Introduction
Cancer cachexia, a syndrome characterized by severe
weight loss and muscle depletion, is of significant concern in
patients with advanced cancer. Its incidence rate ranges from
50-80%, and markedly affects mortality, accounting for up to 20% of
cancer-related deaths (1,2). In Japan, the European Palliative Care
Research Collaborative criteria and European Society for Medical
Oncology criteria are used in cancer cachexia management as no
specific guidelines exist for this condition in the country
(3,4).
Cancer cachexia is a complex syndrome involving
various factors, including skeletal muscle loss, activation of
inflammatory cytokines, metabolic disturbances due to insulin
resistance, and increased catabolism of hyperlipidemia (5,6).
Cancer cachexia progression may result in irreversible
malnutrition, significantly reducing the activities of daily living
and quality of life, leading to worse prognosis. As metabolic
disorders progress, effective nutrient utilization is impaired;
hence, multidisciplinary interventions, such as nutritional
therapy, exercise, and pharmacotherapy are essential from the early
stages of cancer cachexia (6,7).
Promising pharmacological approaches for cachexia management
include ghrelin agonists, beta-blockers, beta-adrenergic agonists,
androgen receptor agonists, and antimyostatin peptides (8). However, their clinical application in
cancer cachexia management remains limited, and their use is
associated with potential adverse effects, necessitating cautious
application.
Anamorelin, the first oral small-molecule
ghrelin-like agent worldwide, became available in Japan in 2021 and
was anticipated to alleviate cancer cachexia-related weight loss
and anorexia (9). Ghrelin, a
growth hormone secretagogue primarily secreted in the stomach, acts
on appetite-promoting neurons in the hypothalamus and indirectly
stimulates muscle protein synthesis by stimulating the secretion of
the growth hormone and insulin-like growth factor 1 in the liver
(10-12).
Additionally, ghrelin is believed to counteract cancer cachexia
through multiple mechanisms, including its anti-inflammatory
effects and inhibition of muscle atrophy (13,14).
Anamorelin is indicated for cancer cachexia management in patients
with non-small cell lung, gastric, pancreatic, and colorectal
cancers. Notably, its use necessitates caution owing to potential
adverse events, including fatal arrhythmias and hyperglycemia
(15).
Considering that anamorelin is a Japan-specific
medicine, real-world data on its use remain limited. Furthermore,
although early intervention is recommended for cancer cachexia,
research on the optimal timing of anamorelin administration is
scarce, highlighting the need for further investigation. Therefore,
the aim of this retrospective study was to investigate the use of
anamorelin in cancer cachexia treatment and examine the
relationship between treatment duration and patient survival.
Patients and methods
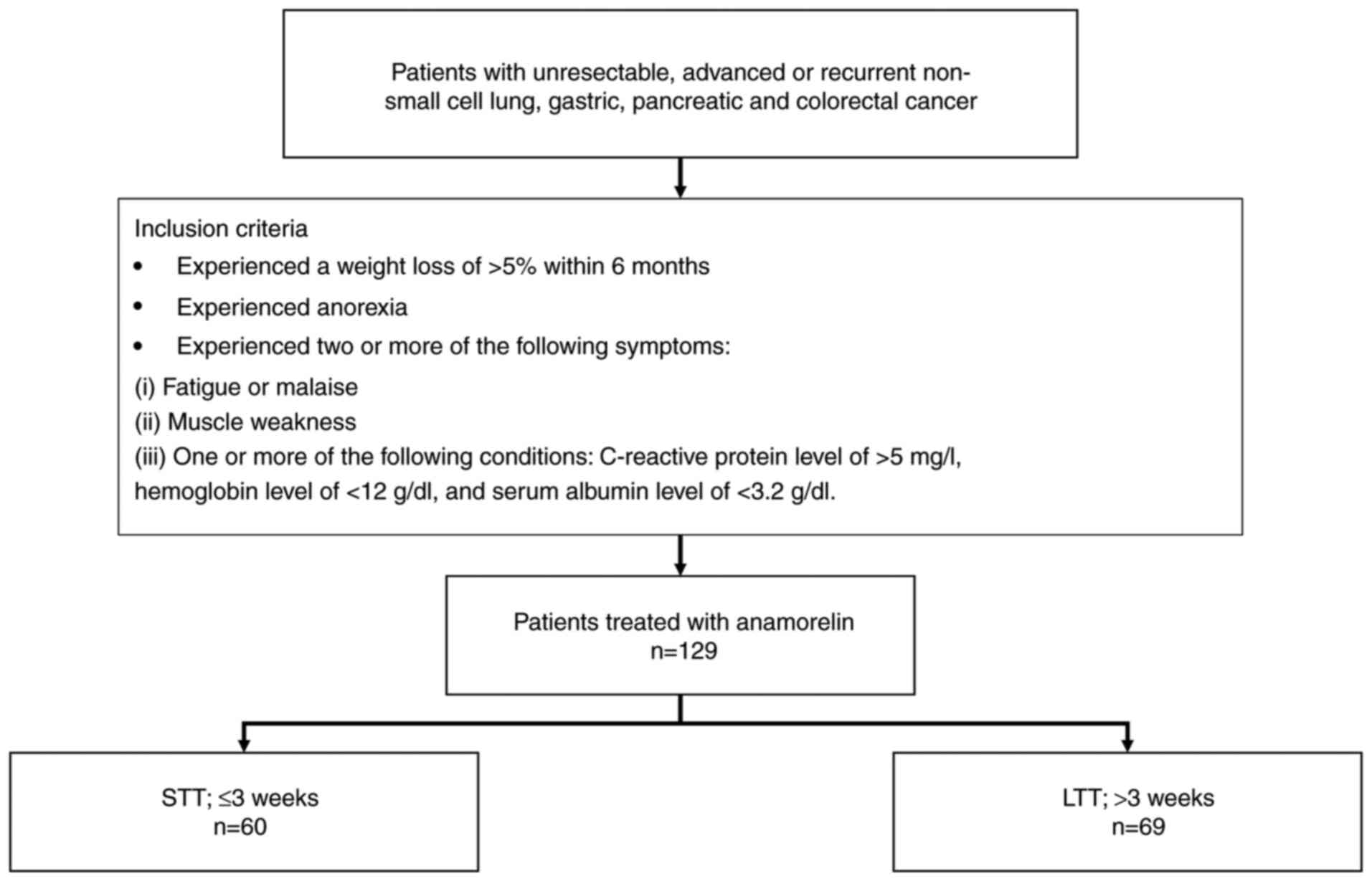
Study design and end point
This retrospective study included patients who were
prescribed anamorelin between August 2021 and January 2024 at the
Saitama Cancer Center, Japan. Eligible patients had unresectable,
advanced, or recurrent non-small cell lung, gastric, pancreatic, or
colorectal cancers with cachexia, defined by >5% weight loss and
anorexia within 6 months, along with at least two of the following
symptoms: i) fatigue or malaise; ii) muscle weakness; and iii) one
or more of the following conditions: C-reactive protein (CRP) level
>5 mg/l, hemoglobin level <12 g/dl, and serum albumin level
<3.2 g/dl. The inclusion criteria align with the clinical
indications for prescribing anamorelin. Inclusion criteria were
retrospectively assessed using electronic medical records,
including documented weight loss percentages, clinical notes on
anorexia, and laboratory test results for cachexia-related symptoms
(e.g., CRP, hemoglobin, and serum albumin levels). This review
ensured consistent application of the eligibility criteria.
Fig. 1 depicts the study
flowchart. Because the anamorelin package insert states that its
efficacy is determined in the third week of administration,
patients were classified into two groups: short-term treatment
(STT; discontinuation within 3 weeks) and long-term treatment (LTT;
>3 weeks). The primary endpoint was to evaluate the effect of
treatment duration on the survival of patients with cancer
cachexia. The secondary endpoint was to investigate the time from
diagnosis to treatment initiation for each type of cancer and the
duration of anamorelin administration.
Data extraction
The following baseline patient characteristics at
the initiation of anamorelin treatment were extracted from the
patient medical records: age; sex; primary cancer site; Eastern
Cooperative Oncology Group (ECOG) performance status (PS) score;
albumin, CRP, and hemoglobin levels; Glasgow Prognostic Score
(GPS); date of diagnosis of stage IV, inoperable advanced, or
recurrent cancer; history of surgery for gastric cancer; reason for
discontinuation of anamorelin; start and end dates of anamorelin
treatment; reason for completion of anamorelin treatment; and date
of death or last hospital visit. The ECOG PS 0-2 classification,
which was previously used as a selection criterion in national and
international phase III trials, was used as the standard in this
study (16). The GPS was
calculated using the following formula: CRP level <10 mg/l and
albumin level ≥3.5 g/dl, GPS=0; CRP level >10 mg/l, GPS=1; and
CRP level >10 mg/l and albumin level <3.5 g/dl,
GPS=2(17).
Reasons for discontinuation of anamorelin were
categorized as treatment ineffectiveness, difficulty in medication
intake, psychological burden of medicine intake, death, and other
reasons. Treatment ineffectiveness was determined clinically,
primarily on weight changes and patient-reported symptoms, such as
persistent anorexia, despite continued treatment. Difficulty in
medication intake was identified from medical records describing
swallowing difficulties (dysphagia), nausea, or a general decline
in physical condition hindering oral intake. The psychological
burden of taking the medicine was assessed through medical records
of patient-reported concerns, including reluctance to take oral
medication owing to anxiety or subjective distress related to
medication intake.
Statistical analysis
The Fisher's exact test was used to compare nominal
variables, whereas the Mann-Whitney U test was used to
compare continuous variables. Outliers were defined as values
exceeding the third quartile by more than 1.5 times the
interquartile range. Overall survival curves were generated using
the Kaplan-Meier method, and differences in the curves were
compared using a log-rank test. Patients were censored if they met
one of the following criteria: i) overall survival of >365 days
or ii) data not traceable owing to other reasons, including
hospital transfer. Statistical analyses were performed using EZR
ver. 1.66(18), with a P-value of
<0.05 considered statistically significant.
Results
Patient characteristics
In total, 129 patients were included in this study,
with 60 and 69 patients in the STT and LTT groups, respectively
(Table I). Compared with the LTT
group, the STT group had significantly more patients with gastric
cancer (P=0.013) or an albumin level of <3.5 g/dl (P=0.044). In
contrast, the LTT group had a significantly higher number of older
patients (P=0.021) and those with an ECOG PS of 0-2 (P=0.008) than
the STT group. No significant differences were observed between the
two groups with respect to other patient characteristics. A
comparison of the number of patients who underwent surgery for
gastric cancer showed no significant differences between the groups
(STT group: 23.1% vs. LTT group: 20.0%, P=1.0). The most common
reason for termination of anamorelin treatment in both groups was
treatment ineffectiveness (STT group, 33.3%; LTT group, 21.7%). In
the STT group alone, the reasons for termination were difficulty in
medication intake (23.3%) and the psychological burden of
medication intake (11.7%), while in the LTT group, the reasons were
death (13.0%) and the psychological medication intake (10.1%).
Among patients in the STT group who discontinued owing to
difficulty in medication intake, the primary factors were
dysphagia, nausea, and general deterioration in physical condition.
Importantly, no discontinuations were attributed to side effects of
anamorelin.
 | Table ICharacteristics of the study
patients. |
Table I
Characteristics of the study
patients.
| Characteristics | STT group (n=60) | LTT group (n=69) | P-value |
|---|
| Median age, years
(range)a | 71 (36-84) | 73 (50-90) | 0.021 |
| Male, n
(%)b | 40 (66.7) | 52 (75.4) | 0.331 |
| Primary cancer, n
(%)b | | | |
|
Gastric
cancer | 26 (43.3) | 15 (21.7) | 0.013 |
|
Colorectal
cancer | 5 (8.3) | 6 (8.7) | >0.999 |
|
Pancreatic
cancer | 14 (23.3) | 22 (31.9) | 0.328 |
|
NSCLC | 15 (25.0) | 26 (37.7) | 0.134 |
| ECOG PS score, n
(%)b | | | |
|
0-2 | 39 (65.0) | 59 (85.5) | 0.008 |
|
3-4 | 21 (35.0) | 10 (14.5) | 0.008 |
| Median serum albumin
level, g/dl (range)a | 3.1 (2.0-4.7) | 3.4 (1.5-4.8) | 0.095 |
| Serum albumin level
<3.5 g/dl, n (%)b | 43 (71.7) | 36 (52.2) | 0.044 |
| Median C-reactive
protein level, mg/l (range)a | 20.7 (0.1-305.2) | 14.7 (0-257.9) | 0.227 |
| C-reactive protein
level, n (%)b | | | |
|
>10
mg/l | 39 (65.0) | 39 (58.0) | 0.468 |
|
>5
mg/l | 46 (76.7) | 45 (65.2) | 0.242 |
| Median hemoglobin
level, g/dl (range)a | 11.0 (7.2-15.2) | 10.4 (7.7-15.7) | 0.404 |
| Hemoglobin level
<12.0 g/dl, n (%)b | 41 (68.3) | 52 (75.4) | 0.326 |
| Glasgow prognostic
scorec, n
(%)b | | | |
|
0 | 21 (35.0) | 29 (42.0) | 0.468 |
|
1 | 8 (13.3) | 14 (20.3) | 0.350 |
|
2 | 31 (51.7) | 25 (36.2) | 0.109 |
| Median time from
diagnosis to treatment initiation, days (range)a | 279 (0-2486) | 193 (0-3122) | 0.286 |
| Median administration
period, days (range)a | 9.5 (0-19) | 44.5 (21-444) | <0.0001 |
Timing of anamorelin initiation and
duration of treatment
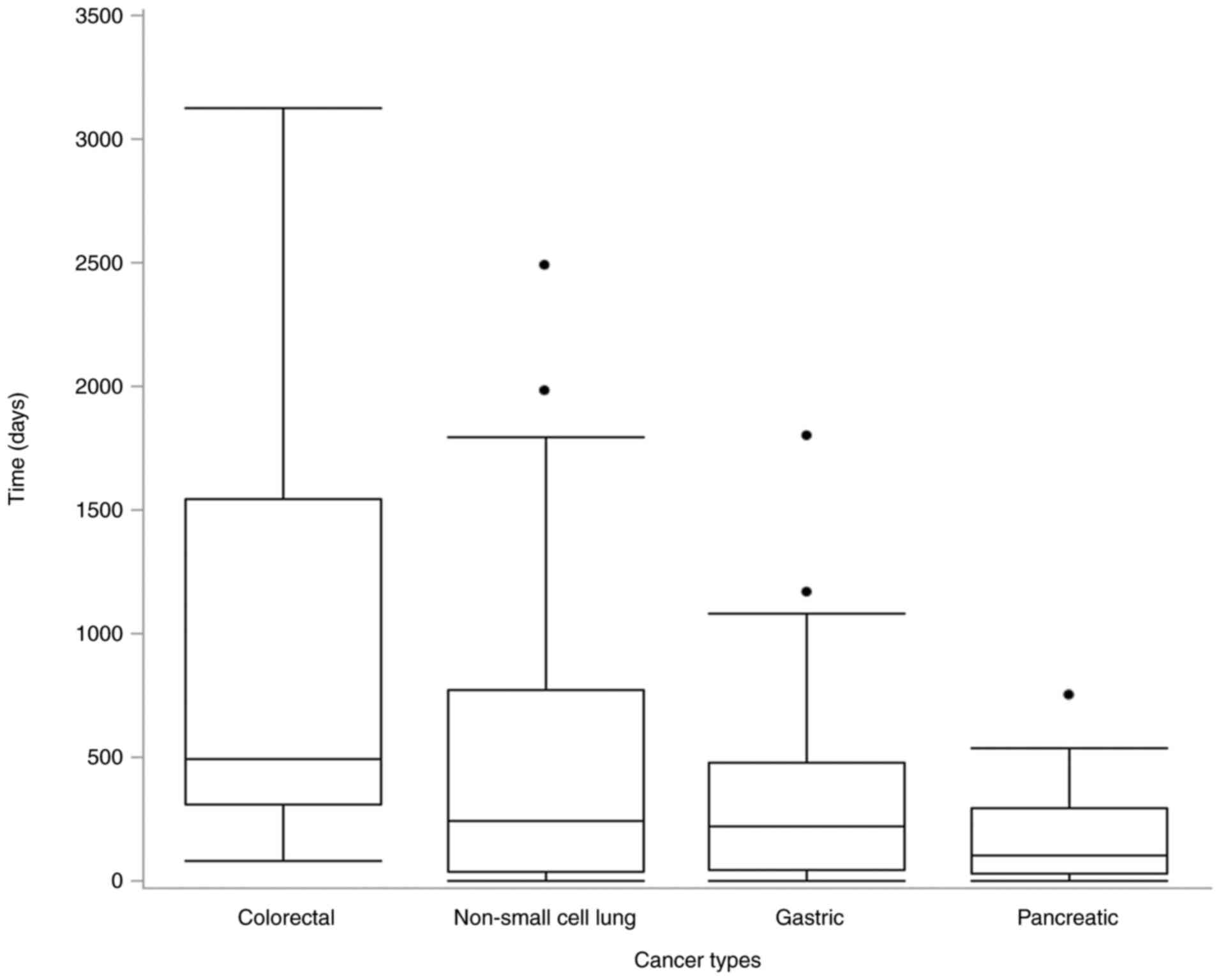
Fig. 2 shows the
time between diagnosis and anamorelin treatment initiation. The
median time from the date of diagnosis to the start of anamorelin
treatment was the longest among patients with colorectal cancer
(489 days), followed by those with non-small cell lung cancer (244
days), gastric cancer (222 days), and pancreatic cancer (99 days).
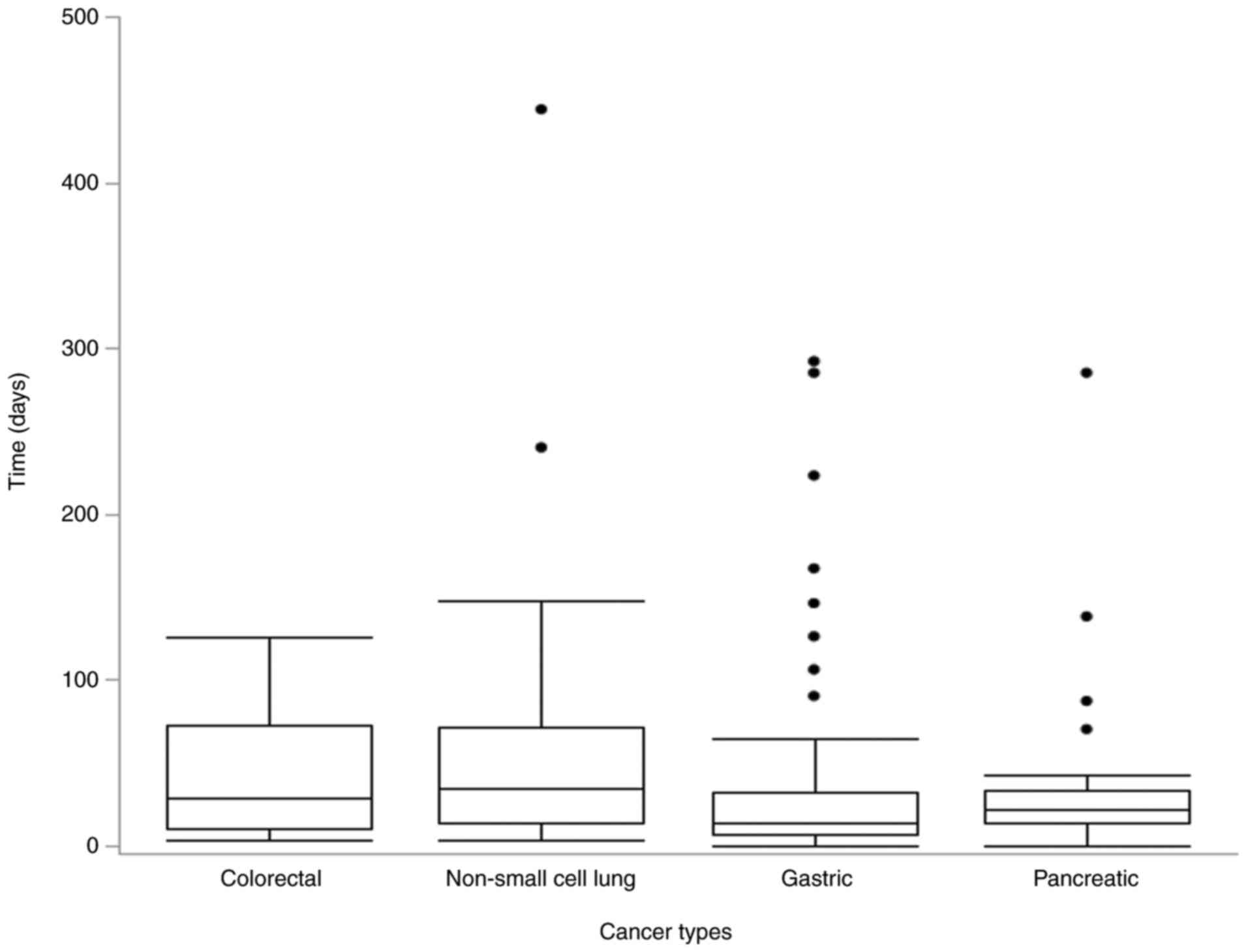
The anamorelin treatment period is shown in Fig. 3. The median duration of anamorelin
treatment was the longest for lung cancer (34 days), followed by
that for colon cancer (28 days), pancreatic cancer (22 days), and
gastric cancer (14 days). Three cases with non-small cell lung
cancer (treatment durations: 240, 444, and 773 days), eight with
stomach cancer (treatment durations: 90, 106, 126, 146, 167, 223,
285, and 292 days), and four with pancreatic cancer (treatment
durations: 70, 87, 138, and 285 days) were analyzed as
outliers.
Period from anamorelin treatment
initiation to death or last hospital visit
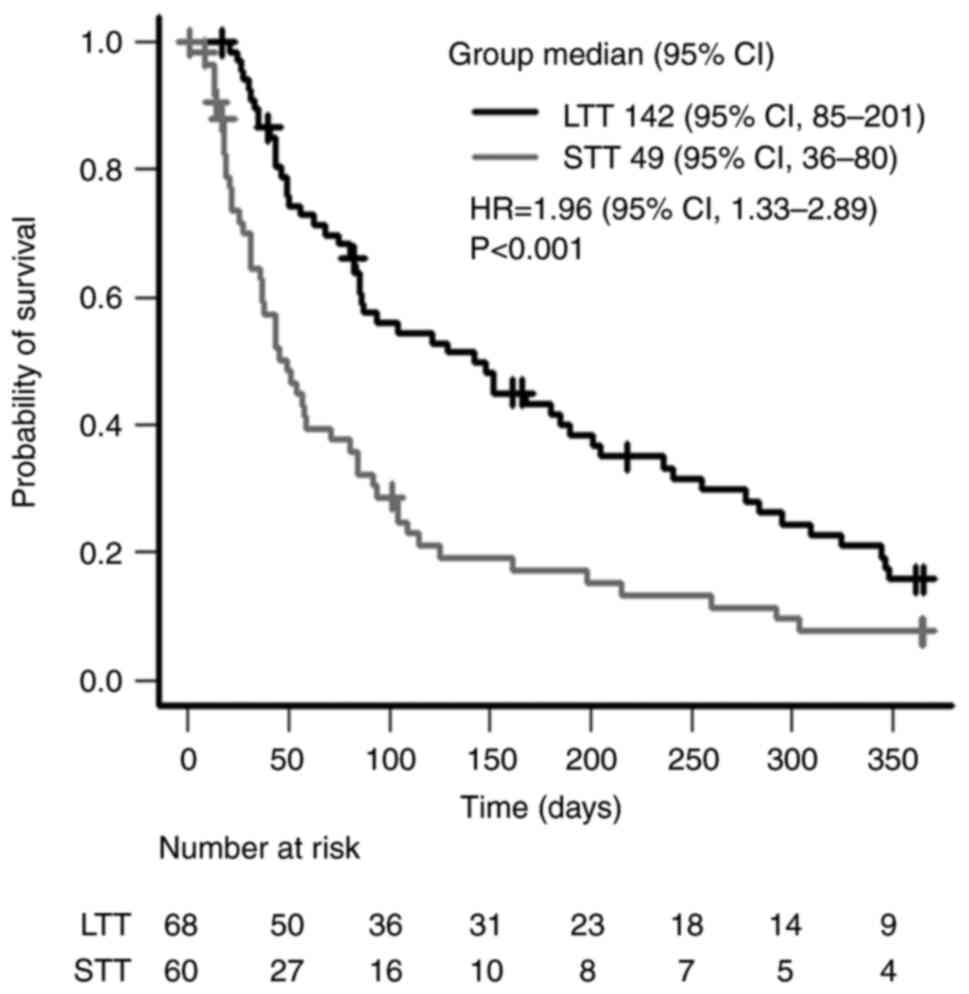
The overall survival rate from the start of
anamorelin treatment to death or last hospital visit is shown in
Fig. 4. The median survival
durations were 49 days [95% confidence interval (CI), 36-80] and
142 days (95% CI, 85-201) in the STT and LTT groups, respectively,
with the duration in the LTT group being significantly longer
(P<0.001). The data of a patient in the LTT group were excluded
from the analysis as the treatment end date was unknown.
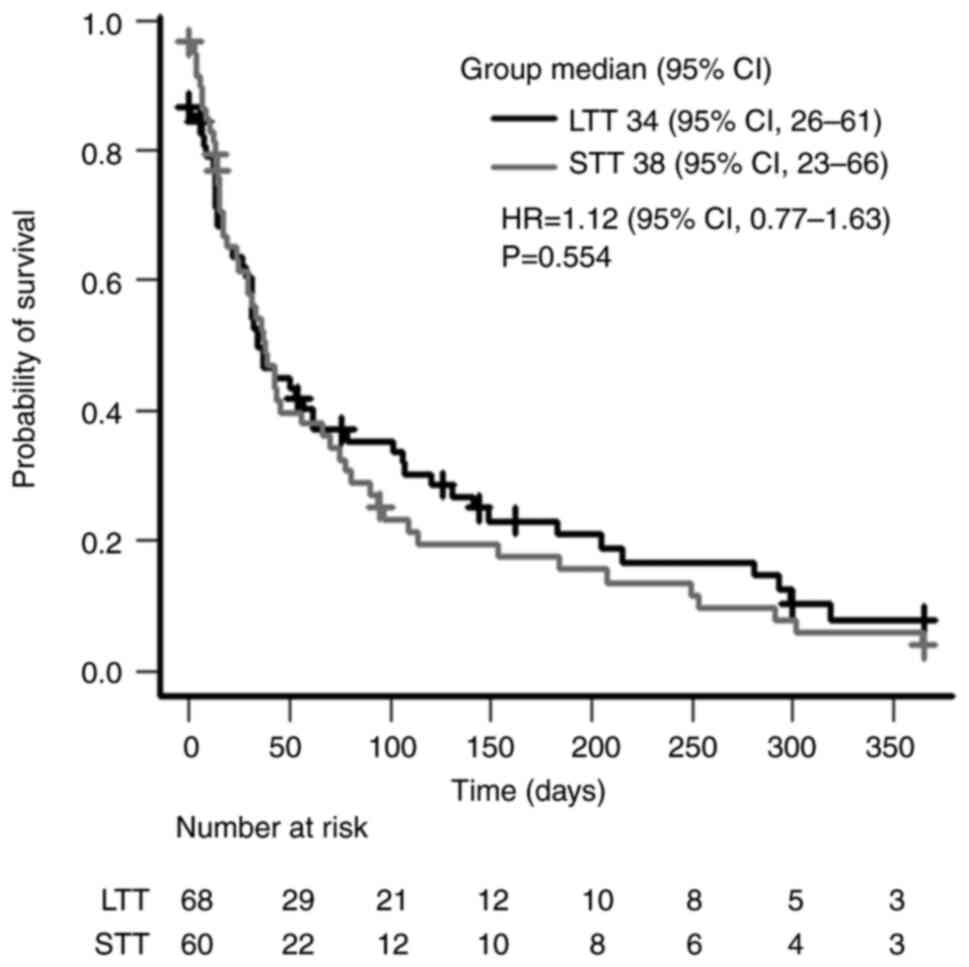
Survival after completion of
anamorelin treatment
The overall survival rate was defined as the number
of days from the day after the last day of anamorelin treatment to
the day of death or last hospital visit (Fig. 5). The median survival durations
were 38 days (95% CI, 23-66 days) and 34 days (95% CI, 26-61 days)
in the STT and LTT groups, respectively (P=0.554). The data of a
patient in the LTT group were excluded from the analysis as the
treatment end date was unknown.
Discussion
In this retrospective study, the use of anamorelin
in managing cancer cachexia was examined, focusing on patient
characteristics and survival during and after treatment. Age,
gastric cancer, hypoalbuminemia, and ECOG PS score were identified
as patient factors affecting treatment duration. While patients in
the LTT group had significantly longer survival duration during
treatment than those in the STT group, post-discontinuation
survival did not differ significantly among groups, regardless of
treatment duration. These findings suggested that anamorelin
treatment duration can be a predictor of survival during
treatment.
In this study, anamorelin was administered long term
to patients with an ECOG PS score of 0-2. Our findings align with
those of studies examining the efficacy of anamorelin in patients
with pancreatic cancer with a poor ECOG PS score and studies
investigating factors associated with the early discontinuation of
anamorelin (19,20). This result suggests that patients
with good ECOG PS scores may tolerate long-term anamorelin
treatment. In this study, the LTT group had a higher number of
older patients, possibly owing to the role of anamorelin in
increasing sensitivity to ghrelin, which declines with age and may
activate relevant signaling pathways. Blood ghrelin levels
generally decrease with age; however, the relationship between
ghrelin and aging remains unclear, as existing studies have yielded
conflicting results, warranting further investigation (21). Regarding cancer type, the STT group
had a higher number of patients with gastric cancer. Ghrelin is a
peptide hormone produced and secreted mainly in the gastric body.
Total gastrectomy reduces blood ghrelin levels by 10-30% and distal
gastrectomy decreases these levels by 50-70% (22). In the present study, the surgical
history of patients with gastric cancer was compared between the
two groups; however, no effect of surgery on treatment duration was
observed. This result suggests that factors beyond gastric
preservation may have affected the duration of anamorelin
treatment; however, further studies with a larger sample size are
warranted to validate this. In addition, hypoalbuminemia was more
common in the STT group, suggesting its potential as a predictive
factor for treatment duration. Given that hypoalbuminemia is
associated with reduced survival rate of patients with gastric
cancer, it may contribute to considerably shorter treatment
duration observed in this patient population (23). Further investigation into the
interplay between cancer type and hypoalbuminemia using larger
cohorts is warranted.
The median treatment duration tended to be shorter
for non-small cell lung, colorectal, pancreatic, and gastric
cancers, in that order. The results, except that of gastric cancer,
were similar to those of the interim analysis of the post-marketing
survey for anamorelin. Notably, the median treatment duration for
gastric cancer was 14 days shorter in this study than the 29 days
reported in the post-marketing survey (24). In addition, gastric cancer was
associated with a high frequency of abnormal values compared with
other cancer types, suggesting that additional factors may
influence the duration of anamorelin administration in this
context. Further detailed studies are warranted to investigate
this.
Survival during anamorelin treatment was
significantly shorter in the STT group than in the LTT group.
Similar results were reported in a study involving a small number
of cases (20). In this study, the
reason for treatment discontinuation was also investigated,
revealing that more patients in the STT group discontinued
treatment because of medication intake difficulty. Therefore, it is
recommended that anamorelin be prescribed to patients with a good
ECOG PS score who can tolerate prolonged treatment. We found that
the median survival duration after the completion of anamorelin
treatment was approximately 1 month, regardless of the treatment
duration. To the best of our knowledge, this is a novel finding.
The predicted survival time for refractory cachexia is generally
considered to be less than 3 months (3). However, this period has been
established by experts as an empirical guideline and is not based
on real-world data. Moreover, our study is the first to report
survival after the discontinuation of anamorelin for cachexia.
Based on our findings, determining whether anamorelin can be
effective against refractory cachexia or whether the predicted
survival period has been overestimated remains a subject for future
research.
This study had several limitations. First, this was
a single-center, retrospective, observational study conducted among
Japanese patients, which may limit the generalizability and
validity of its findings to a broader population. Second,
limitations inherent to retrospective studies, such as selection
and reporting bias cannot be ruled out. Third, the observed
prolonged survival in the LTT group may have been influenced by
immortal time bias, which is an inherent limitation of the study
design. Although time-dependent analyses or sensitivity analyses
could potentially address this issue, such approaches were not
feasible in the present study owing to the retrospective nature of
the data and limitations in the dataset structure. Specifically,
making multiple assumptions regarding variable selection and the
handling of missing data was difficult. Furthermore, prior studies
have provided limited evidence regarding causal relationships in
this context. Therefore, we decided not to perform sensitivity
analyses, as they were unlikely to improve the robustness or
reliability of our findings. We acknowledge this as a
methodological limitation, and future studies should consider more
refined analytical strategies to minimize the impact of immortal
time bias. Fourth, patient characteristics and prognosis differ
according to cancer type. Therefore, a cancer type-specific
analysis would have been preferable. However, in this study,
stratified analysis was challenging owing to statistical
limitations owing to the limited number of patients with each
cancer type. Additionally, although factors such as patient age,
presence of gastric cancer, PS, and serum albumin levels may
independently affect survival, considering the scope of the study,
adjustments using propensity score matching or subgroup analysis
were not feasible owing to the small sample size. Fifth, prognostic
factors such as exercise therapy, nutritional support, and
chemotherapy were not examined in this study. Hence, future studies
addressing these limitations are warranted. Sixth, the
applicability of our findings may be influenced by cultural,
healthcare, and demographic differences. Variations in dietary
habits, patient preferences, and perceptions of appetite loss could
affect treatment adherence and efficacy. Differences in healthcare
systems, including access to supportive care and nutritional
interventions, may also impact real-world outcomes. Additionally,
racial differences in drug metabolism and cachexia progression
could influence treatment response. Finally, anamorelin is
currently approved only in Japan, making it a drug unique to the
Japanese healthcare system. Its clinical use remains geographically
limited, and its applicability to other healthcare settings is
unclear. Given these limitations, further studies are warranted to
evaluate its effectiveness in diverse patient populations and
different healthcare environments.
Notably, anamorelin is only approved in Japan,
limiting its clinical applicability to other healthcare settings.
To the best of our knowledge, no single-center study with a larger
number of patients has been conducted, making our findings an
important source of clinical information. Nevertheless, further
investigation through a larger multicenter prospective study is
warranted.
In conclusion, the study findings indicated that
older patients with better ECOG PS scores exhibited a longer
treatment duration, whereas those with gastric cancer and
hypoalbuminemia had a shorter treatment duration. In addition, the
duration of anamorelin treatment was reflected in the survival of
patients; however, the main novel finding of this study was that
the survival time after treatment discontinuation remained
approximately one month, regardless of the length of treatment.
Considering the limited information on the efficacy and safety of
anamorelin, future multicenter prospective studies with relatively
larger sample sizes are warranted.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MoS was the principal investigator. MiS, DT, TA, KT,
TN and MoS planned the study protocol and performed data
collection. MH, MiS and MoS performed data analysis. MiS and MoS
wrote the first draft of the manuscript. MiS, MO and MoS supervised
the study, contributed to the interpretation of data and approved
the final draft. All authors commented on the previous versions of
the manuscript. TA, KT, TN and MO confirmed the authenticity of the
raw data. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board (IRB) of Saitama Cancer Center (IRB no. 1398; Ina,
Japan), and all procedures were performed in accordance with The
Declaration of Helsinki. The requirement for informed consent was
waived by the IRB due to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Gullett N, Rossi P, Kucuk O and Johnstone
PAS: Cancer-induced cachexia: A guide for the oncologist. J Soc
Integr Oncol. 7:155–169. 2009.PubMed/NCBI
|
|
2
|
Argilés JM, Busquets S, Stemmler B and
López-Soriano FJ: Cancer cachexia: Understanding the molecular
basis. Nat Rev Cancer. 14:754–762. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Arends J, Strasser F, Gonella S, Solheim
TS, Madeddu C, Ravasco P, Buonaccorso L, De Van Der Schueren MA,
Baldwin C, Chasen M, et al: Cancer cachexia in adult patients: ESMO
clinical practice guidelines*. ESMO Open. 6(100092)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Argilés JM, Moore-Carrasco R, Busquets S
and López-Soriano FJ: Catabolic mediators as targets for cancer
cachexia. Drug Discov Today. 8:838–844. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Argilés JM, Busquets S and López-Soriano
FJ: Cytokines as mediators and targets for cancer cachexia. Cancer
Treat Res. 130:199–217. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Japanese Society for Palliative Medicine:
Clinical guidelines for infusion therapy in advanced cancer
patients. KANEHARA & Co., Ltd., Tokyo, 2013.
|
|
8
|
Argilés JM, Busquets S and López-Soriano
FJ: Cancer cachexia, a clinical challenge. Curr Opin Oncol.
31:286–290. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nakanishi Y, Higuchi J, Honda N and Komura
N: Pharmacological profile and clinical efficacy of anamorelin HCl
(ADLUMIZ®Tablets), the first orally available drug for
cancer cachexia with ghrelin-like action in Japan. Nihon Yakurigaku
Zasshi. 156:370–381. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-releasing
acylated peptide from stomach. Nature. 402:656–660. 1999.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Chen HY, Trumbauer ME, Chen AS, Weingarth
DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM,
et al: Orexigenic action of peripheral ghrelin is mediated by
neuropeptide Y and agouti-related protein. Endocrinology.
145:2607–2612. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Velloso CP: Regulation of muscle mass by
growth hormone and IGF-I. Br J Pharmacol. 154:557–568.
2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li WG, Gavrila D, Liu X, Wang L,
Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C and
Weintraub NL: Ghrelin inhibits proinflammatory responses and
nuclear factor-kappaB activation in human endothelial cells.
Circulation. 109:2221–2226. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen JA, Splenser A, Guillory B, Luo J,
Mendiratta M, Belinova B, Halder T, Zhang G, Li YP and Garcia JM:
Ghrelin prevents tumour- and cisplatin-induced muscle wasting:
Characterization of multiple mechanisms involved. J Cachexia
Sarcopenia Muscle. 6:132–143. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wakabayashi H, Arai H and Inui A:
Anamorelin in Japanese patients with cancer cachexia: An update.
Curr Opin Support Palliat Care. 17:162–167. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Naito T, Uchino J, Kojima T, Matano Y,
Minato K, Tanaka K, Mizukami T, Atagi S, Higashiguchi T, Muro K, et
al: A multicenter, open-label, single-arm study of anamorelin
(ONO-7643) in patients with cancer cachexia and low body mass
index. Cancer. 128:2025–2035. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
de Paula Pantano N, Paiva BSR, Hui D and
Paiva CE: Validation of the modified Glasgow prognostic score in
advanced cancer patients receiving palliative care. J Pain Symptom
Manage. 51:270–277. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takeda T, Sasaki T, Okamoto T, Fukuda K,
Hirai T, Yamada M, Nakagawa H, Mie T, Furukawa T, Kasuga A, et al:
Efficacy of amamorelin in advanced pancreatic cancer patients with
a poor performance status. Intern Med. 64:351–358. 2025.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tsukiyama I, Iwata T, Takeuchi T, Kato RI,
Sakuma M, Tsukiyama S, Kato M, Ikeda Y, Ohashi W, Kubo A, et al:
Factors associated with early discontinuation of anamorelin in
patients with cancer-associated cachexia. Support Care Cancer.
31(621)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yin Y and Zhang W: The role of ghrelin in
senescence: A mini-review. Gerontology. 62:155–162. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Takiguchi S, Takata A, Murakami K,
Miyazaki Y, Yanagimoto Y, Kurokawa Y, Takahashi T, Mori M and Doki
Y: Clinical application of ghrelin administration for gastric
cancer patients undergoing gastrectomy. Gastric Cancer. 17:200–205.
2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ouyang X, Dang Y, Zhang F and Huang Q: Low
serum albumin correlates with poor survival in gastric cancer
patients. Clin Lab. 64:239–245. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Takayama K, Kojima A, Honda C, Nakayama M,
Kanemata S, Endo T and Muro K: Real-world safety and effectiveness
of anamorelin for cancer cachexia: Interim analysis of
post-marketing surveillance in Japan. Cancer Med.
13(e7170)2024.PubMed/NCBI View Article : Google Scholar
|