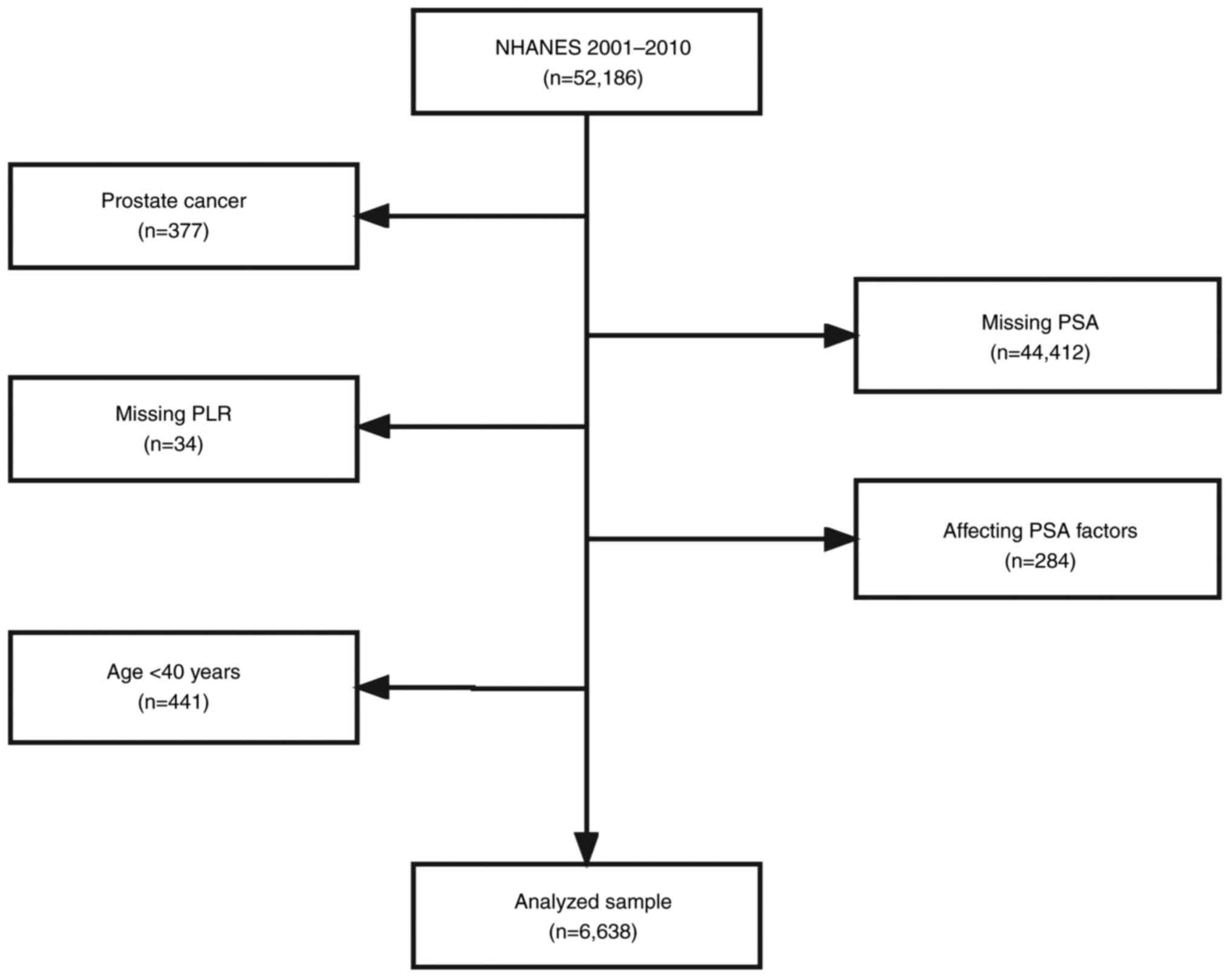
present study based on the following exclusion criteria: i)
Participants diagnosed with PCa (n=377); ii) missing PSA
(n=44,412); iii) missing PLR (n=34); iv) factors affecting PSA
concentration: Diagnosed with prostatitis, stain drug user,
received prostate biopsy within one week and had urinary system
surgery within one month (n=284); and v) Age <40 years (n=441).
After screening, 6,638 out of 52,186 participants were suitable for
the present study after thorough screening (Fig. 1). It is important to note that the
present study was a survey regarding the relationship between a
specific clinical indicator and PSA in the general male population
in USA. Patients with PCa which have significantly different PSA
levels compared with the general population and patients with PCa
should be excluded as a confounding factor affecting PSA (19,20).
In addition, the present study complied with the Declaration of
Helsinki of the World Medical Association in the design and conduct
of the present study. In the present study, data analysis based on
NHANES was utilized.
Statistical analysis
All statistical analyses were performed using
Package R and EmpowerStats (http://www.empowerstats.com), with a complex weighted
sampling design from NHANES. Participants were characterized
according to the quartiles of PLR (Category 1: 2.252-96.116;
Category 2: 96.116-122.198; Category 3: 122.198-156.667; Category
4: >156.667). Percentages were used for categorical variables
and mean ± standard deviation for continuous variables. For
comparing the differences between groups, categorical and
continuous variables were analyzed by using weighted χ2
tests and linear regression models, respectively. The link between
PLR and PSA was assessed using a weighted multivariate linear
regression model. An unadjusted model (Model 1) was created first,
and then a minimally adjusted model (Model 2) was constructed after
adjusting for age, family income, ethnicity, military status,
marital status and education. Finally, fully adjusted models (Model
3) were calculated after adjusting for age, household income,
ethnicity, military status, marital status, education, monocyte
count, neutrophil count, platelet count, lymphocyte-to-monocyte
ratio (LMR) and systemic immune inflammation index. The analysis
was then stratified by age, family income, ethnicity, military
status, marital status and education and tested for interactions.
In the present study, a P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics of
participants
The weighted distribution of baseline
characteristics is shown in Table
I, including socio-demographic data and laboratory data of
chosen participants selected from the NHANES (2001-2010) survey. In
the present study, the average age of the chosen participants was
58.563±11.848 years. Then, different PLR were divided into four
quartiles (Q1-Q4). The distribution of neutrophil and basophil
count in Q1-Q4 of PLR revealed no statistical difference
(P>0.05). Compared with the different groups in Table I, the distribution of PLR
demonstrated an age difference, where aged participants had higher
PLR than younger ones, had higher family income, higher platelet
count, higher C-reactive protein, higher NLR, higher systemic
immune inflammation index and were more likely to have a higher
education level. On the other hand, participants with more elevated
PLR had lower leukocyte count, lower mononuclear count, lower
eosinophil count, lower red cell count, lower hemoglobin and lower
LMR. In the present study, non-Hispanic whites were the main
participants.
 | Table IBaseline characteristics of the
selected participants. |
Table I
Baseline characteristics of the
selected participants.
|
Platelet-to-lymphocyte ratio quartile | Q1 | Q2 | Q3 | Q4 | P-value |
|---|
| N | 1660 | 1659 | 1656 | 1663 | |
| Total prostate
specific antigen (ng/ml) | 1.531±2.321 | 1.678±2.529 | 1.681±3.114 | 1.958±3.213 | <0.001 |
| Age, years | 58.033±11.583 | 58.458±18.333 | 58.001±11.667 | 59.751±12.083 | <0.001 |
| Family income | 2.628±1.616 | 2.787±1.632 | 2.952±1.635 | 2.935±1.628 | <0.001 |
| Leukocyte count
(1,000 cells/µl) | 8.017±3.344 | 7.243±1.844 | 6.839±1.874 | 6.510±3.077 | <0.001 |
| Lymphocyte count
(1,000 cells/µl) | 2.824±2.514 | 2.155±0.482 | 1.831±0.408 | 1.425±0.378 | <0.001 |
| Mononuclear count
(1,000 cells/µl) | 0.628±0.221 | 0.577±0.183 | 0.556±0.180 | 0.536±0.184 | <0.001 |
| Neutrophils count
(1,000 cells/µl) | 4.270±1.661 | 4.241±1.520 | 4.198±1.591 | 4.289±2.644 | 0.208 |
| Eosinophil count
(1,000 cells/µl) | 0.253±0.195 | 0.230±0.168 | 0.220±0.179 | 0.229±0.290 | <0.001 |
| Basophils count
(1,000 cells/µl) | 0.112±0.063 | 0.109±0.038 | 0.107±0.034 | 0.120±0.221 | 0.259 |
| Red cell count
(million cells/µl) | 4.877±0.486 | 4.893±0.457 | 4.907±0.464 | 4.813±0.488 | <0.001 |
| Hemoglobin
(g/µl) | 15.144±1.352 | 15.092±1.246 | 15.086±1.233 | 14.744±1.395 | <0.001 |
| Platelet count
(1,000 cells/µl) | 204.312±51.978 | 233.80±50.624 | 251.395±54.792 | 280.114±71.685 | <0.001 |
| C-reactive
protein(mg/µl) | 0.377±0.742 | 0.386±0.968 | 0.396±0.923 | 0.528±1.141 | <0.001 |
|
Lymphocyte-to-monocyte ratio | 4.746±2.153 | 4.031±1.369 | 3.572±1.244 | 2.932±1.222 | <0.001 |
|
Neutrophil-to-lymphocyte ratio | 1.652±0.707 | 2.031±0.783 | 2.359±0.934 | 3.183±1.807 | <0.001 |
| Systemic immune
inflammation index | 329.43±140.228 | 461.38±167.68 | 578.697±222.92 | 890.905±869.83 | <0.001 |
| Military
status | | | | | 0.008 |
|
Yes | 536 (32.309%) | 539 (32.489%) | 566 (34.179%) | 620 (37.282%) | |
|
No | 1,123
(67.691%) | 1,120
(67.511%) | 1,090
(65.821%) | 1,043
(62.718%) | |
| Education | | | | | <0.001 |
|
Less than
9th grade | 327 (19.723%) | 287 (17.310%) | 244 (14.752%) | 229 (13.770%) | |
|
9-11th
grade | 272 (16.405%) | 242 (14.596%) | 198 (11.971%) | 238 (14.311%) | |
|
High school
grad | 381 (22.979%) | 387 (23.341%) | 408 (24.667%) | 364 (21.888%) | |
|
Some college
or AA degree | 275 (16.586%) | 370 (22.316%) | 379 (22.914%) | 426 (25.616%) | |
|
College
graduate or above | 403 (24.306%) | 372 (22.437%) | 425 (25.695%) | 406 (24.414%) | |
| Marital status | | | | | 0.035 |
|
Married | 1,374
(82.821%) | 1,403 (84.67%) | 1,437
(86.933%) | 1,421
(85.448%) | |
|
Single | 183 (11.031%) | 167 (10.078%) | 151 (9.135%) | 165 (9.922%) | |
|
Living with
a partner | 102 (6.148%) | 87 (5.250%) | 65 (3.932%) | 77 (4.630%) | |
| Ethnicity | | | | | <0.001 |
|
Mexican
American | 346 (20.843%) | 312 (18.807%) | 305 (18.418%) | 246 (14.793%) | |
|
Other
hispanic | 110 (6.627%) | 136 (8.198%) | 89 (5.374%) | 76 (4.570%) | |
|
Non-hispanic
white | 800 (48.193%) | 842 (50.753%) | 947 (57.186%) | 975 (58.629%) | |
|
Non-hispanic
black | 354 (21.325%) | 298 (17.963%) | 267 (16.123%) | 315 (18.942%) | |
|
Other
ethnicity | 50 (3.012%) | 71 (4.280%) | 48 (2.899%) | 51 (3.067%) | |
The connection between PSA
concentrations and serum PLR
The results of the univariate and multivariate
analyses by the weighted linear model are presented in Table II. In the non-adjusted model, PSA
concentrations increased by 0.003 ng/ml (0.002, 0.004) with each
increase in PLR, with a statistically significant trend indicated
by a P<0.001. After minimal adjustment for age, household
income, ethnicity, military status, marital status and education,
PSA concentration increased by 0.002 ng/ml (0.001, 0.003) with each
increase in PLR, with a statistically significant trend indicated
by a P<0.001. The fully adjusted model that adjusts for age,
family income, ethnicity, military status, marital status,
education, mononuclear count, neutrophils count, platelet count,
LMR and SII indicated that the PSA concentrations were increased by
0.004 ng/ml (0.001, 0.007) with each increase in PLR, with a
statistically significant trend indicated by a P<0.004.
 | Table IIUnivariate and multivariate analyses
by the weighted linear model. |
Table II
Univariate and multivariate analyses
by the weighted linear model.
| Exposure | Non-adjusted
model | Minimally adjusted
model | Fully adjusted
model |
|---|
| PLR | 0.003
(0.002,0.004), <0.001 | 0.002
(0.001,0.003), <0.001 | 0.004(0.001,0.007)
<0.004 |
| PLR quartile | | | |
|
Q1 | Ref | Ref | Ref |
|
Q2 | 0.160 (-0.025,
0.345) 0.08975 | 0.112 (-0.068,
0.292) 0.22344 | 0.133 (-0.074,
0.339) 0.20817 |
|
Q3 | 0.165 (-0.020,
0.350) 0.08091 | 0.208 (0.027,
0.389) 0.02455 | 0.243 (0.001,0.486)
0.04935 |
|
Q4 | 0.402 (0.216,
0.588) 0.00002 | 0.298 (0.117,
0.480) 0.00127 | 0.355 (0.043,
0.667) 0.02593 |
| P for trend | <0.001 | <0.001 | 0.028 |
Stratified associations between PSA
concentrations and PLR
As demonstrated in Table III, a stratified analysis was
conducted by age, ratios of family income, ethnicity, military
status, marital status and education to assess the associations
between PLR and PSA concentrations. It is likely that those aged
>80 years, a low group of ratios of family income, those who had
not served in the military, had married, had an education level
less than 9th grade and had higher PSA concentrations, with
increasing PLR displaying a significant trend (p for trend=0.0148,
p for trend=0.0027, p for trend=0.0192, p for trend=0.0373 and p
for trend=0.0003). However, no interactive effects were
observed.
 | Table IIIEffect size of PLR on
prostate-specific antigen in the prespecified and exploratory
subgroup. |
Table III
Effect size of PLR on
prostate-specific antigen in the prespecified and exploratory
subgroup.
| PLR | N | β | 95% CI low | 95% CI high | P-value | p for
interaction |
|---|
| Stratified by
age | | | | | | 0.4961 |
|
<60 | 2058 | 0.001 | -0.006 | 0.007 | 0.8635 | |
|
60-80 | 2072 | 0.002 | -0.002 | 0.007 | 0.2425 | |
|
>80 | 2073 | 0.005 | 0.001 | 0.009 | 0.0148 | |
| Stratified by ratio
of family income | | | | | | 0.0646 |
|
Low
group | 2064 | 0.007 | 0.002 | 0.012 | 0.0027 | |
|
Median
group | 2071 | 0.003 | -0.001 | 0.006 | 0.1352 | |
|
High
group | 2068 | -0.003 | -0.01 | 0.004 | 0.4659 | |
| Stratified by
ethnicity | | | | | | 0.846 |
|
Mexican
American | 1118 | 0.003 | -0.004 | 0.01 | 0.3537 | |
|
Other
hispanic | 360 | 0.011 | -0.01 | 0.032 | 0.3173 | |
|
Non-hispanic
white | 3365 | 0.002 | -0.002 | 0.005 | 0.3039 | |
|
Non-hispanic
black | 1155 | 0.005 | -0.002 | 0.012 | 0.1332 | |
|
Other
ethnicity/ethnicity | 205 | 0 | -0.028 | 0.028 | 0.9923 | |
| Stratified by
military status | | | | | | 0.2802 |
|
Yes | 2124 | 0.002 | -0.002 | 0.005 | 0.3745 | |
|
No | 4079 | 0.004 | 0.001 | 0.008 | 0.0192 | |
| Stratified by
marital status | | | | | | 0.9962 |
|
Married | 5281 | 0.003 | 0 | 0.006 | 0.0373 | |
|
Single | 615 | 0.003 | -0.004 | 0.01 | 0.3613 | |
|
Living with
a partner | 307 | 0.003 | -0.013 | 0.018 | 0.7538 | |
| Stratified by
education | | | | | | 0.0504 |
|
Less than
9th grade | 997 | 0.012 | 0.005 | 0.018 | 0.0003 | |
|
9-11th
grade | 889 | 0.003 | -0.005 | 0.011 | 0.4316 | |
|
High school
grad | 1442 | 0 | -0.006 | 0.007 | 0.944 | |
|
Some college
or AA degree | 1366 | 0.002 | -0.006 | 0.01 | 0.6173 | |
|
College
graduate or above | 1509 | 0 | -0.005 | 0.005 | 0.9856 | |
Identification of sensitivity
analysis
A sensitivity analysis was conducted to confirm the
accuracy and stability of the results. First, the PLR was converted
as a continuous variable to the categorical variable in the
quartile value, and then the P-value was calculated for the trend
(Table II). Surprisingly, the
result of the categorical variable was consistent with the effect
of the PLR as a continuous variable. A smooth curve was constructed
based on the fully adjusted model to investigate the possible
linear relationship between the PLR and PSA concentrations.
According to the fully adjusted model, there was a linear
relationship between PLR and PSA concentration after adjusting for
other covariates (Fig. 2). The
results revealed that for each increase in PLR, the PSA
concentrations were elevated by 0.004 ng/ml. These results
indicated a positive association between PLR and PSA
concentrations.
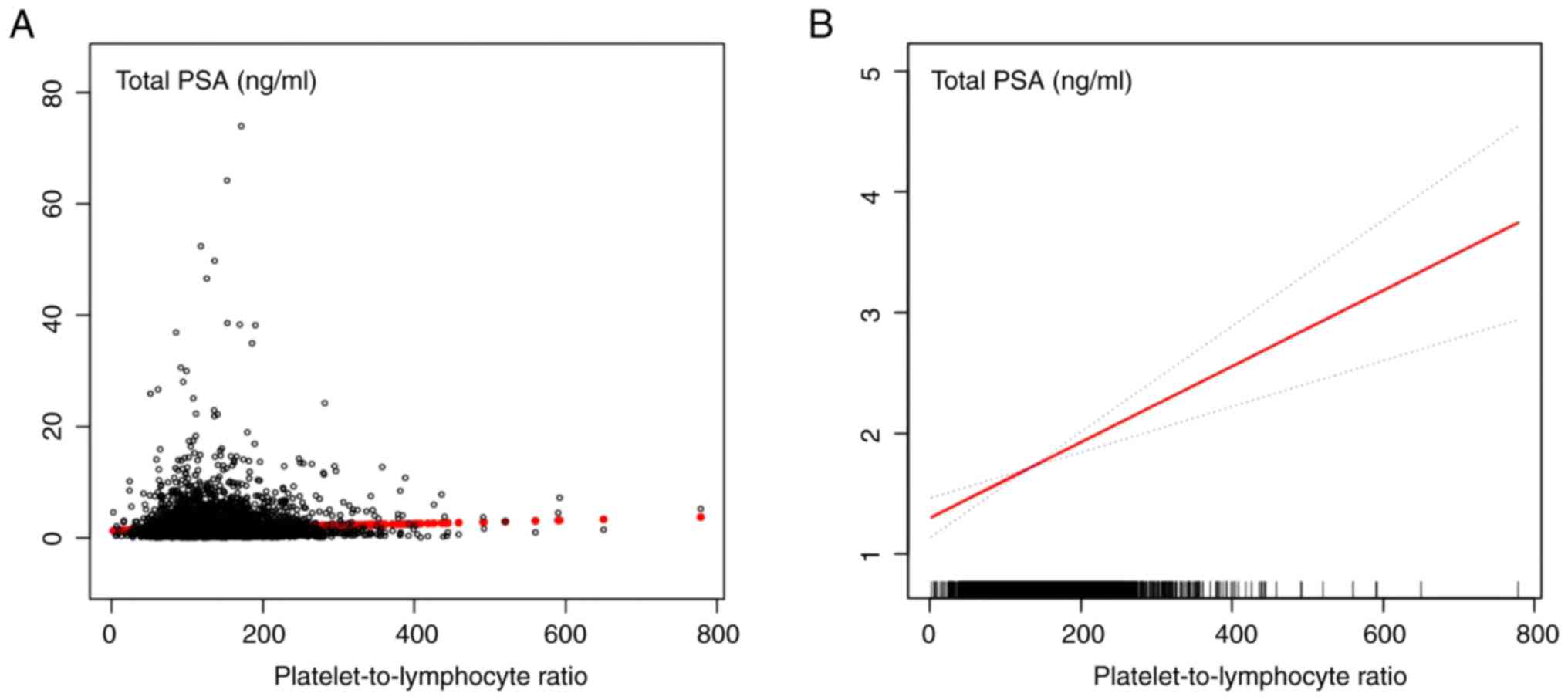 | Figure 2The relationship between
platelet-to-lymphocyte ratio and serum PSA connections. (A) Each
black point represents a sample. (B) Red line represents the smooth
curve fit between variables. Blue lines represent the 95% of
confidence interval from the fit. Age, family income, ethnicity,
military status, marital status, education, mononuclear count,
neutrophils count, platelet count, lymphocyte to monocyte ratio,
and systemic immune inflammation index were adjusted. PSA,
prostate-specific antigen. |
Discussion
PLR and PSA exhibited a favorable connection in the
present study. To the best of the authors' knowledge, the present
study is the first to examine and discover this link among men from
USA without a history of cancer using the NHANES database. Although
PLR and PSA have been studied previously, an association between
them has not been discovered, and previous studies have suffered
from small sample sizes and missing data (21). Accordingly, the connection between
PLR and PSA necessitates additional research to clarify their
relationship. Therefore, it is essential to further comprehend the
individual variability in PSA concentrations that may emerge from
PLR to prevent the bias of PSA testing during the diagnosis of
prostate-related disorders. The present study population was drawn
from NHANES (2001-2010), excluding 45,548 ineligible participants.
The results of the present study revealed that with every increment
of PLR, the PSA concentration increased by 0.004 ng/ml, which means
that if the PLR increased by 100, the PSA concentration would
increase by 0.4 ng/ml. Sensitivity analysis confirmed the results,
which are robust.
Platelet and lymphocyte counts are routinely
measured as parameters based on blood tests. PLR represents a
marker of inflammation. High PLR reflects elevated
platelet-dependent pro-tumor responses and reduced
lymphocyte-mediated anti-tumor immune responses, which could
potentially lead to cancer progression and a poor prognosis.
Platelets have been shown to promote cancer cell growth and
metastasis through direct and indirect actions (22,23).
On PCa, on the one hand, platelets adhere to tumor cells with the
help of fibrinogen; at the same time, they promote more fibrinogen
aggregation around tumor cells by forming thrombin, thus protecting
them from the cytotoxicity of natural killer cells (24); on the other hand, platelet-derived
microparticles promote the invasiveness of PCa cells through
upregulation of MMP-2 production (25). Currently, a considerable amount of
evidence indicates that lymphocytes are the cellular basis of
cancer immunosurveillance and can inhibit tumor cell proliferation
and metastasis (26). Huang et
al (27) revealed that high
pre-treatment levels of circulating lymphocytes are associated with
longer relapse-free survival and slightly improved overall survival
(OS) in patients with oropharyngeal cancer. Sznurkowski et
al (28) concluded that the
increased number of tumor-infiltrating lymphocytes is associated
with an improved prognosis in various cancers, including breast and
colorectal. As a parameter combining platelet count and lymphocyte
count, PLR can provide relatively accurate prognostic information
about cancer patients (29,30).
It is widely accepted that there is a strong correlation between
the development and prognosis of tumors and a systemic inflammatory
response (31-33).
As a commonly used marker of systemic inflammation, the prognostic
value of NLR has also been powerfully demonstrated in PCa (34-36).
However, the significance of PLR in PCa prognosis remains
conflicting (17,18).
A previous study provided evidence that PLR is an
independent prognostic factor for progression-free survival and OS
in PCa patients (37). Similarly,
Yuksel et al (17) reported
that PLR has the potential to differentiate between benign
prostatic hyperplasia and PCa. This can ultimately serve as a
diagnostic tool for PCa. By contrast, Lee et al (18) concluded that pre-biopsy PLR cannot
significantly predict CSPCa, rendering it inadequate for PLR
diagnosis. Similar results were reported by Sun et al
(16), revealing that there is no
significant correlation between PLR and either PCa or PSA after
comparing the predictive effects of several inflammatory markers on
PCa. Therefore, the aforementioned study concluded that PLR has
little diagnostic and prognostic value for PCa. Because most
studies involved men from Asia at relatively low risk of developing
PCa and the conclusions were not definitive, studies are still
needed to assess the relationship between PLR and PSA levels.
Therefore, it was hypothesized that PLR affects PSA concentrations
and may create testing bias, which could result in inconsistent
interpretations. Further cohort trials are necessary to further
comprehend the function of PLR as either a protective or risk
factor in the progression of PCa.
The findings of the present study, support a
positive correlation between PLR and PSA. A positive correlation
between PLR and PSA can lead to detection bias, which may have
implications for PCa screening. Since PLR preferentially elevates
PSA concentrations in men without PCa, PSA testing for PCa
screening in men with high PLR can lead to the overdiagnosis of
asymptomatic PCa. Therefore, if PLR can elevate the PSA produced by
prostate tumors or enhance the ability of tumor-derived PSA to
enter the serum, it is necessary to adjust the PSA threshold for
further examine platelets as well as lymphocytes to ultimately rule
out interference with PSA by PLR. For example, in a high PLR
population, the actual PSA value should be used as the screening
diagnostic criterion. Actual PSA=PSA measurement-PLR *
0.004. Further studies are needed to explore the mechanisms by
which the PLR affects PSA concentration and its impact on PCa
screening. In addition, prospective cohort studies are still needed
to confirm the causal relationship and serum platelets and
lymphocytes are involved in both the genesis and development of
PCa, which also needs to be verified by in vitro and in
vivo experiments.
Compared with prior research, the present study
boasts several noteworthy findings. Firstly, it is the first
large-scale cross-sectional study to find a positive association
between PLR and PSA in men from USA with a non-tumor history.
Secondly, the present study utilized a highly reliable,
standardized, and multilayer random sample, providing a
representative portrayal of the general USA population. Then, a
sensitivity analysis was performed and a smoothing curve was
constructed based on a fully adjusted model to investigate the
possible linear relationship between PLR and PSA concentration.
Nevertheless, there are certain limitations to the interpretation
of the findings of the present study. Primarily, it is challenging
to distinguish causality in the present study due to the inherent
limitations of the NHANES database as a cross-sectional survey.
Although prospective studies have demonstrated that PLR has an
important predictive role in the diagnosis and prognosis of PCa
(38,39), prospective cohort studies are still
needed for further validation because these studies are single
center with small sample sizes. To further validate the accuracy
and applicability of the findings of the present study, a
prospective cohort study is being designed based on a Chinese
population, and the authors are working towards a multicenter
study. Furthermore, participants diagnosed with PCa were excluded
and those with factors impacting PSA concentrations or missing
data. Consequently, the findings of the present study cannot be
generalized to the aforementioned population. Lastly, the survey is
based on the NHANES database, which is limited to the individuals
from USA. As a result, generalizability is geographically limited.
Nonetheless, in conjunction with the existing studies in China,
Italy, Austria, and other regions (16,40,41),
there are favorable reasons to hypothesize that the association
between PLR and PSA, or PCa, is geospatially generalizable.
In conclusion, in men from USA, there is an
independent and positive association between PLR and PSA, which
could potentially result in overdiagnosis of asymptomatic PCa in
populations with higher PLR levels.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Zhuhai Science
and Technology Plan Projects in the Field of Social Development
Foundation (grant no. 20191210E030071).
Availability of data and materials
All data are available at NHANES website https://www.cdc.gov/nchs/nhanes/index.htm (accessed on
October 7, 2022).
Authors' contributions
WL, HL and BH conceptualized and designed the study.
BH and SH acquired and analyzed the data. MY interpreted the data.
BH and SH wrote the original draft of the manuscript. WL and HL
reviewed the manuscript. All authors read and approved the final
version of the manuscript. BH, SH and MY confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vickers AJ: Prostate cancer screening:
Time to question how to optimize the ratio of benefits and harms.
Ann Intern Med. 167:509–510. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Alarcón-Zendejas AP, Scavuzzo A,
Jiménez-Ríos MA, Álvarez-Gómez RM, Montiel-Manríquez R,
Castro-Hernández C, Jiménez-Dávila MA, Pérez-Montiel D,
González-Barrios R, Jiménez-Trejo F, et al: The promising role of
new molecular biomarkers in prostate cancer: From coding and
non-coding genes to artificial intelligence approaches. Prostate
Cancer Prostatic Dis. 25:431–443. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fenton JJ, Weyrich MS, Durbin S, Liu Y,
Bang H and Melnikow J: Prostate-Specific Antigen-Based screening
for prostate cancer: Evidence report and systematic review for the
US preventive services task force. JAMA. 319:1914–1931.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
US Preventive Services Task Force.
Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB,
Davidson KW, Doubeni CA, Ebell M, Epling JW Jr, et al: Screening
for prostate cancer: US preventive services task force
recommendation statement. JAMA. 319:1901–1913. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gudmundsson J, Sigurdsson JK,
Stefansdottir L, Agnarsson BA, Isaksson HJ, Stefansson OA,
Gudjonsson SA, Gudbjartsson DF, Masson G, Frigge ML, et al:
Genome-wide associations for benign prostatic hyperplasia reveal a
genetic correlation with serum levels of PSA. Nat Commun.
9(4568)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhao Y, Zhang Y, Wang X, Lin D and Chen Z:
Relationship between body mass index and concentrations of prostate
specific antigen: A cross-sectional study. Scand J Clin Lab Invest.
80:162–167. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu Y, Xiao G, Zhou JW, Yang JK, Lu L,
Bian J, Zhong L, Wei QZ, Zhou QZ, Xue KY, et al: Optimal starting
age and baseline level for repeat tests: Economic concerns of PSA
screening for Chinese Men-10-Year experience of a single center.
Urol Int. 104:230–238. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tan GH, Nason G, Ajib K, Woon DTS,
Herrera-Caceres J, Alhunaidi O and Perlis N: Smarter screening for
prostate cancer. World J Urol. 37:991–999. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Misra-Hebert AD, Hu B, Klein EA,
Stephenson A, Taksler GB, Kattan MW and Rothberg MB: Prostate
cancer screening practices in a large, integrated health system:
2007-2014. BJU Int. 120:257–264. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cortellini A, Ricciuti B, Borghaei H,
Naqash AR, D'Alessio A, Fulgenzi CAM, Addeo A, Banna GL and Pinato
DJ: Differential prognostic effect of systemic inflammation in
patients with non-small cell lung cancer treated with immunotherapy
or chemotherapy: A post hoc analysis of the phase 3 OAK trial.
Cancer. 128:3067–3079. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cao Y, Zheng X, Hu Y, Li J, Huang B, Zhao
N, Liu T, Cai K and Tian S: Levels of systemic inflammation
response index are correlated with tumor-associated bacteria in
colorectal cancer. Cell Death Dis. 14(69)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Song M, Zhang Q, Song C, Liu T, Zhang X,
Ruan G, Tang M, Xie H, Zhang H, Ge Y, et al: The advanced lung
cancer inflammation index is the optimal inflammatory biomarker of
overall survival in patients with lung cancer. J Cachexia
Sarcopenia Muscle. 13:2504–2514. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cui S, Cao S, Chen Q, He Q and Lang R:
Preoperative systemic inflammatory response index predicts the
prognosis of patients with hepatocellular carcinoma after liver
transplantation. Front Immunol. 14(1118053)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sawada R, Akiyoshi T, Kitagawa Y, Hiyoshi
Y, Mukai T, Nagasaki T, Yamaguchi T, Konishi T, Yamamoto N, Ueno M
and Fukunaga Y: Systemic inflammatory markers combined with
tumor-infiltrating lymphocyte density for the improved prediction
of response to neoadjuvant chemoradiotherapy in rectal cancer. Ann
Surg Oncol. 28:6189–6198. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun Z, Ju Y, Han F, Sun X and Wang F:
Clinical implications of pretreatment inflammatory biomarkers as
independent prognostic indicators in prostate cancer. J Clin Lab
Anal. 32(e22277)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yuksel OH, Urkmez A, Akan S, Yldirim C and
Verit A: Predictive value of the platelet-To-Lymphocyte ratio in
diagnosis of prostate cancer. Asian Pac J Cancer Prev.
16:6407–6412. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee JW, Jeong H, Son H and Cho MC:
Platelet-to-lymphocyte ratio is not a predictor of clinically
significant prostate cancer at the prostate biopsy: A large cohort
study. Sci Rep. 11(14240)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wei C, Tian L, Jia B, Wang M, Xiong M, Hu
B, Deng C, Hou Y, Hou T, Yang X and Chen Z: Association between
serum triglycerides and prostate specific antigen (PSA) among U.S.
Males: National Health and Nutrition Examination Survey (NHANES),
2003-2010. Nutrients. 14(1325)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu Z, Chen C, Yu F, Yuan D, Wang W, Jiao
K, Yang S, Zhang Y, Wang Y, Liu L, et al: Association of total
dietary intake of sugars with Prostate-Specific antigen (PSA)
concentrations: Evidence from the National Health and Nutrition
Examination Survey (NHANES), 2003-2010. Biomed Res Int.
2021(4140767)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
McDonald AC, Vira MA, Vidal AC, Gan W,
Freedland SJ and Taioli E: Association between systemic
inflammatory markers and serum prostate-specific antigen in men
without prostatic disease-the 2001-2008 National Health and
Nutrition Examination Survey. Prostate. 74:561–567. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liao K, Zhang X, Liu J, Teng F, He Y,
Cheng J, Yang Q, Zhang W, Xie Y, Guo D, et al: The role of
platelets in the regulation of tumor growth and metastasis: The
mechanisms and targeted therapy. MedComm (2020).
4(e350)2023.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Wang J, Zhou X, He Y, Chen X, Liu N, Ding
Z and Li J: Prognostic role of platelet to lymphocyte ratio in
prostate cancer: A meta-analysis. Medicine (Baltimore).
97(e12504)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li Y, Wang H, Zhao Z, Yang Y, Meng Z and
Qin L: Effects of the interactions between platelets with other
cells in tumor growth and progression. Front Immunol.
14(1165989)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tao DL, Tassi Yunga S, Williams CD and
McCarty OJT: Aspirin and antiplatelet treatments in cancer. Blood.
137:3201–3211. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li L, Yu R, Cai T, Chen Z, Lan M, Zou T,
Wang B, Wang Q, Zhao Y and Cai Y: Effects of immune cells and
cytokines on inflammation and immunosuppression in the tumor
microenvironment. Int Immunopharmacol. 88(106939)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Huang SH, Waldron JN, Milosevic M, Shen X,
Ringash J, Su J, Tong L, Perez-Ordonez B, Weinreb I, Bayley AJ, et
al: Prognostic value of pretreatment circulating neutrophils,
monocytes, and lymphocytes in oropharyngeal cancer stratified by
human papillomavirus status. Cancer. 121:545–555. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sznurkowski JJ, Zawrocki A, Emerich J and
Biernat W: Prognostic significance of CD4+ and CD8+ T cell
infiltration within cancer cell nests in vulvar squamous cell
carcinoma. Int J Gynecol Cancer. 21:717–721. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ropponen KM, Eskelinen MJ, Lipponen PK,
Alhava E and Kosma VM: Prognostic value of tumour-infiltrating
lymphocytes (TILs) in colorectal cancer. J Pathol. 182:318–324.
1997.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Adams S, Gray RJ, Demaria S, Goldstein L,
Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et
al: Prognostic value of tumor-infiltrating lymphocytes in
triple-negative breast cancers from two phase III randomized
adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin
Oncol. 32:2959–2966. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schuettfort VM, D'Andrea D, Quhal F,
Mostafaei H, Laukhtina E, Mori K, König F, Rink M, Abufaraj M,
Karakiewicz PI, et al: A panel of systemic inflammatory response
biomarkers for outcome prediction in patients treated with radical
cystectomy for urothelial carcinoma. BJU Int. 129:182–193.
2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xie H, Ruan G, Wei L, Deng L, Zhang Q, Ge
Y, Song M, Zhang X, Lin S, Liu X, et al: The inflammatory burden
index is a superior systemic inflammation biomarker for the
prognosis of non-small cell lung cancer. J Cachexia Sarcopenia
Muscle. 14:869–878. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shafique K, Proctor MJ, McMillan DC,
Qureshi K, Leung H and Morrison DS: Systemic inflammation and
survival of patients with prostate cancer: Evidence from the
Glasgow Inflammation Outcome Study. Prostate Cancer Prostatic Dis.
15:195–201. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jang WS, Cho KS, Kim MS, Yoon CY, Kang DH,
Kang YJ, Jeong WS, Ham WS and Choi YD: The prognostic significance
of postoperative neutrophil-to-lymphocyte ratio after radical
prostatectomy for localized prostate cancer. Oncotarget.
8:11778–11787. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kwon YS, Han CS, Yu JW, Kim S, Modi P,
Davis R, Park JH, Lee P, Ha YS, Kim WJ and Kim IY: Neutrophil and
lymphocyte counts as clinical markers for stratifying low-risk
prostate cancer. Clin Genitourin Cancer. 14:e1–e8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang S, Ji Y, Chen Y, Du P, Cao Y, Yang X,
Ma J, Yu Z and Yang Y: The values of systemic Immune-Inflammation
index and Neutrophil-Lymphocyte ratio in the localized prostate
cancer and benign prostate hyperplasia: A retrospective clinical
study. Front Oncol. 11(812319)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang Y, Xu F, Pan J, Zhu Y, Shao X, Sha J,
Wang Z, Cai Y, Liu Q, Dong B, et al: Platelet to lymphocyte ratio
as an independent prognostic indicator for prostate cancer patients
receiving androgen deprivation therapy. BMC Cancer.
16(329)2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li F, Hu H, Gu S, Chen X and Sun Q:
Platelet to lymphocyte ratio plays an important role in prostate
cancer's diagnosis and prognosis. Int J Clin Exp Med.
8:11746–11751. 2015.PubMed/NCBI
|
|
39
|
Chong W, Zhang Z, Luo R, Gu J, Lin J, Wei
Q, Li B, Myers R, Lu-Yao G, Kelly WK, et al: Integration of
circulating tumor cell and neutrophil-lymphocyte ratio to identify
high-risk metastatic castration-resistant prostate cancer patients.
BMC Cancer. 21(655)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bauckneht M, Rebuzzi SE, Signori A,
Frantellizzi V, Murianni V, Lodi Rizzini E, Lavelli V, Donegani MI,
Ponzano M, Gaudiano A, et al: The prognostic power of inflammatory
indices and clinical factors in metastatic castration-resistant
prostate cancer patients treated with radium-223 (BIO-Ra study).
Eur J Nucl Med Mol Imaging. 49:1063–1074. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Langsenlehner T, Pichler M, Thurner EM,
Krenn-Pilko S, Stojakovic T, Gerger A and Langsenlehner U:
Evaluation of the platelet-to-lymphocyte ratio as a prognostic
indicator in a European cohort of patients with prostate cancer
treated with radiotherapy. Urol Oncol.
33(201.e9-e16)2015.PubMed/NCBI View Article : Google Scholar
|