Introduction
There is a worldwide increase in the incidence of
colorectal cancer and diverticulosis on the left side of the colon.
Hartmann's procedure (HP) is a surgery used to reduce the risk of
postoperative complications in patients undergoing surgery for
left-sided colonic benign disease, patients with colorectal cancer
who have oncologic emergencies, and those with poor general
condition (frail or older adults) (1-3).
Although HP remains an important surgical option, the achievement
of stoma reversal is significantly lower than that of stoma closure
after primary anastomosis (4). A
permanent stoma significantly impairs patient quality of life and
increases medical costs. Furthermore, previous studies have
demonstrated that the achievement of stoma reversal is
significantly different between benign and malignant diseases
(5,6). The reason for this may be that the
long-term course after stoma reversal varies depending on the
underlying disease conditions.
Colostomy reversal after HP is considered a
challenging surgery with a high risk of anastomotic complications
and mortality (7,8). However, even in successful reversal
cases in which the patient is stoma-free, several complications,
such as cancer recurrence or death, can occur during follow-up,
particularly in case of malignant diseases. Only a few studies have
examined the long-term outcomes after reversal (9,10).
To the best of our knowledge, no study has investigated long-term
outcomes after reversal in benign and malignant diseases.
Therefore, in this study, we aimed to compare the
achievement of stoma reversal and examine the long-term outcomes
after reversal between benign and malignant disease groups in a
cohort from a single institution.
Patients and methods
Study design and patient
population
This retrospective observational study was conducted
at the Hakodate Municipal Hospital (Hakodate, Japan). The study and
manuscript adheres to the STROBE guidelines for observational
studies. Patients who underwent HP for any disease between January
2005 and December 2021 in our hospital were included. HP was
defined as the removal of a damaged colonic segment with the
abandonment of the sutured distal colon stump and creation of an
end colostomy in the upstream colonic segment (3). We excluded patients who underwent HP
for gynecological or recurrent malignant diseases (because there
were no cases of colostomy reversal) and died during
hospitalization after HP. The patients were classified into benign
and malignant disease groups according to the disease for which HP
was indicated. In our department, both colorectal and general
surgeons performed HP, and indications for colostomy reversal after
HP were good patient condition and no major pelvic complications
after HP (such as dehiscence of the rectal stump). The following
situations were also considered in cases of malignant disease: no
disease recurrence for at least 6 months from HP after adjuvant
chemotherapy in patients who underwent curative cancer surgery, and
no disease for which systemic treatment was being received in
patients with distant metastasis. After HP, patients with malignant
disease were regularly followed up for treatment or examination.
Conversely, patients with benign disease were followed up by
irregular visits alone for various reasons (including visit for any
other disease and visit by emergency) or were contacted during the
anorectal function survey.
Study endpoints
The primary endpoint was the difference in
stoma-reversal rate between benign and malignant diseases. The
secondary endpoints were predictive factors for the achievement of
stoma reversal and stoma-free survival (SFS) and anorectal function
after reversal in patients with benign and malignant diseases.
Data collection and assessments
Data on patient characteristics collected from the
medical records included age, sex, body mass index (BMI), American
Society of Anesthesiologists physical status (ASA-PS), Charlson
comorbidity index (CCI), indication for HP, type of surgery for HP,
surgical approach for HP, adjacent organ resection during HP,
length of residual stump, postoperative complications
(intra-abdominal abscess and dehiscence of residual stump), and
discharge location. Surgical outcomes of stoma reversal included
the surgical approach, anastomotic method, and diverting loop
ileostomy. Data on postoperative complications and mortality rates
were also collected. The calculation of CCI has been previously
reported (11). The CCI cut-off
values for predicting stoma reversal were determined based on the
receiver operating characteristic curves in the benign disease and
malignant disease groups separately. The CCI was classified into
low and high scores according to the cut-off values (4 and 6 points
in benign and malignant diseases, respectively). Residual stumps
were classified as located above the sacral promontory, between the
sacral promontory and peritoneal reflection, and below the
peritoneal reflection. Postoperative complications were assessed
using the Clavien-Dindo classification (12). The observational period was
calculated from HP or stoma reversal to the last follow-up date.
The SFS rate was measured based on the time from reversal.
Anorectal function was evaluated before HP (pre-HP)
and after reversal (post-reversal) using the low anterior resection
syndrome (LARS) score, a categorical scoring system. The patients'
LARS scores were graded into three categories: No LARS (LARS score
<20), minor LARS (LARS score between 21 and 29) and major LARS
(LARS score >20). The survey targeted patients who were alive in
September 2022. Pre-HP function was retraced and assessed at the
time of the survey.
Statistical analysis
Categorical variables are summarized as frequencies
and percentages. Continuous variables are summarized as mean ±
standard deviation or median (interquartile range). Between-group
comparisons were performed using Welch's t-test for continuous
variables or Mann-Whitney U test for categorical variables. In
addition, Fisher's exact test was used to compare the frequencies
of categorical variables. Comparisons of two measurements between
pre-HP and post-reversal functions from the same patient were
performed using Wilcoxon signed rank test. Comparisons between the
three groups were performed using Kruskal-Wallis rank sum test. The
cumulative stoma-reversal rate was estimated and compared between
the groups using the Gray test. Predictive factors for stoma
reversal were analyzed using univariate and multivariate analyses
with the Fine-Gray regression model. Variables with P<0.05 in
the univariate analysis were entered into the multivariate analysis
as covariates. The SFS was calculated using the Kaplan-Meier method
and compared between the groups using the log-rank test. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using EZR (version 1.61;
Saitama Medical Center, Jichi Medical University, Saitama, Japan),
a graphical user interface for R (version 4.2.2; The R Foundation
for Statistical Computing, Vienna, Austria) and a modified version
of R Commander (version 2.8-0) designed to add statistical
functions frequently used in biostatistics (13).
Results
Patient flow and characteristics
between the benign and malignant disease groups
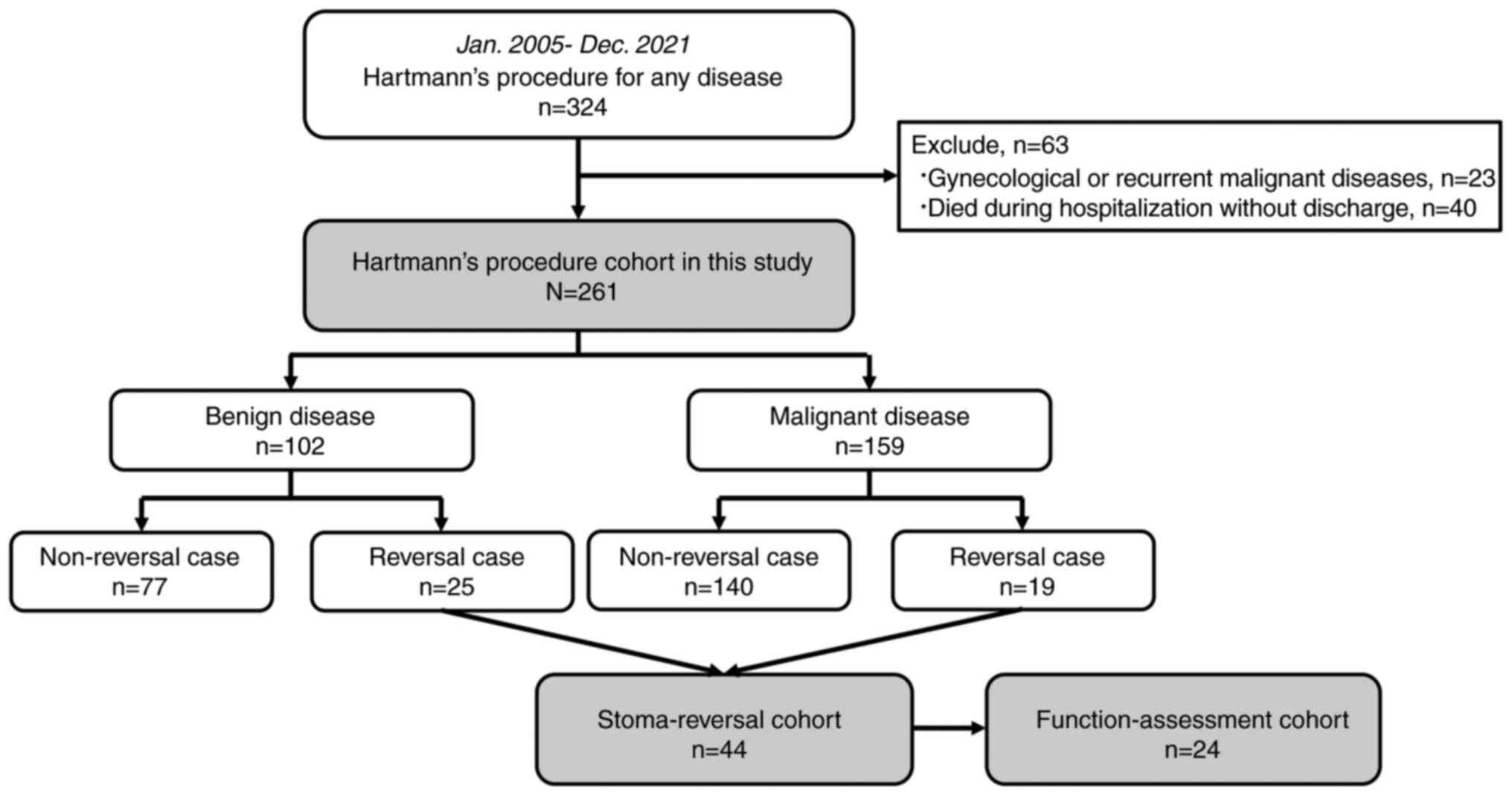
A total of 324 patients who underwent HP for any
disease between January 2005 and December 2021 were eligible for
the study. However, 23 patients who underwent HP for gynecological
or recurrent malignant diseases and 40 who died during
hospitalization were excluded. Eventually, 261 patients were
included in this study. Among these, 102 and 159 patients were
included in the benign disease and malignant disease groups,
respectively. The stoma reversal was performed in 44 patients
(16.9%). Among them, 24 (77.4%) of the 31 survivors in September
2022 underwent assessments of anorectal function. The patient
flowchart is shown in Fig. 1. The
characteristics of the 261 patients are presented in Table I. There were significant
differences in sex, ASA-PS, type of surgery for HP, surgical
approach for HP, adjacent organs resection during HP, length of the
residual stump, and discharge location between the benign and
malignant disease groups.
 | Table IComparison of patient characteristics
between the benign and malignant disease groups. |
Table I
Comparison of patient characteristics
between the benign and malignant disease groups.
| Characteristic | Hartmann's procedure
cohort (n=261) | Benign disease
(n=102) | Malignant disease
(n=159) | P-value (benign
disease vs. malignant disease) |
|---|
| Mean age, years
(SD) | 72.9 (12.3) | 74.0 (13.3) | 72.2 (11.6) | 0.249 |
| Sex, n (%) | | | | 0.043 |
|
Male | 141 (54.0) | 47 (46.1) | 94 (59.1) | |
|
Female | 120 (46.0) | 55 (53.9) | 65 (40.9) | |
| Mean BMI,
kg/m2 (SD) | 21.2 (4.0) | 21.4 (4.3) | 21.1 (3.7) | 0.580 |
| No data for BMI, n
(%) | 2 (0.8) | 1 (1.0) | 1 (0.6) | |
| ASA-PS, n (%) | | | | <0.001 |
|
ASA-I | 10 (3.8) | 3 (2.9) | 7 (4.4) | |
|
ASA-II | 84 (32.2) | 14 (13.7) | 70 (44.0) | |
|
ASA-III | 131 (50.2) | 62 (60.8) | 69 (43.4) | |
|
ASA-IV | 32 (12.3) | 19 (18.6) | 13 (8.2) | |
|
ASA-V | 1 (0.4) | 1 (1.0) | 0 (0) | |
|
No data | 3 (1.2) | 3 (2.9) | 0 (0) | |
| Median Charlson
comorbidity indexa
(IQR) | 7 (5-9) | 5 (4-6) | 8 (6-10) | <0.001 |
|
Low score, n
(%)b | 81 (31.0) | 34 (33.3) | 47 (29.6) | 0.584 |
|
High score,
n (%)c | 180 (69.0) | 68 (66.7) | 112 (70.4) | |
| Type of surgery for
HP, n (%) | | | | <0.001 |
|
Elective | 118 (45.2) | 10 (9.8) | 108 (67.9) | |
|
Urgent | 143 (54.8) | 92 (90.2) | 51 (32.1) | |
| Surgical approach
for HP, n (%) | | | | 0.024 |
|
Open | 144 (55.2) | 63 (61.8) | 81 (50.9) | |
|
Laparoscopic | 99 (37.9) | 29 (28.4) | 70 (44.0) | |
|
Conversion | 18 (6.9) | 10 (9.8) | 8 (5.0) | |
| Adjacent organs
resection during HP, n (%) | 65 (24.9) | 6 (5.9) | 59 (37.1) | <0.001 |
| Length of residual
stump, n (%) | | | | <0.001 |
|
Above sacral
promontory | 26 (10.0) | 18 (17.6) | 8 (5.0) | |
|
Between
sacral promontory and peritoneal reflection | 203 (77.8) | 79 (77.5) | 124 (78.0) | |
|
Below
peritoneal reflection | 32 (12.3) | 5 (4.9) | 27 (17.0) | |
| Postoperative
complication, n (%) | | | | |
|
Intra-abdominal
abscess | 20 (7.7) | 11 (10.8) | 9 (5.7) | 0.154 |
|
Dehiscense | 8 (3.1) | 2 (2.0) | 6 (3.8) | 0.488 |
| Discharge location,
n (%) | | | | <0.001 |
|
Home | 144 (55.2) | 39 (38.2) | 105 (66.0) | |
|
Different
hospital | 109 (41.8) | 59 (57.8) | 50 (31.4) | |
|
Others | 8 (3.1) | 4 (3.9) | 4 (2.5) | |
| Mean follow-up from
HP, months (SD) | 40.8 [40.1] | 45.8 [45.4] | 37.7 [36.0] | 0.130 |
Predictive factors for stoma reversal
and cumulative stoma-reversal rate between the benign and malignant
disease groups
The results of univariate and multivariate analyses
of the predictive factors for stoma reversal are summarized in
Table II. CCI, indication for HP,
type of surgery for HP, and discharge location were independent
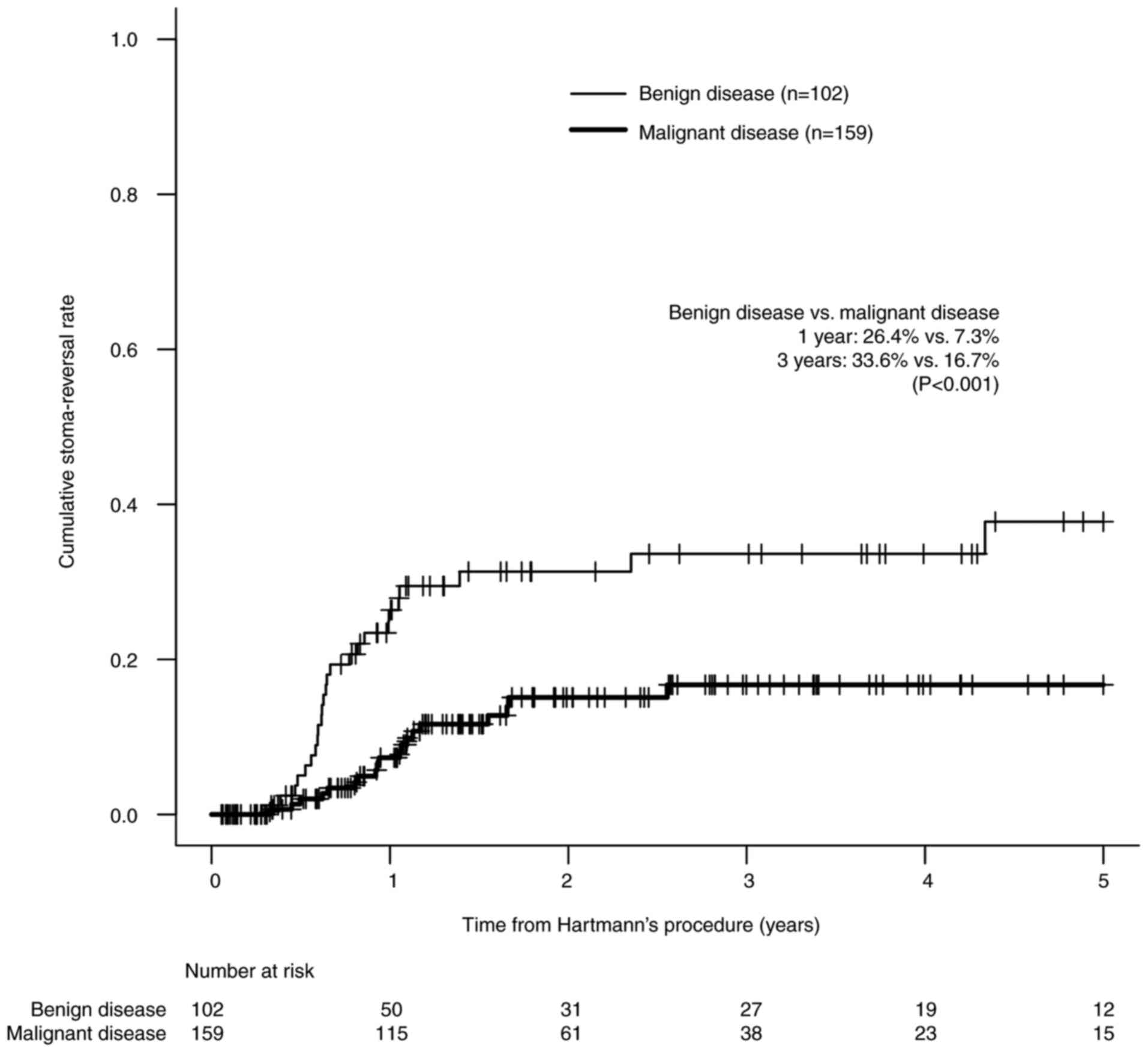
predictive factors for stoma reversal. Fig. 2 shows that the cumulative
stoma-reversal rate in the malignant disease group was
significantly lower than that in the benign disease group (26.4%
vs. 7.3% at 1 year after HP and 33.6% vs. 16.7% at 3 years after
HP, P<0.001 by the Gray test). The indications for HP,
stoma-reversal rate, and time-to-reversal are shown in Table III. The most common indications
for HP were diverticular disease and colorectal cancer with
obstruction in patients with benign and malignant diseases,
respectively. The mean time-to-reversal for benign and malignant
diseases were 11.0 and 12.6 months, respectively (P=0.526). Among
159 patients with malignant diseases, very few patients with
distant metastasis underwent stoma reversal compared to those
without distant metastasis (4.5% vs. 17.2%, P=0.023). External
causes had a high reversal rate (57.1%) regardless of the primary
cause.
 | Table IIPredictive factors associated with
stoma reversal in univariate and multivariate analyses. |
Table II
Predictive factors associated with
stoma reversal in univariate and multivariate analyses.
| | Univariate | Multivariate |
|---|
| Variable | n (%) | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | | | | | |
|
<70
years | 93 (35.6) | 1.615
(0.893-2.922) | 0.110 | | |
|
>70
years | 168 (64.4) | Reference | | | |
| Sex | | | | | |
|
Male | 141 (54.0) | 1.244
(0.688-2.251) | 0.470 | | |
|
Female | 120 (46.0) | Reference | | | |
| BMI | | | | | |
|
BMI >21
kg/m2 | 132 (50.6) | 1.088
(0.605-1.957) | 0.780 | | |
|
BMI <21
kg/m2 and no data | 129 (49.4) | Reference | | | |
| ASA-PS | | | | | |
|
ASA
>III | 164 (62.8) | 1.034
(0.564-1.895) | 0.910 | | |
|
ASA-I or II
and no data | 97 (37.2) | Reference | | | |
| Charlson
comorbidity index | | | | | |
|
Low
score | 81 (31.0) | 6.131
(3.117-12.06) | <0.001 | 3.684
(1.720-7.888) | <0.001 |
|
High
score | 180 (69.0) | Reference | | Reference | |
| Indication for
HP | | | | | |
|
Malignant
disease | 159 (60.9) | 0.363
(0.201-0.656) | <0.001 | 0.523
(0.278-0.983) | 0.044 |
|
Benign
disease | 102 (39.1) | Reference | | Reference | |
| Type of surgery for
HP | | | | | |
|
Urgent | 143 (54.8) | 5.755
(2.588-12.79) | <0.001 | 4.121
(1.602-10.60) | 0.003 |
|
Elective | 118 (45.2) | Reference | | Reference | |
| Surgical approach
for HP | | | | | |
|
Laparoscopic | 99 (37.9) | 0.470
(0.237-0.933) | 0.031 | 1.113
(0.500-2.478) | 0.790 |
|
Open and
conversion | 162 (62.1) | Reference | | Reference | |
| Adjacent organs
resection during HP | | | | | |
|
Yes | 65 (24.9) | 0.638
(0.304-1.343) | 0.240 | | |
|
No | 196 (75.1) | Reference | | | |
| Length of residual
stump | | | | | |
|
Below
peritoneal reflection | 32 (12.3) | 0.634
(0.220-1.830) | 0.400 | | |
|
Above
peritoneal reflection | 229 (87.7) | Reference | | | |
| Postoperative
complicationa | | | | | |
|
Yes | 23 (8.8) | 0.719
(0.235-2.202) | 0.560 | | |
|
No | 238 (91.2) | Reference | | | |
| Discharge
location | | | | | |
|
Home | 144 (55.2) | 4.280
(1.823-10.05) | <0.001 | 4.454
(1.859-10.67) | <0.001 |
|
Different
hospital and others | 117 (44.8) | Reference | | Reference | |
 | Table IIIStoma-reversal rates and intervals
depending on indication for Hartmann's procedure. |
Table III
Stoma-reversal rates and intervals
depending on indication for Hartmann's procedure.
| Indication for
Hartmann's procedure | n (%) | Reversal rate n
(%) | Mean
time-to-reversal, months (SD) |
|---|
| Benign disease | 102(100) | 25/102 (24.5) | 11.0 (9.9) |
|
Diverticular
disease | 45 (44.1) | 12/45 (26.7) | 11.8 (13.2) |
|
Stercoral
perforation | 16 (15.7) | 2/16 (12.5) | 8.9 (4.3) |
|
Bowel
ischemia | 14 (13.7) | 3/14 (21.4) | 9.5 (2.6) |
|
Others | 17 (16.7) | 2/17 (11.8) | 17.8 (14.7) |
|
External
causes (trauma, iatrogenic perforation, operative
complication) | 10 (9.8) | 6/10 (60.0) | 8.6 (2.4) |
| Malignant
disease | 159(100) | 19/159 (11.9) | 12.6 (6.3) |
|
Colorectal
cancer with obstruction | 58 (36.5) | 9/58 (15.5) | 11.1 (4.7) |
|
Colorectal
cancer with perforation | 53 (33.3) | 8/53 (15.1) | 13.3 (8.3) |
|
Others | 44 (27.7) | 0/44 (0) | NA |
|
External
causes (operative complication) | 4 (2.5) | 2/4 (50.0) | 16.3 (3.2) |
| Status of malignant
disease | | | |
|
Absence of
distant metastasis | 93 (58.5) | 16/93 (17.2) | 12.5 (6.9) |
|
Presence of
distant metastasis | 66 (41.5) | 3/66 (4.5) | 12.8 (1.5) |
Surgical outcomes of stoma reversal
and stoma-free survival after reversal
The surgical outcomes in the 44 patients who
underwent stoma reversal are shown in Table IV. The laparoscopic approach was
the most common surgical approach in the benign disease group, and
open surgery the most common strategy in the malignant disease
group. The length of the residual stump, anastomotic method,
diverting stoma, postoperative complication grade, mortality rate,
and duration of postoperative follow-up were not significantly
different between the benign and malignant disease groups. One
patient with diverting loop ileostomy during reversal underwent
ileostomy closure approximately 4 months after colostomy reversal.
Owing to anastomotic complications on day 1 after reversal, one of
the 25 patients with benign disease underwent stoma recreation
(second Hartmann's operation). However, the patient's stoma was not
reversed. Among the 19 patients with malignant disease, 1 underwent
stoma recreation (ileostomy) because of anastomotic complications
on day 7 after reversal, and the stoma was closed approximately 3
years after the recreation. Three patients underwent stoma
recreation owing to cancer recurrence in the pelvis (transverse
colostomy for peritoneal recurrence in one patient with stage III
cancer with a 7.9-month interval to reversal, a second Hartmann's
operation for local recurrence in a patient with stage II cancer
with an 11.3-month interval, and Mile's operation for local
recurrence in a patient with stage IV cancer with an interval of 10
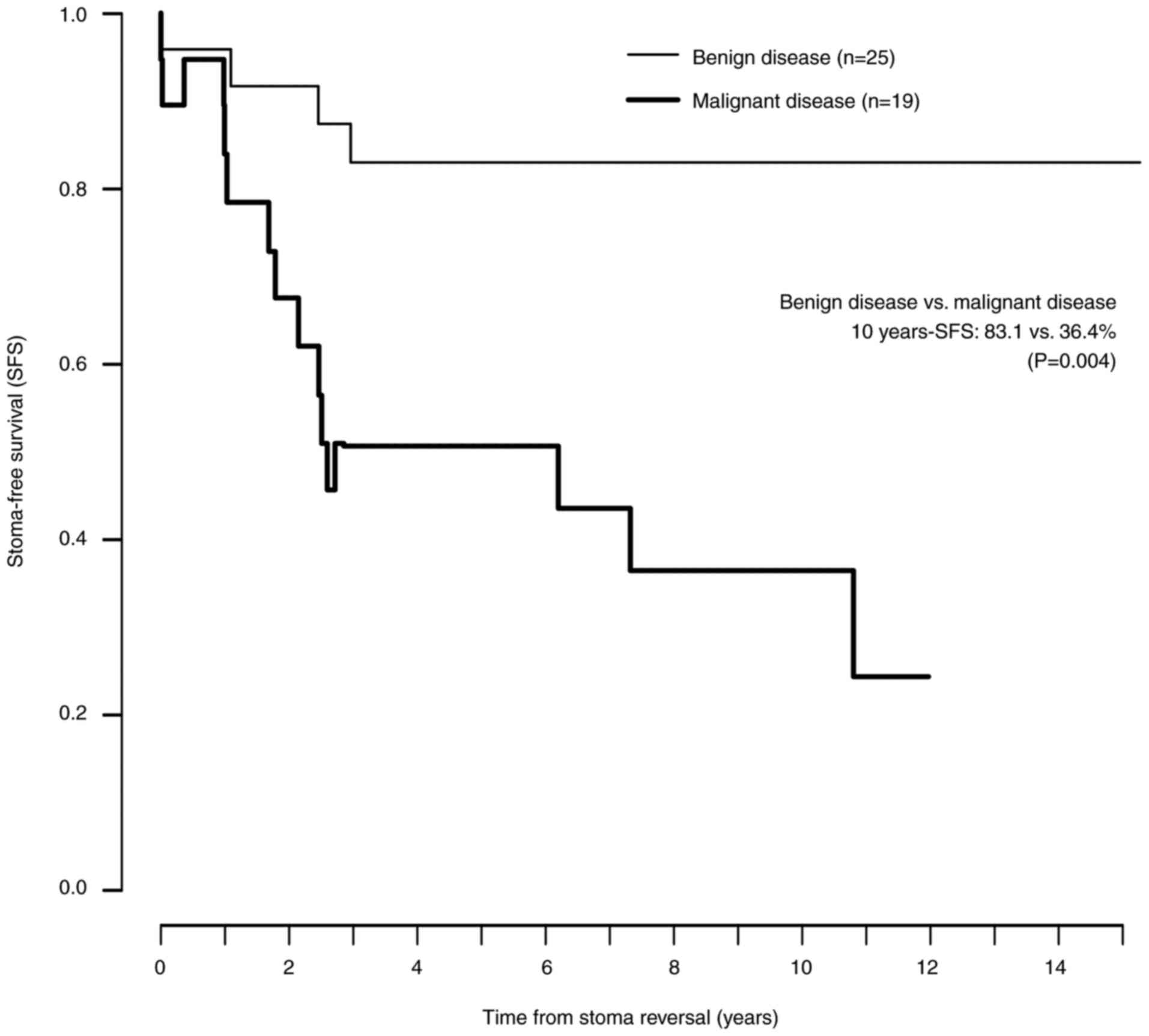
months). Their stomas became permanent. Fig. 3 shows the Kaplan-Meier curves for
SFS after reversal in the benign and malignant disease groups. The
SFS was significantly decreased in the malignant disease group with
time elapsing from reversal, compared with the benign disease group
(benign disease vs. malignant disease using the log-rank test,
1-year SFS: 96.0% vs. 83.9%, P=0.214; 3-year SFS: 83.1% vs. 50.6%,
P=0.036; 5-year SFS: 83.1% vs. 50.6%, P=0.036, 10-year SFS: 83.1%
vs. 36.4%, P=0.004).
 | Table IVSurgical outcomes of stoma
reversal. |
Table IV
Surgical outcomes of stoma
reversal.
| Variable | Stoma-reversal
cohort (n=44) | Benign disease
(n=25) | Malignant disease
(n=19) | P-value (benign
disease vs. malignant disease) |
|---|
| Surgical approach
for stoma reversal, n (%) | | | | 0.034 |
|
Open | 17 (38.6) | 6 (24.0) | 11 (57.9) | |
|
Laparoscopic | 25 (56.8) | 18 (72.0) | 7 (36.8) | |
|
Conversion | 2 (4.6) | 1 (4.0) | 1 (5.3) | |
| Length of residual
stump, n (%) | | | | 0.116 |
|
Above sacral
promontory | 13 (29.6) | 10 (40.0) | 3 (15.8) | |
|
Between
sacral promontory and peritoneal reflection | 27 (61.4) | 12 (48.0) | 15 (78.9) | |
|
Below
peritoneal reflection | 4 (9.1) | 3 (12.0) | 1 (5.3) | |
| Anastomotic method,
n (%) | | | | 0.766 |
|
Circular
stapler | 34 (77.3) | 19 (76.0) | 15 (78.9) | |
|
Linear
stapler | 4 (9.1) | 3 (12.0) | 1 (5.3) | |
|
Hand-sewn | 3 (6.8) | 2 (8.0) | 1 (5.3) | |
|
No data | 3 (6.8) | 1 (4.0) | 2 (10.5) | |
| Diverting loop
ileostomy, n (%) | 1 (2.3) | 0 (0) | 1 (5.3) | 0.432 |
| Postoperative
complication (<30 days), n (%) | | | | |
|
All
grade | 13 (29.5) | 7 (28.0) | 6 (31.6) | >0.999 |
|
Major
complication (CD>III) | 9 (20.5) | 4 (16.0) | 5 (26.3) | 0.467 |
|
Anastomotic
complication | 3 (6.8) | 2 (8.0) | 1 (5.3) | >0.999 |
| 30-day mortality, n
(%) | 0 (0) | 0 (0) | 0 (0) | NA |
| Mean follow-up from
stoma reversal, months (SD) | 74.0 (46.4) | 81.2 (45.1) | 64.5 (47.7) | 0.247 |
Assessments of anorectal function
The post-reversal LARS scores of the 24 patients
were significantly worse than their pre-HP scores (P=0.017).
Post-reversal minor LARS occurred in four patients (16.7%), and
post-reversal major LARS in four patients (16.7%). The
post-reversal LARS scores and the occurrence of post-reversal LARS
were not significantly different between the benign and malignant
disease groups. Subsequent analysis based on the length of the
residual stump revealed that post-reversal LARS scores were
significantly lower when the residual stump was short by
Kruskal-Wallis rank sum test (P=0.023). Residual stumps below the
peritoneal reflection were associated with major LARS. The results
of anorectal function are summarized in Table V.
 | Table VAssessments of anorectal
function. |
Table V
Assessments of anorectal
function.
| | Indication for
Hartmann's procedure | Length of residual
stump |
|---|
| Variable | Function-assessment
cohort (n=24) | Benign disease
(n=17) | Malignant disease
(n=7) | P-value (benign
disease vs. malignant disease) | Above sacral
promontory (n=5) | Between sacral
promontory and peritoneal reflection (n=17) | Below peritoneal
reflection (n=2) | P-value (among
three groups according to length of residual stump) |
|---|
| Median LARS score
(IQR) | | | | | | | | |
|
Pre-HP | 6 (0-14) | 7 (5-14) | 5 (0-16.5) | 0.747a | 5 (0-5) | 9 (0-20) | 9.5
(7.25-11.75) | 0.403b |
|
Post-reversal | 11 (6.5-26.75) | 11 (7-20) | 23 (5.5-27.5) | 0.725a | 5 (5-7) | 11 (9-26) | 37.5
(36.75-38.25) | 0.023b |
|
P-value
(pre-HP vs. post-reversal) | 0.017c | 0.120c | 0.059c | |
>0.999c | 0.066c | 0.500c | |
| Post-reversal | | | | 0.118d | | | | 0.043d |
| LARS, n (%) | | | | | | | | |
|
No LARS | 16 (66.7) | 13 (76.5) | 3 (42.9) | | 5(100) | 11 (64.7) | 0 (0) | |
|
Minor
LARS | 4 (16.7) | 1 (5.9) | 3 (42.9) | | 0 (0) | 4 (23.5) | 0 (0) | |
|
Major
LARS | 4 (16.7) | 3 (17.6) | 1 (14.3) | | 0 (0) | 2 (11.8) | 2(100) | |
Discussion
This study revealed that malignant disease was an
independent significant predictive factor for stoma reversal in the
multivariate analysis. Moreover, the cumulative stoma-reversal rate
was lower for malignant than for benign disease, although After
reversal, SFS was significantly reduced in the malignant disease
group compared to benign disease group as the time from reversal
elapsed. The was comparable in terms of functional outcomes between
the groups.
The HP remains an important surgical option due to
the increasing incidence of colorectal cancer and diverticulosis of
the left colon worldwide and in Asian countries, respectively
(14). The rate of stoma closure
was reported to be lower after HP than after primary anastomosis
(4). In particular, the stoma
closure rate after HP was different between benign and malignant
diseases (5,6). Our findings showed that the
cumulative stoma-reversal rate was significantly different between
benign and malignant diseases. Additionally, among malignant
diseases, advanced cancers with distant metastasis have
significantly lower rates of stoma reversal. This may be because
the timing of stoma reversal is missed owing to ongoing treatment
for distant metastasis after HP.
Furthermore, our findings demonstrated that low CCI,
urgent surgery, and home discharge were independently associated
with a higher stoma-reversal rate. The CCI score reflects the age
and comorbidities of the individual and represents their background
(11). Royo-Aznar et al
(5) reported that patients with a
low CCI had a higher rate of stoma reversal. In this study, the
achievement of stoma reversal was not significantly associated with
ASA-PS at the time of HP while it was significantly associated with
CCI. If patients with severe systemic disease at the time of HP
(high ASA-PS) recovered, had a low CCI, and were discharged home,
they had a good chance of reversal. Urgent surgery meant that HP
had to be performed and it was a lifesaving procedure. In such
cases, the patient has a good chance of reversal if they recover.
Our data could help surgeons provide accurate information for
patients and their families about the prospect of colostomy closure
or a permanent stoma prior to obtaining informed consent.
The colostomy reversal after HP is a difficult
surgery with a high risk of anastomotic complications and mortality
(7,8). However, with the introduction of
minimally invasive techniques (14-18),
the incidence of complications after reversal has lowered (19-22).
Therefore, the indications of colostomy reversal after HP can now
be expanded. Although there are many reports on short-term outcomes
after reversal (22,23), only a few studies have examined its
long-term outcomes (9,10). We speculate that the long-term
follow-up of patients after reversal may result in the
identification of complications such as cancer recurrence in
patients with malignant disease and anorectal disorders. These
issues require further investigation.
Because patients with malignant disease undergoing
HP often have colorectal obstruction or perforation, subsequent
disease recurrence may occur even if the reversal is successful
(24,25). In this study, stoma recreation was
required in three cases of cancer recurrence in the pelvis. For
malignant diseases, an appropriate indication for colostomy
reversal, such as setting an adequate interval between surgeries,
may avoid unnecessary challenging surgery. As mentioned earlier, in
our center, colostomy reversal after HP is indicated when there is
no disease recurrence for at least 6 months post-HP and after
adjuvant chemotherapy in patients who underwent curative cancer
surgery, and there is no disease progression in patients with
distant metastasis controlled by systemic therapy. Although there
was no significant difference, the interval from HP to reversal in
the 3 patients who underwent stoma recreation owing to cancer
recurrence was shorter than that in the remaining 16 patients in
the malignant disease group (9.6 months vs. 13.1 months, P=0.090).
A previous study reported the time-to-reversal of 282 days (9.3
months) for malignant disease (6).
Determining the appropriate interval for reversal could be the
object of future research. Additionally, it is important to obtain
informed consent before stoma reversal in patients with malignant
disease owing to the risk of recurrence.
Few studies have examined anorectal function after
reversal. In their study of 64 patients with colostomy reversal,
Van Hoof et al (10)
reported that 15.6 and 17.2% of patients had minor LARS and major
LARS, respectively. Caille et al (9) stated that among 21 patients who
underwent reversal after HP due to failure of the previous
anastomosis, 33.3% reported minor LARS and 23.8% reported major
LARS. In our study, 16.7% of 24 patients reported minor LARS and
16.7% reported major LARS. This result is comparable to those shown
in previous studies. Furthermore, the present study revealed no
significant differences in anorectal function between those with
benign and malignant diseases. However, a short residual rectal
stump could be associated with poor function. Although there were
only two patients with residual stumps below the peritoneal
reflection, they experienced major LARS. Interestingly, a previous
study demonstrated that the length of the rectal stump did not
differ significantly among patients with no, minor, and major LARS
(10). Further studies will be
required to investigate the association between anorectal function
and the length of residual stump. Based on our finding of
predictive factors for stoma reversal, we suggest that
interventions such as pelvic floor muscle exercises should be
considered before reversal for patients with a high likelihood of
reversal and a short residual stump.
This study had some limitations. First, we
retrospectively collected data from the surgical database and
medical records in a single center. Second, the number of
participants was small and the rate of stoma reversal was low to
examine each variable related to the outcomes. Multicenter studies
with large samples and minimal bias are required for more reliable
statistical analyses. Third, due to the retrospective nature of
this study, it was difficult to obtain data on whether patients
desired stoma reversal. Some patients may have been satisfied with
a permanent stoma and therefore did not seek reversal, which could
have resulted in an underestimation of the stoma reversal rate.
Fourth, both colorectal and general surgeons performed HP, and
stoma reversal decisions were primarily based on patient condition
rather than surgeon specialty. However, colorectal surgeons
predominantly treated malignant diseases, and their surgical
strategies may have influenced the observed trends. Fifth, the time
between stoma reversal and the survey for post-reversal anorectal
function varied from case to case, which may have influenced the
functional results. Sixth, because pre-HP anorectal function was
retraced and assessed at the time of the survey, the actual
function may not have been accurately represented. Further
prospective studies are required to overcome these limitations.
Seventh, follow-up methods differed between benign and malignant
diseases. In particular, patients with benign diseases were not
followed up regularly. This may underestimate the stoma-reversal
rate in patients with benign diseases. However, because the
follow-up period for benign diseases was relatively long and there
was no significant difference in the follow-up period, we believe
that this impact was not so large. The reason for this is that our
hospital is located in the southern area of Hokkaido, which has few
healthcare resources, and patients have few opportunities to visit
other medical institutions.
In conclusion, our findings indicated that the
stoma-reversal rate and SFS after reversal could be worse in
patients with malignant disease than in those with benign disease,
although anorectal function after reversal was not significantly
different. The postoperative long-term course of HP may vary
between patients according to the indication for HP. Enforcement of
HPs should be carefully considered, taking these circumstances into
account.
Acknowledgements
The authors would like to thank Professor Yohei
Kawasaki, Ph.D. (Institute for Assistance of Academic and
Education) for the advice on statistical analysis.
Funding
Funding: This study was supported by the Japanese Society for
the Promotion of Science KAKENHI (grant no. 23K19492).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KIm, HK, AS, KS, KIt, TF, KIc, TO, DY, YT, MU, MK
and KN conceptualized and designed the study. KIm and HK confirm
the authenticity of all the raw data. KIm wrote the manuscript and
performed the statistical analyses. KIm, HK, AS, KS, KIt, TF, KIc,
TO, DY, YT, MU, MK and KN performed the operations and collected
clinicopathological data. KN supervised the study. KIm, HK, AS, KS,
KIt, TF, KIc, TO, DY, YT, MU, MK and KN interpreted the results and
wrote the report. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Research
Ethics Committee of Hakodate Municipal Hospital (Hakodate, Japan;
approval nos. 2021-84 and 2022-228). Furthermore, the study was
conducted in accordance with the tenets of the 1964 Declaration of
Helsinki and its later amendments. All patients gave their consent
through the opt-out method.
Patient consent for publication
All patients gave their consent through the opt-out
method.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID IDs: KEN IMAIZUMI, 0000-0002-7751-6270; AYA
SATO, 0000-0002-4231-2315; KENTARO SATO, 0000-0002-1765-7477;
KENTARO ICHIMURA, 0000-0002-2838-4240.
References
|
1
|
Albarran SA, Simoens Ch, Van De Winkel N,
da Costa PM and Thill V: Restoration of digestive continuity after
Hartmann's procedure: ASA score is a predictive factor for risk of
postoperative complications. Acta Chir Belg. 109:714–719.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Moro-Valdezate D, Royo-Aznar A,
Martín-Arévalo J, Pla-Martí V, García-Botello S, León-Espinoza C,
Fernández-Moreno MC, Espín-Basany E and Espí-Macías A: Outcomes of
Hartmann's procedure and subsequent intestinal restoration. Which
patients are most likely to undergo reversal? Am J Surg.
218:918–927. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Christou N, Rivaille T, Maulat C, Taibi A,
Fredon F, Bouvier S, Fabre A, Derbal S, Durand-Fontanier S, Valleix
D, et al: Identification of risk factors for morbidity and
mortality after Hartmann's reversal surgery-a retrospective study
from two French centers. Sci Rep. 10(3643)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Edomskis PP, Hoek VT, Stark PW, Lambrichts
DPV, Draaisma WA, Consten ECJ, Bemelman WA and Lange JF: LADIES
trial collaborators. Hartmann's procedure versus sigmoidectomy with
primary anastomosis for perforated diverticulitis with purulent or
fecal peritonitis: Three-year follow-up of a randomised controlled
trial. Int J Surg. 98(106221)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Royo-Aznar A, Moro-Valdezate D,
Martín-Arévalo J, Pla-Martí V, García-Botello S, Espín-Basany E and
Espí-Macías A: Reversal of Hartmann's procedure: A single-centre
experience of 533 consecutive cases. Colorectal Dis. 20:631–638.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Katsura M, Fukuma S, Chida K, Saegusa Y,
Kanda S, Kawasaki K, Tsuzuki Y and Ie M: Which factors influence
the decision to perform Hartmann's reversal in various causative
disease situations? A retrospective cohort study between 2006 and
2021. Colorectal Dis. 25:305–314. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Banerjee S, Leather AJ, Rennie JA, Samano
N, Gonzalez JG and Papagrigoriadis S: Feasibility and morbidity of
reversal of Hartmann's. Colorectal Dis. 7:454–459. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Toro A, Ardiri A, Mannino M, Politi A, Di
Stefano A, Aftab Z, Abdelaal A, Arcerito MC, Cavallaro A, Cavallaro
M, et al: Laparoscopic reversal of Hartmann's procedure: State of
the art 20 years after the first reported case. Gastroent Res
Pract. 2014(530140)2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Caille C, Collard M, Moszkowicz D, Prost À
la Denise J, Maggiori L and Panis Y: Reversal of Hartmann's
procedure in patients following failed colorectal or coloanal
anastomosis: An analysis of 45 consecutive cases. Colorectal Dis.
22:203–211. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Van Hoof S, Sels T, Patteet E, Hendrickx
T, Van den Broeck S, Hubens G and Komen N: Functional outcome after
Hartmann's reversal surgery using LARS, COREFO & QoL scores. Am
J Surg. 225:341–346. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: development and validation. J Chronic Dis.
40:373–383. 1987.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Oh HK, Han EC, Ha HK, Choe EK, Moon SH,
Ryoo SB, Jeong SY and Park KJ: Surgical management of colonic
diverticular disease: Discrepancy between right- and left-sided
diseases. World J Gastroenterol. 20:10115–10120. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Trépanier JS, Arroyave MC, Bravo R,
Jiménez-Toscano M, DeLacy FB, Fernandez-Hevia M and Lacy AM:
Transanal Hartmann's colostomy reversal assisted by laparoscopy:
Outcomes of the first 10 patients. Surg Endosc. 31:4981–4987.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Giuliani G, Formisano G, Milone M, Salaj
A, Salvischiani L and Bianchi PP: Full robotic Hartmann's reversal:
Technical aspects and preliminary experience. Colorectal Dis.
22:1734–1740. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
van Loon YT, Clermonts SHEM, Wasowicz DK
and Zimmerman DDE: Reversal of left-sided colostomy utilizing
single-port laparoscopy: Single-center consolidation of a new
technique. Surg Endosc. 34:332–338. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sato K, Kasajima H, Yamana D, Imaizumi K
and Nakanishi K: Technique of the Single-port laparoscopic
Hartmann's reversal via the colostomy Site-A video vignette.
Colorectal Dis. 25:1050–1051. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Siddiqui MR, Sajid MS and Baig MK: Open vs
laparoscopic approach for reversal of Hartmann's procedure: A
systematic review. Colorectal Dis. 12:733–741. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
van de Wall BJ, Draaisma WA, Schouten ES,
Broeders IA and Consten EC: Conventional and laparoscopic reversal
of the Hartmann procedure: A review of literature. J Gastrointest
Surg. 14:743–752. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ng DC, Guarino S, Yau SL, Fok BK, Cheung
HY, Li MK and Tang CN: Laparoscopic reversal of Hartmann's
procedure: Safety and feasibility. Gastroenterol Rep (Oxf).
1:149–152. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Horesh N, Lessing Y, Rudnicki Y, Kent I,
Kammar H, Ben-Yaacov A, Dreznik Y, Avital S, Mavor E, Wasserberg N,
et al: Comparison between laparoscopic and open Hartmann's
reversal: Results of a Decade-long multicenter retrospective study.
Surg Endosc. 32:4780–4787. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pei KY, Davis KA and Zhang Y: Assessing
trends in laparoscopic colostomy reversal and evaluating outcomes
when compared to open procedures. Surg Endosc. 32:695–701.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen HS and Sheen-Chen SM: Obstruction and
perforation in colorectal adenocarcinoma: An analysis of prognosis
and current trends. Surgery. 127:370–376. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ogawa K, Miyamoto Y, Harada K, Eto K,
Sawayama H and Iwagam S: Evaluation of clinical outcomes with
propensity-score matching for colorectal cancer presenting as an
oncologic emergency. Ann Gastroenterol Surg. 6:523–530.
2022.PubMed/NCBI View Article : Google Scholar
|