Introduction
Neuroendocrine neoplasms (NENs) are tumors that
originate from neuroendocrine cells and are classified into two
categories: i) Neuroendocrine organs, such as the pituitary, thymus
and adrenal glands, and ii) non-neuroendocrine organs, including
the gastrointestinal tract, pancreas, lungs, thymus, skin and
genitourinary tract. According to the 5th edition of the World
Health Organization (WHO) Classification of Thoracic Tumors (May
2021), NENs account for ~1-2% of primary lung tumors and primarily
originate from bronchial mucosal epithelial K-cells, which are
distributed in large bronchial tubes and bifurcation mucosa. Lung
NENs are classified into four subtypes: Typical carcinoid tumors,
atypical carcinoid tumors, large-cell neuroendocrine carcinoma
(NEC), and small-cell lung carcinomas (1). These tumors can metastasize to the
lymph nodes, liver, lungs and bones, with brain metastases
occurring in ~1-5% of cases (2-4).
The incidence of pituitary metastases is extremely low, accounting
for ~1% of all metastatic brain lesions (5). However, simultaneous metastases to
both the pituitary gland and the pineal region are exceedingly
rare. In the present study, a case of a pulmonary neuroendocrine
tumor with simultaneous metastases to these regions was reported,
aiming to provide a reference for clinical diagnosis and
treatment.
Case report
A 68-year-old man first presented to the
neurosurgery clinic of Gansu Provincial Hospital (Lanzhou, China)
with intermittent headaches, fever and anorexia that had persisted
for 1 month. The patient had been a smoker for >30 years, at 20
cigarettes per day. A neurological examination revealed no positive
signs. Hormone levels and other laboratory results are presented in
Table I; demonstrating that
pituitary hormone secretion was significantly suppressed with
enlargement of the pituitary and pineal region lesions. Ancillary
examinations revealed the following findings: X-ray chest
radiographs showed no obvious abnormalities (chest CT was not
performed). MRI of the pituitary gland showed that it was full in
shape, with a maximum height of ~1.1 cm in the coronal position. A
slightly hyperintense T1 signal shadow, round in shape and
measuring ~0.57 cm in diameter, was observed in the middle and
upper part of the pituitary gland. Enhancement scans showed early
peripheral enhancement of the lesion, with inconspicuous
enhancement in the center and sustained enhancement of the lesion.
The pituitary stalk was shifted and showed enhancement (Fig. 1A-C). The preliminary diagnosis of
pituitary microadenoma was made based on the patient's history and
auxiliary examinations. It was recommended that a pituitary MRI be
repeated every 3 months to observe dynamic changes in the
lesion.
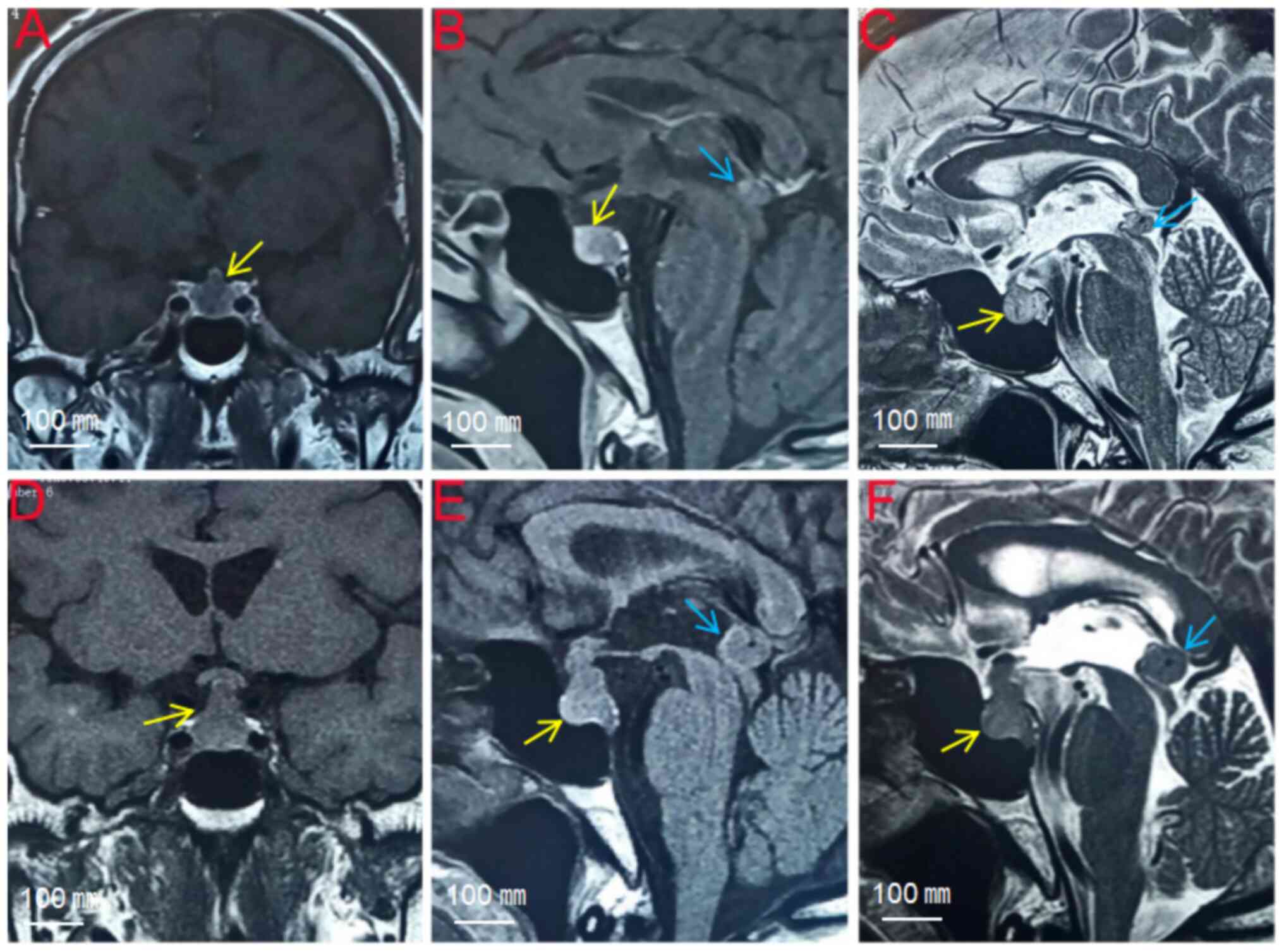 | Figure 1Magnetic resonance imaging. (A-C)
First pituitary MRI: The pituitary gland is full, the maximum
height of the pituitary gland is ~1.1 cm in the coronal position,
and a slightly longer T1 signal shadow can be observed in the
middle and upper part of the pituitary gland, which is ~0.57 cm in
diameter. The enhancement scan shows peripheral enhancement of the
lesion. (D-F) Second pituitary MRI: The pituitary gland is full,
the maximum height of the pituitary gland in the coronal position
is ~1.5 cm, the corset sign can be observed in the middle and upper
part of the pituitary gland, and the pituitary stalk is thickened
in the form of a nodule, which is obviously strengthened after
enhancement. The pineal region can be seen in the form of a nodular
abnormality, with a diameter of ~1.2 cm, with clear borders, and it
is obviously and uniformly strengthened after enhancement. The
signals of the lesion are the same as that of pituitary tumor
(metastatic pituitary NEC: yellow arrows; metastatic pineal region
NEC: blue arrows). |
 | Table ILaboratory results. |
Table I
Laboratory results.
| Indexes | First lab test | Second lab test | Reference range |
|---|
| Urinary free cortisol
(nmol/24 h) | 0 | 0 | 100-379 |
| Cortisol
(nmol/l) | 60.2 | 16.9 | 102.0-536.0 |
| Human growth hormone
(ng/ml) | 0.485 | 0.329 | 0.970-4.700 |
| Insulin-like growth
factor-1 (ng/ml) | 62 | 58 | 127-424 |
| Thyroid stimulating
hormone (mIU/l) | 0.75 | 0.02 | 0.35-4.94 |
| T3 (nmol/l) | 1.53 | 1.07 | 0.98-2.33 |
| T4 (nmol/l) | 82.35 | 91.91 | 62.80-150.84 |
| Adrenocorticotropic
hormone (pg/ml) | 15.57 | 4.37 | 7.00-64.00 |
| Testosterone
(nmol/l) | <0.45 | <0.45 | 4.94-32.01 |
|
Dehydroepiandrosterone sulfate
(µg/dl) | 12.5 | 7.0 | 48.8-361.8 |
| Prolactin
(ng/ml) | 115.19 | 76.70 | 3.46-19.40 |
| Luteinizing hormone
(IU/l) | 0.45 | 0.33 | 0.57-12.07 |
| Gastrin-releasing
peptide precursor (pg/ml) | 393.0 | 317.5 | <63.7 |
| Neuron-specific
enolase (ng/ml) | 22.10 | 25.27 | <17.00 |
| α-fetoprotein
(ng/ml) | - | 1.41 | <8.78 |
More than 4 months after the first consultation, the
patient reported a progressive worsening of headache symptoms,
accompanied by deteriorating vision and diabetes insipidus;
therefore, the patient visited the Department of Neurosurgery of
Gansu Provincial Hospital (Lanzhou, China) for further evaluation.
The patient was admitted with blurred vision in both eyes and
diabetes insipidus, with no other significant findings. Auxiliary
examinations revealed that visual evoked potentials indicated a
delayed P100 latency in both eyes and a peripheral visual field
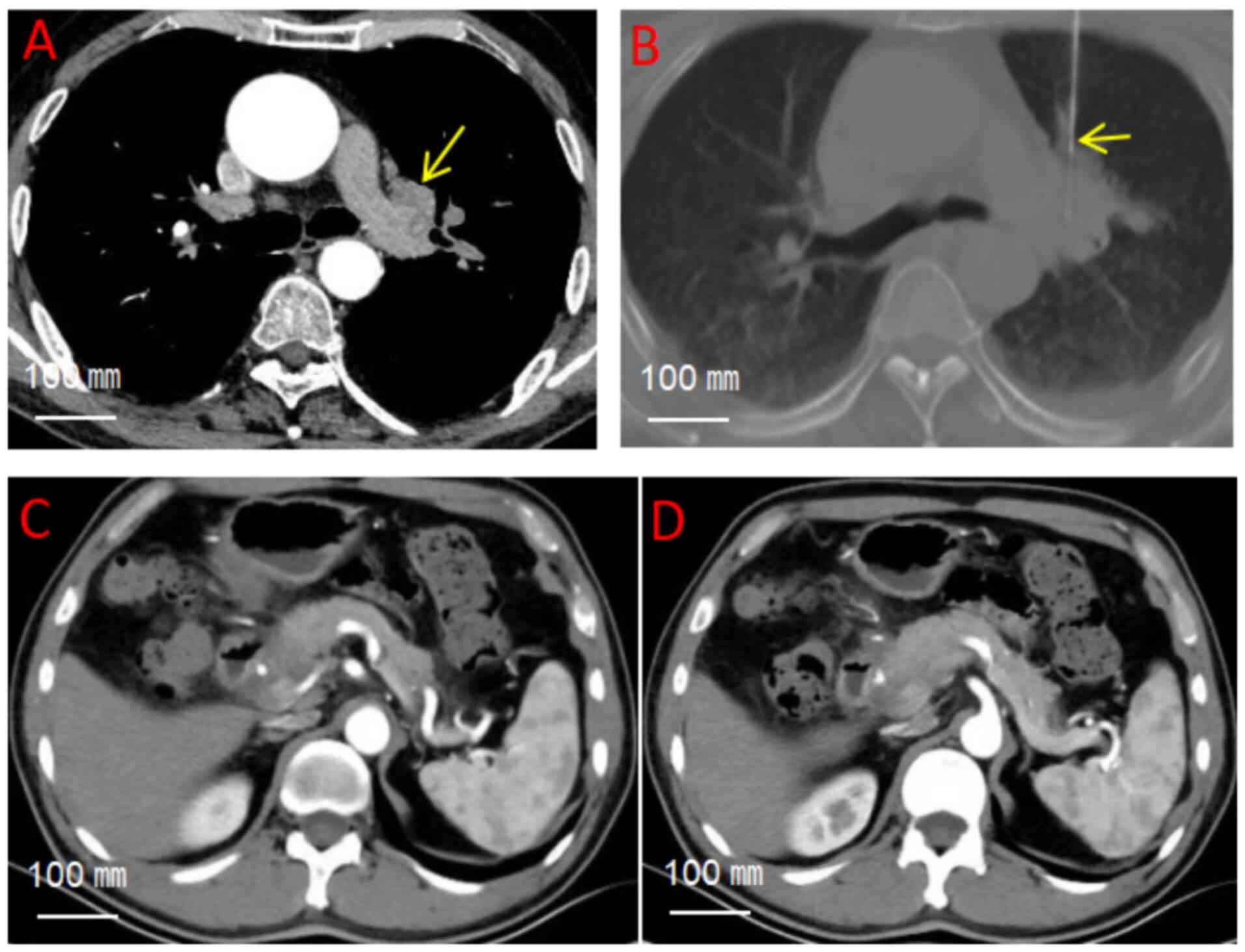
defect in the right eye was observed. Chest CT revealed a nodular
soft tissue density shadow in the hilar region of the upper lobe of
the left lung, measuring ~1.8x3.2 cm (Fig. 2A). Abdominal CT image revealed no
significant abnormalities in the pancreas, spleen, or
gastrointestinal tract, with preserved anatomical architecture and
absence of focal lesions (Fig. 2C
and D). MRI of the pituitary
gland, both on plain scan and with contrast enhancement, showed a
full pituitary morphology, with a maximum height of ~1.5 cm in the
coronal position. The corset sign was observed in the middle and
upper portion of the pituitary gland. The stalk of the pituitary
gland was thickened in a nodular shape and showed significant
enhancement after contrast administration. The pituitary MRI foci
size had notably increased compared with 4 months ago. In the
pineal region, a nodular abnormal signal measuring ~1.2 cm in
diameter with clear borders was observed, which demonstrated
significant and uniform enhancement after contrast administration
(Fig. 1D-F). Based on the
patient's medical history and auxiliary tests, the preliminary
diagnoses included tumors of the sellar region, pineal gland and
lung.
After multidisciplinary diagnosis and treatment
(neurosurgery/imaging center/thoracic surgery) discussions, it was
decided that the pituitary lesion should be resected first to
relieve optic nerve compression and alleviate symptoms of blurred
vision and diabetes insipidus. After ruling out contraindications
to surgery, the trans-nasal butterfly approach
neuro-endoscopic-assisted resection of the sellar region lesion was
performed under general anesthesia. The tumor tissue was soft,
grayish-red in color and had a general blood supply. Postoperative
pathology suggested metastatic NEC (atypical carcinoid tumor).
Hematoxylin and eosin (H&E) staining was performed as follows:
Samples (thickness, 3.5 µm) were fixed using neutral buffered
formalin (pH 7.2-7.4) at 25˚C for 12 h. Hematoxylin staining was
performed for 8 min and eosin staining for 2 min (both at 24˚C).
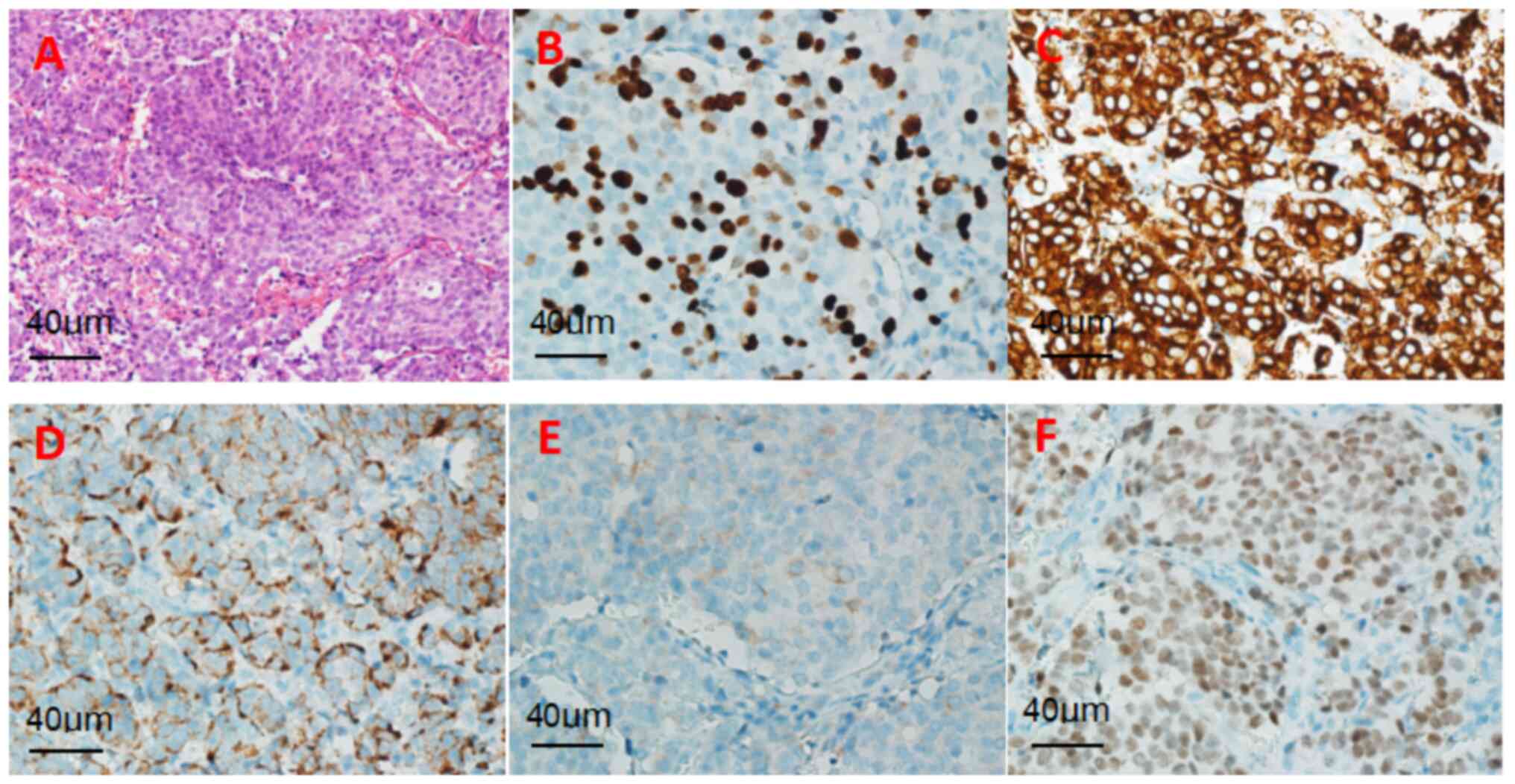
(H&E) staining revealed a solid tumor involving the pituitary
gland, characterized by small cells with high-grade nuclear atypia
and a mitotic index >30/2 mm2 (magnification, x400;
light microscope) (Fig. 3A).
Immunohistochemical analysis was then performed. Normal goat serum
(10%, diluted in PBS pH 7.4) was used for blocking at 25˚C for 45
min.
Primary antibodies included CKP (1:100; cat. no.
M3515; Agilent; temperature and duration of incubation: 60 min at
25˚C), TTF-1 (1:100; cat. no. M3575; Agilent; temperature and
duration of incubation: 4˚C for 16 h), Syn (1:200; cat. no.
790-4464; Roche Diagnostics; temperature and duration of
incubation: 60 min at 25˚C) and CgA (1:500; cat. no. 760-2539;
Roche Diagnostics; temperature and duration of incubation: 60 min
at 25˚C). Secondary antibodies included anti-mouse IgG (H+L)
antibody (biotin-conjugated; 1:200; cat. no. BA-9200; Vector
Laboratories, Inc.; temperature and duration of incubation: 30 min
at 25˚C) and anti-rabbit IgG (H+L) antibody (biotin-conjugated;
1:200, cat. no. BA-1000; Vector Laboratories, Inc.; temperature and
duration of incubation: 30 min at 25˚C). A light microscope
(Olympus Corporation) was used for observation (scale bar, 40
µm).
Immunohistochemistry showed the following results:
Pan cytokeratin (CKP)(+), synaptophysin (Syn)(+), chromogranin A
(CgA)(+), thyroid transcription factor (TTF-1)(+) and Ki-67
expression (index 40%) (Fig.
3B-F). Given the presence of multiple nodules in the patient's
lungs, the pituitary NEC was considered to have originated from the
lungs. To further confirm the primary tumor, a percutaneous
CT-guided aspiration biopsy of the left hilar mass was performed
under local anesthesia (Fig. 2B).
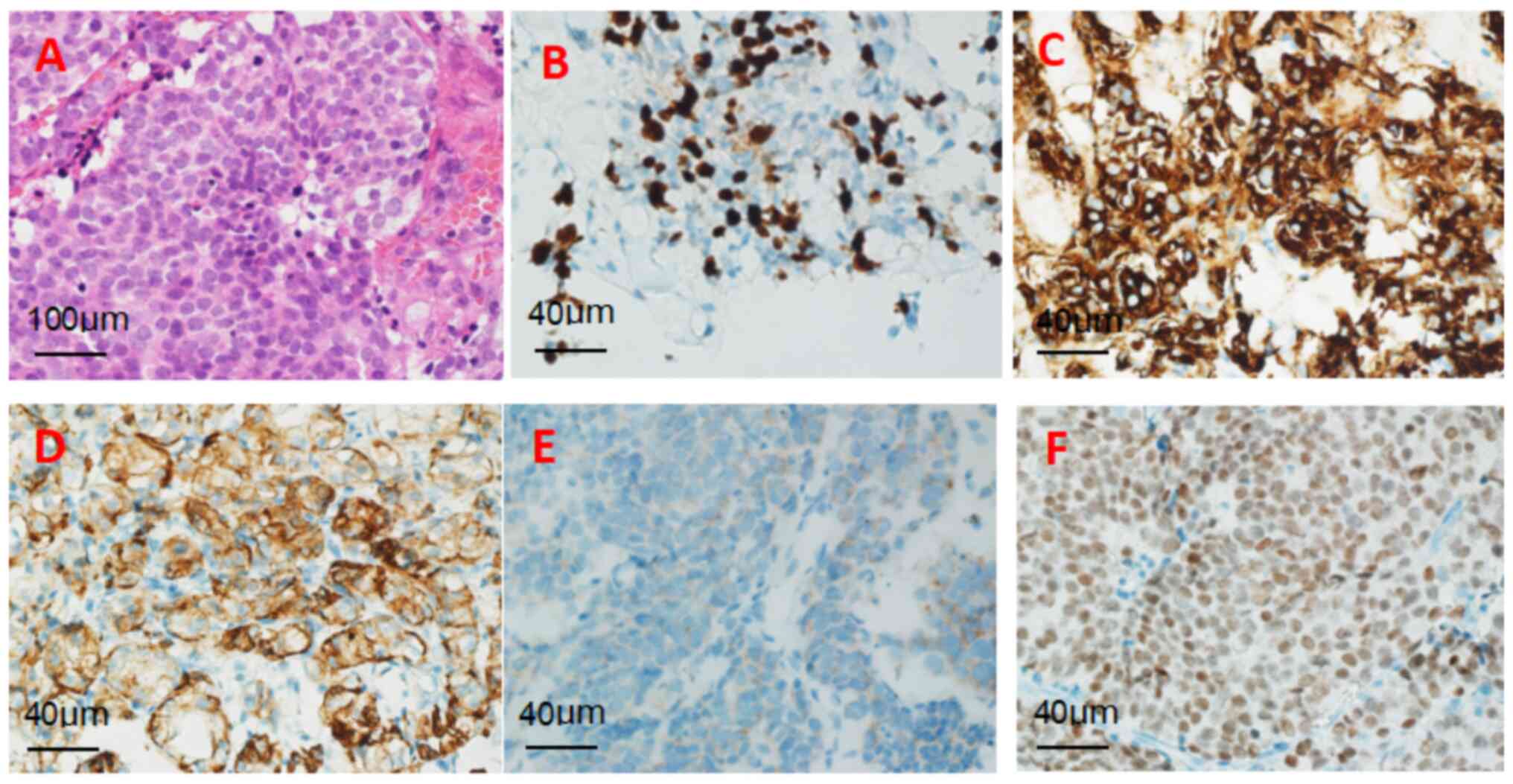
The pathological results of the lung mass indicated that the tumor
was a primary pulmonary neuroendocrine tumor (atypical carcinoid
tumor). Histopathological evaluation of the pulmonary
neuroendocrine tumor is presented in Fig. 4A-F, encompassing H&E staining
and immunohistochemical profiles [CKP(+), Syn(+), CgA(+), TTF-1(+),
Ki-67 (index 40%)].
The histopathological concordance between the
pulmonary and pituitary lesions (Figs.
3 and 4) provides robust
diagnostic evidence supporting pituitary metastasis originating
from a pulmonary NEC. Specifically, both lesions exhibited
identical histomorphological features. Immunohistochemical
profiling demonstrated strong diffuse positivity for Syn, CgA and
CKP in both lesions, with a Ki-67 proliferation index of >30%.
Crucially, nuclear TTF-1 immuno-expression was unequivocally
detected in tumor cells at both anatomical sites, definitively
establishing metastatic dissemination from a primary lung origin.
TTF-1, a lineage-specific nuclear transcription factor expressed in
thyroid follicular epithelium and pulmonary respiratory epithelium,
serves as a diagnostic marker for adenocarcinomas of lung/thyroid
origin while being absent in neoplasms arising from other organs.
In summary, the co-expression of Syn, CgA and CKP, coupled with
nuclear TTF-1 immunoreactivity, provides robust evidence for the
metastatic nature of the sellar tumor and its derivation from a
primary pulmonary NEC.
Therefore, the final diagnosis was established by
combining the medical history with the pathological results,
confirming that the pituitary and pineal region metastases
originated from a pulmonary neuroendocrine tumor. Due to the small
size and deep location of the pineal region lesion, the patient did
not undergo further surgical treatment or pathological tests.
However, from the imaging analysis, the MRI signal characteristics
of the lesion were consistent with those of the sellar region.
Following the monist approach to diagnosis and treatment, the
pineal region lesion was considered homologous to the metastatic
tumor of the pituitary gland. Hormone replacement therapy
(hydrocortisone 100 mg once daily via intravenous infusion, with
dosage adjustment guided by serial hormonal level monitoring) was
administered after surgery, and after a favorable recovery, further
radiotherapy and chemotherapy were recommended. However, the
patient refused additional treatment, was discharged from the
hospital and succumbed 4 months after surgery.
Discussion
Neuroendocrine tumors are a heterogeneous group of
malignant tumors that originate from the diffusely distributed
neuroendocrine system. Pulmonary neuroendocrine tumors are
classified into two categories based on their histomorphology, the
Ki-67 proliferation index and differences in necrosis (6,7). The
first category consists of well-differentiated neuroendocrine
tumors, including low-grade G1 typical carcinoids and
intermediate-grade G2 atypical carcinoids. These tumors are
commonly found in young women without a history of smoking. The
second group consists of poorly differentiated NEC, including
high-grade small-cell lung carcinoma and high-grade large-cell NEC.
These tumors are common in older men with a history of smoking
(6,8,9). In
the present case, the patient had a history of smoking, and the
lesion progressed rapidly, had a poor prognosis and exhibited
atypical carcinoid morphology. Moreover, H&E staining showed
only small patchy areas of necrosis, and immunohistochemical
profiling demonstrated strong diffuse positivity for Syn, CgA and
CKP in both lesions, with a Ki-67 proliferation index of >30%.
These findings indicate that the metastatic NEC of the pituitary
gland originated from a primary pulmonary tumor.
To date, no reported cases of pulmonary NEC with
synchronous metastases to both the pituitary gland and pineal
region have been identified. In the present case, the patient was
initially misdiagnosed with a primary pituitary adenoma and a
pineal region tumor due to the clinicians' insufficient
preoperative diagnostic experience. The definitive diagnosis of
pituitary and pineal region metastases originating from a pulmonary
neuroendocrine tumor was established based on pathological findings
after surgery. Due to the deep location of the pineal region, its
complex anatomical structure, the small size of the lesion and its
possible homology with pituitary lesions based on imaging analysis,
surgical pathology did not confirm that the pineal region lesion
originated from pulmonary NEC. However, based on a monist
diagnostic and treatment approach, the following evidence supports
the diagnosis of pineal metastasis: i) The possibility that the
same patient suffers from two tumors with different natures in the
same period is extremely low; and ii) the growth rate of the pineal
lesion in this patient was consistent with that of the pituitary
lesion, and the imaging findings were consistent with those of the
pituitary lesion. At present, neuroendocrine metastatic carcinoma
in the pineal region is extremely rare, which may be related to the
fact that, as a highly vascularized endocrine gland, the pineal
gland is not easily seeded by tumor cells through hematogenous
dissemination (10,11). Some scholars have suggested that
this may be related to the following mechanisms (12): i) The pineal gland lacks a
blood-brain barrier; and ii) pineal cells are in direct contact
with the cerebrospinal fluid of the third ventricle, and pineal
metastases are mostly derived from lung cancers, potentially
indicating organ specificity.
As demonstrated in Table I, hormonal levels in the second
pituitary function test exhibited widespread suppression.
Metastatic pituitary NEC typically induces single or multiple
anterior pituitary hormone deficiencies, primarily attributed to
tumor-induced mechanical compression of the anterior pituitary
and/or pituitary stalk. This compression disrupts hypothalamic
regulatory signaling essential for pituitary cell stimulation.
Notably, initial pituitary MRI in this case revealed a microadenoma
without indications for surgical intervention due to the absence of
significant neurological deficits. Subsequent re-evaluation
following a 4-month active surveillance period revealed substantial
progression of the pituitary lesion, characterized by both
volumetric expansion and nodular thickening of the pituitary stalk.
These morphological changes likely intensified compressive effects
on the pituitary stalk, thereby amplifying the inhibitory impact on
hypothalamic-pituitary axis signaling and downstream hormone
secretion.
Since Benjamin discovered the first case of
pituitary metastatic carcinoma through autopsy in 1857, numerous
researchers have studied the condition. A review of national and
international literature reveals that only 34 cases (13-42)
of neuroendocrine metastatic carcinoma of the pituitary gland have
been reported (Table II).
However, no cases of simultaneous pulmonary neuroendocrine tumor
metastasis to both the pituitary gland and pineal region have been
documented.
 | Table IISummary of cases with malignant
neoplasm metastasis within pituitary neuroendocrine tumors. |
Table II
Summary of cases with malignant
neoplasm metastasis within pituitary neuroendocrine tumors.
| | Patient
characteristics | | Outcomes | |
|---|
| First author,
year | Age, years | Sex | Primary tumor
site | Other intracranial
metastasis | Treatment | Pathological
findings | Survival
time/status | (Refs.) |
|---|
| Van der Zwan et
al, 1971 | 73 | F | Breast
adenocarcinoma | No | TCS |
Hormone-negative | POD 28/dead | (13) |
| Richardson and
Katayama, 1971 | 70 | F | Breast
adenocarcinoma | No | TCS | N/A | 3 months/dead | (14) |
| Burns and Kadar,
1973 | 78 | M | Renal pelvis ureter
carcinoma | No | No |
Hormone-negative | 1 month/dead | (15) |
| James et al,
1984 | 77 | M | Renal
carcinoma | No | TSS |
Hormone-negative | N/A | (16) |
| van Seters Arnoud
et al, 1985 | 66 | F | Stomach
adenocarcinoma | No | TCS | PRL-positive | POD 12/dead | (17) |
| Molinatti et
al, 1985 | 71 | M | Lung small cell
carcinoma | No | Radiation |
FSH/LH-positive | 1 month/dead | (18) |
| | 67 | F | Stomach
adenocarcinoma | No | TCS |
PRL/GH-positive | POD 2/dead | (18) |
| Zager and
Hedley-Whyte, 1987 | 56 | F | Breast
adenocarcinoma | No | Radiation |
FSH/LH-positive | 3 weeks/dead | (19) |
| Post et al,
1988 | 61 | F | N/A | No | TSS | ACTH-positive | N/A | (20) |
| | 77 | M | Lung carcinoma | No | TSS | N/A | 6 months/dead | (20) |
| Ramsay et
al, 1988 carcinoma | 67 | M | Prostate | No | No |
Hormone-negative | N/A | (21) |
| | 50 | F | Pancreatic
carcinoma | No | TSS | ACTH-positive | 7 months/dead | (21) |
| Hurley et
al, 1992 | 76 | M | N/A | No | TSS | GH-positive | 6 months/dead | (22) |
| Abe et al,
1997 | 46 | F | Malignant
carcinoid | No | Medication | PRL-positive | 6 months/dead | (23) |
| Bret et al,
2001 | 75 | F | Breast
carcinoma | No | TSS |
FSH/LH/α-SH-positive | 18
months/alive | (24) |
| | 87 | F | N/A | No | TSS |
FSH/LH-positive | 6 months/alive | (24) |
| Noga et al,
2001 | 60 | M | N/A | No | TCS |
Hormone-negative | Few days/dead | (25) |
| Weber et al,
2003 | 62 | F | Renal
carcinoma | No | TSS |
Hormone-negative | 8 months/dead | (26) |
| Nasr et al,
2006 | 44 | F | GHRH producing
carcinoid | No | TSS | GH-positive | N/A | (27) |
| Jung et al,
2007 | 70 | M | Melanoma | No | TSS |
Hormone-negative | N/A | (28) |
| Hoellig et
al, 2009 | 71 | M | Lung small cell
carcinoma | Yes | TSS | N/A | POD 12/dead | (29) |
| Nassiri et
al, 2012 | 55 | F | Neuroendocrine
tumor | No | TSS | GH-positive | 21 months/dead | (30) |
| Rotondo et
al, 2013 | 66 | M | Bronchial non-small
cell carcinoma | Yes | No |
PRL/GH-positive | POD 1/dead | (31) |
| Thewjitcharoen
et al, 2014 | 65 | M | Colorectal
carcinoma | No | TSS | PRL-positive | 9 months/dead | (32) |
| Sogani et
al, 2014 | 64 | M | Lung
adenocarcinoma | Yes | TSS |
ACTH/PRL-positive | >1
month/alive | (33) |
| Magnoli et
al, 2014 | 76 | F | Renal
carcinoma | No | TSS |
FSH/LH/α-SU-positive | 24 months/dead | (34) |
| Fujimori et
al, 2014 | 80 | M | Lung carcinoma | No | TSS | N/A | 7 months/dead | (35) |
| Yang et al,
2017 | 62 | M | Melanoma | No | TSS | PRL-positive | 22
months/alive | (36) |
| Andreev et
al, 2020 | 55 | F | Breast
carcinoma | No | TSS |
FSH/LH-positive | POD 14/dead | (37) |
| Donofrio et
al, 2023 | 68 | M | Colon
adenocarcinoma | No | TSS |
FSH/LH/SF-1-positive | 9 months/dead | (38) |
| Castle-Kirszbaum
et al, 2020 | 51 | F | Breast
adenocarcinoma | Yes | TSS | N/A | 6 months/alive | (39) |
| Liu et al,
2021 | 65 | F | Lung NEC | No | TSS | N/A | POD 7/Dead | (40) |
| Mills et al,
2022 | 65 | F | Breast
carcinoma | Yes | TSS | FSH-positive | POD 12/Dead | (41) |
| Suzuki et
al, 2024 | 75 | M | Lung
adenocarcinoma | No | TSS |
α-SU/SF-1-positive | 12
months/alive | (42) |
| Present study | 68 | F | Lung NEC | Yes | TSS | N/A | 4 months/dead | |
There are several views on the pathogenesis of
metastatic pituitary carcinoma (43-45).
Metastasis to the posterior pituitary lobe occurs through
hematogenous dissemination via the inferior pituitary artery.
Parasellar malignant tumors can invade the perihypopituitary bony
structures and extend into the pituitary fossa, leading to
thickening of the pituitary stalk and metastasis to the anterior
pituitary lobe (43). Pulmonary
NEC with synchronous metastases to both the pituitary gland and
pineal region has a poor prognosis, with survival primarily
dependent on the grade of the primary tumor. Currently, no
standardized guidelines or expert consensus exists for the
treatment of pulmonary NEC with secondary metastases to the
pituitary and pineal regions, and its management remains a topic of
debate (44). It is considered by
some scholars that surgical resection should be followed by
adjuvant radiotherapy, chemotherapy or targeted therapy. Others
consider that surgery does not prolong the patient's survival and
that a biopsy of the primary tumor should be performed to determine
the nature of the lesion, followed by comprehensive treatments such
as stereotactic radiotherapy and chemotherapy (45). In the present case, the patient was
followed up after surgery but succumbed 4 months later, as the
patient's family refused the use of further adjuvant treatments,
such as radiotherapy and chemotherapy, for personal reasons.
The mechanisms underlying the transformation of
adenocarcinoma into NEC within the pituitary gland remain poorly
understood and involve complex interactions among tumor
heterogeneity, phenotypic plasticity and microenvironmental cues.
Based on a comprehensive review of the literature (39,41,43),
potential mechanisms may primarily involve the following: i)
Adenocarcinoma cells may acquire migratory capacity through
epithelial-mesenchymal transition, followed by
trans-differentiation into NEC phenotypes under the influence of
pituitary-specific microenvironmental factors (for example, hypoxia
and neuroendocrine-derived cytokines); ii) the neuroendocrine-rich
pituitary niche may orchestrate phenotypic conversion through
localized activation of developmental signaling pathways and the
secretion of lineage-directing growth factors; and iii) global DNA
methylation remodeling (hypermethylation of CDH1/E-cadherin
promoters) and histone modification shifts (H3K27me3 demethylation)
may drive the transcriptional silencing of
adenocarcinoma-associated genes while activating
neuroendocrine-specific transcriptional programs.
A limitation of the present study is the absence of
serum/plasma arginine vasopressin (AVP) quantification due to
technical constraints in clinical assay availability (AVP testing
is not routinely supported by the institutional laboratory
infrastructure). While direct AVP measurements were unavailable,
the diagnosis of diabetes insipidus was rigorously supported by the
following: i) Concordant clinical manifestations (for example,
polyuria and polydipsia); ii) characteristic pituitary stalk
thickening and loss of the posterior pituitary bright spot on MRI
(Fig. 1); and iii) symptomatic and
biochemical resolution following desmopressin challenge.
Nevertheless, it should be acknowledged that the lack of AVP data
restricts pathophysiological granularity in distinguishing central
vs. nephrogenic etiologies. Future investigations will incorporate
standardized AVP assays to refine diagnostic precision and enhance
mechanistic insights. A further methodological limitation of this
study is the absence of somatostatin receptor scintigraphy (SRS)
assessments. While SRS represents the gold-standard imaging
modality for neuroendocrine tumor characterization, its
implementation was precluded by current institutional constraints
in radiotracer availability and dedicated γ camera instrumentation.
To address this limitation, alternative diagnostic modalities were
utilized to strengthen the diagnostic rationale: Histopathological
and immunohistochemical analyses of the lung lesion confirmed a
neuroendocrine origin [Syn(+), CgA(+) and TTF-1(+)], with no
histological evidence of metastasis from other primary sites.
In conclusion, pulmonary NEC secondary to pituitary
combined with pineal region metastasis is rare, and since most
primary tumors have insidious symptoms, most patients present with
metastases as their first symptom. Therefore, for cases of
pterygoid or pineal tumors with relatively rapid progression of
neurological symptoms, it is necessary to consider the nature of
metastatic tumors that may be highly malignant, and it is
recommended for patients to undergo surgery as early as possible to
obtain sufficient pathological samples to clarify the diagnosis and
to point out the direction of the subsequent treatment, so as to
avoid delaying treatment of the condition.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Gansu Provincial
Natural Science Foundation (grant no. 22JR5RA695) and Lanzhou
Science and Technology Plan Project (grant no. 2023-2-104).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SP was involved in patient management, wrote the
original manuscript, and contributed to conception, design and data
analysis. JZ and JL contributed to supervision, writing,
conception, design, data analysis, and reviewed and edited the
manuscript. YF participated in patient treatment guidance, data
collection, conception, and reviewed the article's final proofs. SP
and JZ confirm the authenticity of all the raw data and
participated in reviewing and approving the final manuscript
proofs. JL guided and reviewed the writing of the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tsao MS, Nicholson AG, Maleszewski JJ,
Marx A and Travis WD: Introduction to 2021 WHO classification of
thoracic tumors. J Thorac Oncol. 17:e1–e4. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pavel M, Grossman A, Arnold R, Perren A,
Kaltsas G, Steinmüller T, de Herder W, Nikou G, Plöckinger U, Lopes
JM, et al: ENETS consensus guidelines for the management of brain,
cardiac and ovarian metastases from neuroendocrine tumors.
Neuroendocrinology. 91:326–332. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lim KHJ, Valle JW and Lamarca A: Unusual
skull base metastasis from neuroendocrine tumor: A case report. J
Med Case Rep. 13(273)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Krug S, Teupe F, Michl P, Gress TM and
Rinke A: Brain metastases in patients with neuroendocrine
neoplasms: Risk factors and outcome. BMC Cancer.
19(362)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
He W, Chen F, Dalm B, Kirby PA and
Greenlee JDW: Metastatic involvement of the pituitary gland: A
systematic review with pooled individual patient data analysis.
Pituitary. 18:159–168. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sazonova O, Manem V, Orain M,
Khoshkrood-Mansoori B, Gaudreault N, Desmeules P, Bossé Y and
Joubert P: Transcriptomic data helps refining classification of
pulmonary carcinoid tumors with increased mitotic counts. Mod
Pathol. 33:1712–1721. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Oka N, Kasajima A, Konukiewitz B, Sakurada
A, Okada Y, Kameya T, Weichert W, Ishikawa Y, Suzuki H, Sasano H
and Klöppel G: Classification and prognostic stratification of
bronchopulmonary neuroendocrine neoplasms. Neuroendocrinology.
110:393–403. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Quinn AM, Chaturvedi A and Nonaka D:
High-grade neuroendocrine carcinoma of the lung with carcinoid
morphology: A study of 12 cases. Am J Surg Pathol. 41:263–270.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rindi G, Klimstra DS, Abedi-Ardekani B,
Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M,
El-Naggar AK, et al: A common classification framework for
neuroendocrine neoplasms: An International Agency for Research on
Cancer (IARC) and World Health Organization (WHO) expert consensus
proposal. Mod Pathol. 31:1770–1786. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Favero G, Bonomini F and Rezzani R: Pineal
gland tumors: A review. Cancers (Basel). 13(1547)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Palmisciano P, Ogasawara C, Nwagwu CD, Bin
Alamer O, Gupta AD, Giantini-Larsen AM, Scalia G, Yu K, Umana GE,
Cohen-Gadol AA, et al: Metastases in the pineal region: A
systematic review of clinical features, management strategies, and
survival outcomes. World Neurosurg. 159:156–167.e2. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Patel S, Rahmani B, Gandhi J, Seyam O,
Joshi G, Reid I, Smith NL, Waltzer WC and Khan SA: Revisiting the
pineal gland: A review of calcification, masses, precocious
puberty, and melatonin functions. Int J Neurosci. 130:464–475.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Van Der Zwan A, Luyendijk W and Bots GT:
Metastasis of mammary carcinoma in a chromophobe adenoma of the
hypophysis. Psychiatr Neurol Neurochir. 74:369–377. 1971.PubMed/NCBI
|
|
14
|
Richardson JF and Katayama I: Neoplasm to
neoplasm metastasis. An acidophil adenoma harbouring metastatic
carcinoma: A case report. Arch Pathol. 91:135–139. 1971.PubMed/NCBI
|
|
15
|
Burns WA and Kadar AT: Unusual metastases
from a transitional-cell carcinoma of the renal pelvis and ureter.
Med Ann Dist Columbia. 42:65–66. 1973.PubMed/NCBI
|
|
16
|
James RL Jr, Arsenis G, Stoler M, Nelson C
and Baran D: Hypophyseal metastatic renal cell carcinoma and
pituitary adenoma. Case report and review of the literature. Am J
Med. 76:337–340. 1984.PubMed/NCBI View Article : Google Scholar
|
|
17
|
van Seters AP, Bots GT, van Dulken H,
Luyendijk W and Vielvoye GJ: Metastasis of an occult gastric
carcinoma suggesting growth of a prolactinoma during bromocriptine
therapy: A case report with a review of the literature.
Neurosurgery. 16:813–817. 1985.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Molinatti PA, Scheithauer BW, Randall RV
and Laws ER Jr: Metastasis to pituitary adenoma. Arch Pathol Lab
Med. 109:287–289. 1985.PubMed/NCBI
|
|
19
|
Zager EL and Hedley-Whyte ET: Metastasis
within a pituitary adenoma presenting with bilateral abducens
palsies: Case report and review of the literature. Neurosurgery.
21:383–386. 1987.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Post KD, McCormick PC, Hays AP and Kandji
AG: Metastatic carcinoma to pituitary adenoma. Report of two cases.
Surg Neurol. 30:286–292. 1988.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ramsay JA, Kovacs K, Scheithauer BW, Ezrin
C and Weiss MH: Metastatic carcinoma to pituitary adenomas: A
report of two cases. Exp Clin Endocrinol. 92:69–76. 1988.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hurley TR, D'Angelo CM, Clasen RA,
DiGianfilippo A and Ryan WG: Adenocarcinoma metastatic to a
growth-hormone-secreting pituitary adenoma: Case report. Surg
Neurol. 37:361–365. 1992.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abe T, Matsumoto K, Iida M, Hayashi M,
Sanno N and Osamura RY: Malignant carcinoid tumor of the anterior
mediastinum metastasis to a prolactin-secreting pituitary adenoma:
A case report. Surg Neurol. 48:389–394. 1997.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bret P, Jouvet A, Madarassy G, Guyotat J
and Trouillas J: Visceral cancer metastasis to pituitary adenoma:
Report of two cases. Surg Neurol. 55:284–290. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Noga C, Prayson RA, Kowalski R, Sweeney PJ
and Mayberg M: Metastatic adenocarcinoma to a pituitary adenoma.
Ann Diagn Pathol. 5:354–360. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Weber J, Gassel AM, Hoch A and Spring A:
Concomitant renal cell carcinoma with pituitary adenoma. Acta
Neurochir (Wien). 145:227–231. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nasr C, Mason A, Mayberg M, Staugaitis SM
and Asa SL: Acromegaly and somatotroph hyperplasia with adenomatous
transformation due to pituitary metastasis of a growth
hormone-releasing hormone-secreting pulmonary endocrine carcinoma.
J Clin Endocrinol Metab. 91:4776–4780. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jung SM, Hsu YY, Chuang CC, Chang CN,
Hsueh C and Kuo TT: A man in his mid-70s with a sellar mass. Brain
Pathol. 17:115–116, 121. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hoellig A, Niehusmann P, Flacke S and
Kristof RA: Metastasis to pituitary adenoma: Case report and review
of the literature. Cent Eur Neurosurg. 70:149–153. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nassiri F, Cusimano M, Rotondo F, Horvath
E and Kovacs K: Neuroendocrine tumor of unknown origin
metastasizing to a growth hormone-secreting pituitary adenoma.
World Neurosurg. 77:201.e9–201.e12. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rotondo F, Kovacs K, Macdonald RL,
Prud'homme GJ, Latta E and Munoz D: Non-small cell bronchial
carcinoma metastasizing into a prolactin-producing pituitary
adenoma. Int J Surg Pathol. 21:68–71. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Thewjitcharoen Y, Shuangshoti S, Lerdlum
S, Siwanuwatn R and Sunthornyothin S: Colorectal cancer manifesting
with metastasis to prolactinoma: Report of a case involving
symptoms mimicking pituitary apoplexy. Intern Med. 53:1965–1969.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sogani J, Yang W, Lavi E, Zimmerman RD and
Gupta A: Sellar collision tumor involving metastatic lung cancer
and pituitary adenoma: Radiologic-pathologic correlation and review
of the literature. Clin Imaging. 38:318–321. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Magnoli F, Finzi G, Riva C and Capella C:
Renal cell carcinoma metastatic to a pituitary FSH/LH adenoma: Case
report and review of the literature. Ultrastruct Pathol.
38:430–437. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fujimori T, Okauchi M, Shindo A, Kawanishi
M, Miyake K, Kawai N and Tamiya T: Intrapituitary adenoma
metastasis from lung cancer with progressive cranial nerve palsies:
A case report and literature review. No Shinkei Geka. 42:943–949.
2014.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
36
|
Yang C, Liu L, Lan X, Zhang S, Li X and
Zhang B: Progressive visual disturbance and enlarging prolactinoma
caused by melanoma metastasis: A case report and literature review.
Medicine (Baltimore). 96(e6483)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Andreev DN, Kim DS, Shishkina LV, Kalinin
PL, Astafieva LI, Tropinskaya OF, Voronina IA, Turkin AM, Nazarov
VV and Kadashev BA: Breast cancer metastasis into a giant
hormone-inactive pituitary adenoma adenoma. (Clinical case and
literature review). Zh Vopr Neirokhir Im N N Burdenko. 84:55–61.
2020.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
38
|
Donofrio CA, Pizzimenti C, Djoukhadar I,
Kearney T, Gnanalingham K and Roncaroli F: Colorectal carcinoma to
pituitary tumour: tumour to tumour metastasis. Br J Neurosurg.
37:1367–1370. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Castle-Kirszbaum M, Beng Phung T, Luen SJ,
Rimmer J, Chandra RV and Goldschlager T: A pituitary metastasis, an
adenoma and potential hypophysitis: A case report of tumour to
tumour metastasis in the pituitary. J Clin Neurosci. 81:161–166.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu X, Wang R, Li M and Chen G: Pituitary
metastasis of lung neuroendocrine carcinoma mimicking pituitary
adenoma: Case report and literature review. Front Endocrinol
(Lausanne). 12(678947)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mills MT, Wharton SB, Connolly DJ, Mirza S
and Sinha S: Pituitary apoplexy secondary to metastatic breast
carcinoma into a gonadotroph cell adenoma of the pituitary. Br J
Neurosurg. 36:643–646. 2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Suzuki K, Tahara S, Hattori Y, Teramoto S,
Ishisaka E, Inomoto C, Osamura RY, Morita A and Murai Y: Lung
adenocarcinoma metastasis within a pituitary neuroendocrine tumor:
A case report with review of literature. Endocr J. 71:295–303.
2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ng S, Fomekong F, Delabar V, Jacquesson T,
Enachescu C, Raverot G, Manet R and Jouanneau E: Current status and
treatment modalities in metastases to the pituitary: A systematic
review. J Neurooncol. 146:219–227. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kameda-Smith MM, Zhang E, Lannon M, Algird
A, Reddy K and Lu JQ: Pituitary metastasis: From pathology to
clinical and radiological considerations. J Clin Neurosci.
93:231–240. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shimon I: Metastatic spread to the
pituitary. Neuroendocrinology. 110:805–808. 2020.PubMed/NCBI View Article : Google Scholar
|