Introduction
Ovarian cancer (OC) remains a critical public health
concern due to its poor prognosis and high mortality rate (1,2).
According to 2022 GLOBOCAN data, OC is the eighth most common
cancer and the eighth leading cause of cancer-related death among
women worldwide, accounting for 206,956 deaths (3). This high mortality may be attributed
to the lack of symptoms in early-stage disease and the absence of
effective screening methods (4,5).
Consequently, OC is frequently diagnosed at an advanced stage,
contributing to its poor prognosis (6,7).
Additionally, the disease is marked by a high recurrence rate, with
over 80% of cases developing resistance to treatment, leading to a
reduced 5-year survival rate (8).
Epithelial ovarian carcinoma (EOC) is the most
common subtype of OC, comprising ~90% of all ovarian malignancies
(4,9). EOCs are classified into Type I and II
based on their histopathological features and clinical behavior
(10-12).
Low-grade serous ovarian carcinoma (LGSOC), a Type I tumor, is
typically indolent, associated with prolonged overall survival, and
presents a relatively favorable prognosis (11,13,14).
It is often linked to serous borderline tumors, which are
considered common precursor lesions (15,16).
LGSOC is frequently associated with mutations in the MAPK signaling
pathway, particularly involving the KRAS and BRAF genes (17,18).
By contrast, high-grade serous ovarian carcinoma (HGSOC) is a Type
II tumor characterized by aggressive growth, rapid progression, and
poor clinical outcomes. It is frequently diagnosed at an advanced
stage (10,11,19,20).
Serous tubal intraepithelial carcinoma, arising from the distal
fimbriae of the fallopian tube, has been identified as a probable
precursor lesion of HGSOC (21-23).
Genomic analyses have revealed that ~96% of HGSOC cases harbor
mutations in the TP53 gene (24-27).
Astrocyte Elevated Gene-1 (AEG-1), also known as
metadherin, is an oncogene that plays a crucial role in cancer
development and progression (28,29).
AEG-1 interacts with various proteins and activates key oncogenic
signaling pathways, including NF-κB, PI3K/Akt, MEK/ERK and
Wnt/β-catenin (28,30). This activation promotes multiple
cancer hallmarks, such as increased proliferation, migration,
invasion, angiogenesis and metastasis (30-32).
Furthermore, AEG-1 expression has been associated with chemotherapy
resistance in several cancer types, including OC (33,34).
It has been previously shown that elevated AEG-1 expression in EOC
correlates with clinicopathological features such as disease stage,
tumor grade, residual tumor size, lymph node metastasis and poor
prognosis (30).
HGSOC and LGSOC differ in their clinical
characteristics and prognoses (14). They also exhibit distinct responses
to chemotherapy (19). As such,
understanding the molecular differences between these two subtypes
of EOC is essential for guiding appropriate treatment strategies.
Given AEG-1's involvement in cancer progression and its potential
influence on chemotherapy response (28,34),
further investigation is warranted. However, the specific role of
AEG-1 in EOC, particularly in HGSOC and LGSOC, remains unclear.
Therefore, the present study aimed to examine differences in AEG-1
expression between HGSOC and LGSOC and to determine whether AEG-1
expression can be used to distinguish between these two
subtypes.
Materials and methods
Study design and data source
The current retrospective analytical study employed
a cross-sectional design. The research was conducted at the
Division of Gynecological Oncology, Department of Obstetrics and
Gynecology, and the Department of Anatomical Pathology, Faculty of
Medicine (Airlangga University, Dr Soetomo Hospital, Surabaya,
Indonesia). Data were obtained from electronic medical records that
were collected between May 2024 and September 2024. The study
population consisted of women diagnosed with HGSOC or LGSOC who
underwent surgical treatment at Dr Soetomo Hospital between January
2021 and December 2023. All diagnoses were confirmed through
histopathological examination.
The inclusion criteria were as follows: i) complete
medical record data; ii) patients who underwent surgical procedures
at Dr Soetomo Hospital, Surabaya; iii) histopathological
confirmation of either low-grade or HGSOC; and iv) availability of
paraffin blocks stored at the Anatomical Pathology Laboratory of Dr
Soetomo Hospital during the study period (2021 to 2023). Patients
who had received neoadjuvant chemotherapy (NAC) or whose
histopathological results originated from institutions other than
Dr. Soetomo Hospital were excluded.
Primary data were obtained from the medical records
of patients meeting the inclusion and exclusion criteria. The study
materials consisted of paraffin-embedded tumor mass tissue blocks
obtained from surgical procedures conducted at Dr Soetomo Hospital.
Immunohistochemical analysis was performed using an AEG-1 antibody
(cat. no. 517220; Santa Cruz Biotechnology, Inc.). Deparaffination
was performed by immersion in xylene and rehydration was achieved
using a descending ethanol series (96, 90 and 80%). To prevent
endogenous peroxidase activity, 3% H2O2 in
methanol was used at room temperature (RT) for 15 min. The tissue
blocks were incubated with AEG-1 antibody at 4˚C overnight, then
stained with DAB at RT for 5 min and finally counterstained with
Meyer's haematoxylin at RT for 5-10 min.
Data extraction and synthesis
The extracted data included demographic, laboratory
and clinicopathological characteristics. Demographic variables
comprised age, parity, age at menarche and menopausal status. The
laboratory variable assessed was the serum CA 125 level.
Clinicopathological factors included the presence of residual
tumors and cancer stage. The present study evaluated key factors
associated with HGSOC, LGSOC and AEG-1 expression.
The primary objective was to compare AEG-1
expression between HGSOC and LGSOC. Additionally, associations
between the extracted variables and the histopathological subtypes
(HGSOC and LGSOC), as well as the relationship between these
variables and AEG-1 expression, were analyzed.
Expression level of AEG-1
AEG-1 expression levels were classified using a
semiquantitative method based on the proportion of tumor cells
exhibiting positive staining and the intensity of that staining.
The proportion of positive cells was scored as follows: 0, no
positive cells; 1, #x003C;10%; 2, 10-50%; and 3, >50%. Staining
intensity was scored as: 0, no staining; 1, weak; 2, moderate; and
3, strong. The total AEG-1 expression score was calculated by
summing the proportion and intensity scores. A total score of 4 or
higher was considered high AEG-1 expression, while a score of 3 or
lower was considered low AEG-1 expression (35,36).
Statistical analysis
Statistical analysis was performed using IBM SPSS
Statistic version 29.0.0.0 (IBM Corp.). The primary statistical
analysis of the collected data was conducted using the Mann-Whitney
test to assess differences in AEG-1 expression between HGSOC and
LGSOC. In addition, the chi-square test or Fisher's exact test were
used to evaluate the relationships between clinical and demographic
characteristics across the two groups. P#x003C;0.05 was considered
to indicate a statistically significant difference.
Results
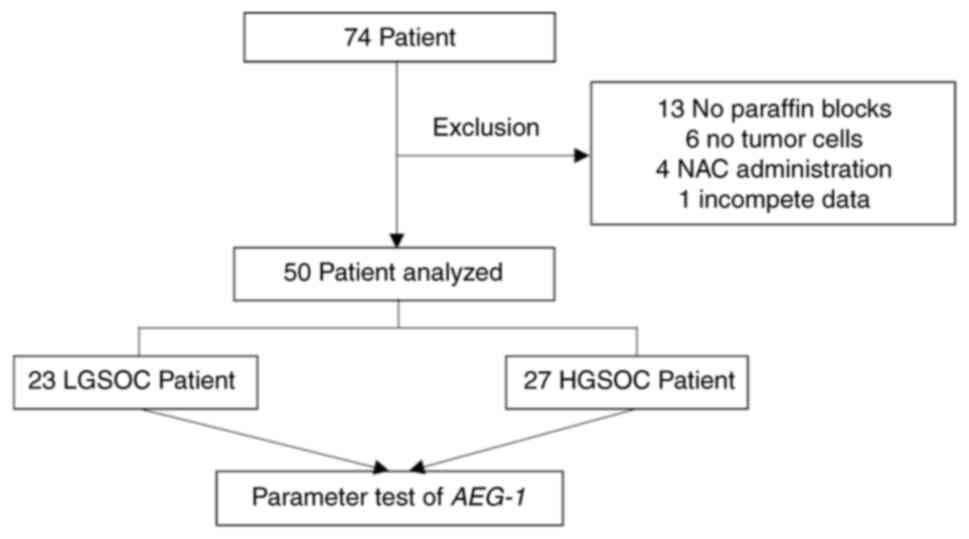
A total of 74 patients were diagnosed with either
HGSOC or LGSOC. Of these, 24 patients met the exclusion criteria,
including 13 patients for whom no paraffin blocks were available,
six patients whose paraffin blocks contained no identifiable tumor
cells, four patients who had received NAC, and one patient with
incomplete data. Based on the inclusion and exclusion criteria, a
final sample of 50 patients was included in the study. The cohort
consisted of 27 patients with HGSOC and 23 with LGSOC (Fig. 1).
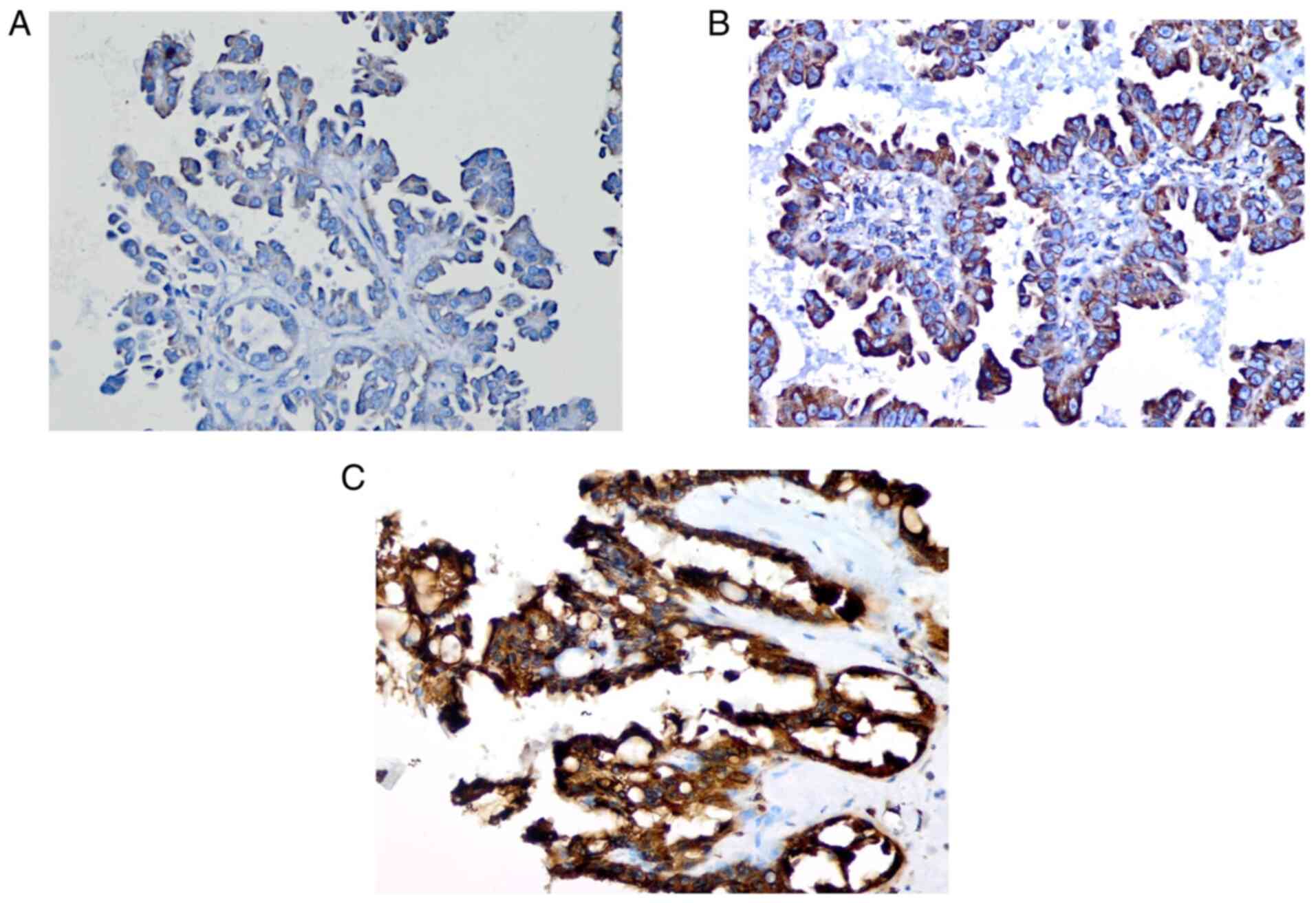
AEG-1 expression was evaluated by
immunohistochemistry. AEG-1 was predominantly localized in the
cytoplasm, nuclear membrane, or cell junctions. Staining was
assessed across 10 randomly selected high-power fields
(magnification, x400) representative of the average tumor size
(Fig. 2).
Characteristics of research
subjects
Among the 50 participants, 34 (68%) were under 55
years of age, and 16 (32%) were over 55. A total of 19 patients
(38%) were nulliparous, while 21 (42%) were multiparous. Early
menarche (age, #x003C;12 years) was reported in 26 participants
(52%), whereas 24 (48%) experienced menarche at age >12 years.
Menopausal status was evenly distributed, with 25 patients (50.0%)
post-menopausal and 25 (50%) pre-menopausal. Normal CA 125 levels
were observed in 5 samples (10%), while elevated levels (>35
ng/ml) were identified in 45 samples (90%). Residual tumor size
#x003C;1 cm was found in 37 patients (74%), and >1 cm in 13
patients (26%). Early-stage disease (FIGO stages I and II) was
diagnosed in 25 patients (50%), and advanced-stage disease (stages
III and IV) in the remaining 25 (50%). AEG-1 expression was low in
15 samples (30%) and high in 35 samples (70%). Regarding
histological subtype, 23 cases (46%) were classified as LGSOC and
27 (54%) as HGSOC (Table I).
 | Table IDescriptive distribution of patient
data. |
Table I
Descriptive distribution of patient
data.
| Patient
characteristics | n (%) |
|---|
| Age, years | |
|
≤55 | 34(68) |
|
>55 | 16(32) |
| Parity | |
|
Nullipara | 19(38) |
|
Primipara | 10(20) |
|
Multipara | 21(42) |
| Menarche | |
|
Early
menarche | 26(52) |
|
Normal
menarche | 24(48) |
| Menopausal
status | |
|
No | 25(50) |
|
Yes | 25(50) |
| CA 125 level | |
|
Normal | 5(10) |
|
High | 45(90) |
| Residual tumor | |
|
≤1 cm | 37(74) |
|
>1
cm | 13(26) |
| Stage | |
|
Early
stage | 25(50) |
|
Advanced
stage | 25(50) |
| AEG-1
expression | |
|
Low | 15(30) |
|
High | 35(70) |
| Ovarian cancer | |
|
Low
grade | 23(46) |
|
High
grade | 27(54) |
Relationship between the
characteristics of research subjects and OC
The characteristics associated with OC incidence
were analyzed to determine whether age, parity, menarche,
menopausal status, CA 125 levels, residual tumor and cancer stage
were related to LGSOC or HGSOC (Table
II).
 | Table IICharacteristic evaluation for ovarian
cancer. |
Table II
Characteristic evaluation for ovarian
cancer.
| | Ovarian cancer | |
|---|
| Patient
characteristics | Total (n=50) | Low grade, n
(%) | High grade, n
(%) | Raw P-value | Adjusted
P-value |
|---|
| Age, years | | | | | |
|
≤55 | 34 | 16 (69.6) | 18 (66.7) | 1.000 | 1.167 |
|
>55 | 16 | 7 (30.4) | 9 (33.3) | | |
| Parity | | | | | |
|
Nullipara | 19 | 7 (30.4) | 12 (44.4) | 0.399 | 0.698 |
|
Primipara | 10 | 4 (17.4) | 6 (22.2) | | |
|
Multipara | 21 | 12 (52.2) | 9 (33.3) | | |
| Menarche | | | | | |
|
Early
menarche | 26 | 14 (60.9) | 12 (44.4) | 0.382 | 0.891 |
|
Normal
menarche | 24 | 9 (39.1) | 15 (55.6) | | |
| Menopausal
status | | | | | |
|
No | 25 | 13 (56.5) | 12 (44.4) | 0.570 | 0.798 |
|
Yes | 25 | 10 (43.5) | 15 (55.6) | | |
| CA 125 level | | | | | |
|
Normal | 5 | 0 (0.0) | 5 (18.5) | 0.054a | 0.189 |
|
High | 45 | 23(100) | 22 (81.5) | | |
| Residual tumor | | | | | |
|
≤1 cm | 37 | 21 (91.3) | 16 (59.3) | 0.024 | 0.168 |
|
>1
cm | 13 | 2 (8.7) | 11 (40.7) | | |
| Stage | | | | | |
|
Early
stage | 25 | 12 (52.2) | 13 (48.1) | 1.000 | 1.000 |
|
Advanced
stage | 25 | 11 (47.8) | 14 (51.9) | | |
As shown in Table
II, patients aged ≤55 years were slightly more common in the
LGSOC group (69.6%) compared with the HGSOC group (66.7%).
Nulliparous and primiparous women were more frequently observed in
the HGSOC group (44.4 and 22.2%, respectively) than in the LGSOC
group (30.4 and 17.4%, respectively). By contrast, multiparous
women were more prevalent in the LGSOC group (52.2%) compared with
the HGSOC group (33.3%). Early menarche (age, #x003C;12 years)
occurred more often in patients with LGSOC (60.9%) than in those
with HGSOC (44.4%). Among post-menopausal women, HGSOC was more
prevalent (55.6%) than LGSOC (43.5%). Elevated CA 125 levels were
observed in all patients with LGSOC (100%) and in 81.5% of those
with HGSOC. Advanced-stage disease was more frequently found in
HGSOC (51.9%) than in LGSOC (47.8%). Tumor residuals >1 cm were
more commonly observed in HGSOC (40.7%) compared with LGSOC
(8.7%).
According to the statistical analysis, only the
variable related to residual tumor size yielded a P-value
#x003C;0.05 (P=0.024), with an odds ratio (OR) of 7.219 [95%
confidence interval (CI): 1.399-37.252], indicating a statistically
significant association. The P-values and ORs for the other
variables were as follows: age, P=1.000, OR=1.143 (95% CI:
0.346-3.777); parity, P=0.399; menarche, P=0.382, OR=1.944 (95% CI:
0.628-6.021); menopausal status, P=0.570, OR=1.625 (95% CI:
0.530-4.984); CA 125 level, P=0.540; and stage, P=1.000, OR =1.175
(95% CI: 0.386-3.576). These results suggest that no statistically
significant associations were found between histological subtype
(HGSOC or LGSOC) and the variables of age, parity, menarche,
menopausal status, CA 125 level, or cancer stage.
Relationship between the
characteristics of research subjects and AEG-1 expression
The association between AEG-1 expression and various
clinical and demographic characteristics including age, parity,
menarche, menopausal status, CA 125 level, residual tumor presence
and cancer stage-was analyzed to explore potential relationships
with low and high AEG-1 expression (Table III).
 | Table IIICharacterization test based on AEG-1
expression. |
Table III
Characterization test based on AEG-1
expression.
| | Expression level of
AEG-1 | |
|---|
| Patient
characteristics | Total (n=50) | Low, n (%) | High, n (%) | Raw P-value | Adjusted
P-value |
|---|
| Age, years | | | | | |
|
≤55 | 34 | 8 (53.3) | 26 (74.3) | 0.191a | 0.668 |
|
>55 | 16 | 7 (46.7) | 9 (25.7) | | |
| Parity | | | | | |
|
Nullipara | 19 | 6 (40.0) | 13 (37.1) | 0.275 | 0.641 |
|
Primipara | 10 | 1 (6.7) | 9 (25.7) | | |
|
Multipara | 21 | 8 (53.3) | 13 (37.1) | | |
| Menarche | | | | | |
|
Early
menarche | 26 | 11 (73.3) | 15 (42.9) | 0.095 | 0.665 |
|
Normal
menarche | 24 | 4 (26.7) | 20 (57.1) | | |
| Menopausal
status | | | | | |
|
No | 25 | 7 (46.7) | 18 (51.4) | 1.000 | 1,167 |
|
Yes | 25 | 8 (53.3) | 17 (48.6) | | |
| CA 125 level | | | | | |
|
Normal | 5 | 0 (0.0) | 5 (14.3) | 0.305a | 0,533 |
|
High | 45 | 15 (100.0) | 30 (85.7) | | |
| Residual tumor | | | | | |
|
≤1 cm | 37 | 11 (73.3) | 26 (74.3) | 1.000a | 1.000 |
|
>1
cm | 13 | 4 (26.7) | 9 (25.7) | | |
| Stage | | | | | |
|
Early
stage | 25 | 9 (60.0) | 16 (45.7) | 0.537 | 0.751 |
|
Advanced
stage | 25 | 6 (40.0) | 19 (54.3) | | |
According to Table
III, patients aged ≤55 years had a higher prevalence of high
AEG-1 expression (74.3%) compared with those with low AEG-1
expression (53.3%). Nulliparous and multiparous women showed a
greater prevalence of low AEG-1 expression (40.0 and 53.3%,
respectively) than high AEG-1 expression, which was observed in
37.1% of patients in both groups. By contrast, primiparous women
exhibited a higher prevalence of high AEG-1 expression (25.7%)
compared with low AEG-1 expression (6.7%). Early menarche (age,
#x003C;12 years) was more frequent among individuals with low AEG-1
expression (73.3%) than among those with high expression (42.9%).
Among pre-menopausal patients, a higher prevalence of high AEG-1
expression was observed (51.4%) compared with low expression
(46.7%). Elevated CA 125 levels were more common among patients
with low AEG-1 expression (100.0%) than those with high expression
(85.7%). Advanced-stage disease was slightly more associated with
high AEG-1 expression (54.3%) than low expression (40.0%). Residual
tumors >1 cm were slightly more common in patients with low
AEG-1 expression (26.7%) compared with those with high AEG-1
expression (25.7%).
The P-values for each variable were as follows: age,
P=0.191, OR=0.396 (95% CI: 0.112-1.403); parity, P=0.275; menarche,
P=0.095, OR=3.667 (95% CI: 0.974-13.806); menopausal status,
P=1.000, OR=0.826 (95% CI: 0.246-2.776); CA 125 level, P=0.305;
residual tumor, P=1.000, OR=0.952 (95% CI: 0.241-3.756); and stage,
P=0.537, OR=1.781 (95% CI: 0.521-6.085). Since all P-values
exceeded the threshold of 0.05, none of the assessed
characteristics revealed a statistically significant association
with AEG-1 expression.
Differential expression of AEG-1 in
OC
AEG-1 expression was analysed to determine whether
there was a significant difference between patients with LGSOC and
those with HGSOC. The comparison was conducted using the
Mann-Whitney U test (Table
IV).
 | Table IVAnalysis of differences in AEG-1
expression in ovarian cancer. |
Table IV
Analysis of differences in AEG-1
expression in ovarian cancer.
| | Expression level of
AEG-1 | |
|---|
| Ovarian cancer | Total (n=50) | Low, n (%) | High, n (%) | Mann-Whitney U | Sig. P-value | Relative risk (95%
confidence interval) | Effect size |
|---|
| Low grade | 23 | 11 (73.3) | 12 (34.3) | 160 | 0.012 | 3.228
(1.188-8.776) | 0.745 |
| High grade | 27 | 4 (26.7) | 23 (65.7) | | | | |
Among the 23 LGSOC samples, low AEG-1 expression was
identified in 11 samples (73.3%), while high AEG-1 expression was
observed in 12 samples (34.3%). By contrast, among the 27 HGSOC
samples, low AEG-1 expression was found in four samples (26.7%),
whereas high AEG-1 expression was detected in 23 samples (65.7%).
Statistical analysis yielded a relative risk (RR) of 3.228, with a
95% CI of 1.188-8.776) and P=0.012.
Discussion
LGSOC and HGSOC are distinct tumor types with
differing morphological features, pathogenesis and molecular
profiles (17). LGSOC generally
has a more favorable prognosis than HGSOC (11,37).
In the present study, the initial cohort included 74 patients
diagnosed with either HGSOC or LGSOC, comprising 45 patients with
HGSOC and 29 with LGSOC. This distribution aligns with existing
literature indicating that HGSOC is more prevalent than LGSOC
(13,38). HGSOC is the most common subtype of
EOC, accounting for ~2/3 of cases and representing ~70% of the
total incidence (21,39). By contrast, LGSOC comprises ~5% to
10% of all serous ovarian carcinoma cases (40,41).
The present study investigated several demographic
characteristics, including age, parity, menarche and menopausal
status. While research exploring the relationship between these
variables and AEG-1 expression is limited, numerous studies suggest
these factors may influence the risk of developing OC (42). The current findings revealed that
younger patients were more frequently diagnosed with LGSOC than
with HGSOC, which is consistent with studies that LGSOC tends to
occur at a younger age than HGSOC (9,19,41,43).
Infertility has been cited as a risk factor for OC
in several studies (1,2,42).
Higher parity is associated with fewer ovulatory cycles, which may
reduce the opportunity for tumor development due to
ovulation-induced injury of the ovarian epithelium (5,44).
Additionally, elevated progesterone levels during pregnancy are
considered to protect against ovarian carcinogenesis by inhibiting
cell proliferation and promoting apoptosis in ovarian epithelial
cells (44). A previous study
reported that increased parity correlates with a sustained
reduction in the risk of EOC (RR=0.81; 95% CI: 0.77-0.86) (5). Specifically, higher parity was linked
to a significant decrease in LGSOC incidence (RR=0.84; 95% CI:
0.76-0.93), while no significant association was found with HGSOC
(RR=0.97; 95% CI: 0.92-1.02) (heterogeneity: P=0.01) (45). Conversely, another study reported
that having more than two pregnancies significantly reduced the
risk of HGSOC (RR=0.25; 95% CI: 0.19-0.50) (46).
Hormonal and reproductive factors are among the most
significant additional risk factors for EOC (44,47).
The total number of menstrual cycles throughout a woman's life is
positively correlated with an increased risk of EOC, highlighting
the potential role of repeated ovulation in ovarian carcinogenesis
(47). As a result, early menarche
and late menopause, which prolong the ovulatory lifespan, can
elevate the risk of developing the disease (2,42).
Although specific literature on the role of menarche in LGSOC is
currently lacking, it has been suggested that both early menarche
and late menopause may increase cumulative estrogen exposure,
potentially contributing to a higher risk of HGSOC (48,49).
CA 125 is widely recognized as the most important
tumor biomarker for the screening and detection of EOC (50). Elevated serum CA 125 levels are
observed in ~50% of early-stage tumors, primarily Type I cancers
such as LGSOC and in ~92% of advanced-stage cases, predominantly
HGSOC (50,51). Higher CA 125 levels have been
associated with increased AEG-1 expression, likely due to AEG-1's
role in promoting tumor cell proliferation and cancer progression
(28,30,34).
However, the present findings did not fully align with previous
studies, as elevated CA 125 levels were more frequently observed
across both groups in our dataset. As a result, the expected cell
counts in the statistical analysis exceeded 20%, introducing
uncertainty in interpreting the association between CA 125 levels
and AEG-1 expression.
HGSOC is characterized by marked aggressiveness in
its proliferation, invasion and metastatic behavior, often
resulting in diagnosis at advanced stages and a higher likelihood
of residual tumor masses >1 cm (52,53).
This understanding supports the current findings, which
demonstrated that a significantly greater number of HGSOC cases
presented with residual tumor sizes >1 cm compared with LGSOC
(OR=7.219; 95% CI: 1.399-37.252). While this result is consistent
with existing literature, the wide CI suggests that the sample size
may have been insufficient. Historically, achieving a maximum
residual tumor diameter of #x003C;2 cm was considered indicative of
successful cytoreductive surgery (54). However, more recent evidence
identified that reducing the residual tumor burden to #x003C;1 cm
provides improved survival outcomes, and it is now widely accepted
that complete macroscopic resection (that is, no visible residual
disease or R0) offers the greatest survival benefit (53).
AEG-1 contributes to cancer development and
progression by activating multiple oncogenic signaling pathways
(28,30). Specifically, AEG-1 enhances the
PI3K/Akt pathway, promoting the phosphorylation of murine double
minute 2 (MDM2) by Akt. This phosphorylation facilitates MDM2's
nuclear translocation, leading to the degradation of p53 and
enabling continued tumor cell proliferation (30,55,56).
Additionally, the PI3K/Akt pathway is involved in angiogenesis, as
Akt activation upregulates HIF-1 expression, which in turn
increases VEGF transcription (35,57,58).
AEG-1 also plays a key role in cancer cell migration, invasion and
metastasis through the activation of the NF-κB pathway (30). Within this pathway, AEG-1 interacts
with multiple components, serving as a crucial mediator in NF-κB
activation and the subsequent induction of inflammation (59,60).
Moreover, by inhibiting retinoid X receptor function, AEG-1 has
emerged as a significant regulator of lipid metabolism and the
tumor microenvironment during cancer development (59).
According to current theoretical frameworks, AEG-1
is associated with the clinicopathological characteristics of
various cancers, including OC. Previous studies investigating AEG-1
expression in OC have demonstrated significant associations with
several clinical variables, including age over 55 years (P=0.031),
advanced FIGO stage (P#x003C;0.001), higher histological grade
(P#x003C;0.001), elevated CA 125 levels (>35 U/ml,
P#x003C;0.001), residual tumor size >1 cm (P#x003C;0.001) and
lymph node metastasis (P=0.027) (30).
By contrast, the present study did not identify a
statistically significant association between AEG-1 expression and
these clinicopathological characteristics. However, this does not
diminish the relevance of the findings. Limitations such as a
relatively small sample size and uneven group distribution likely
impacted the statistical power, underscoring the need for future
studies with larger cohorts or alternative study designs.
Compared with LGSOC, AEG-1 appears to play a role
consistent with the more aggressive biological behavior of HGSOC,
contributing to increased cancer cell proliferation and
invasiveness (28,30,36).
HGSOC is clinically recognized for its aggressive progression and
poor prognosis (10,11,22).
The findings of the present study support this distinction,
revealing that AEG-1 expression was significantly higher in HGSOC
than in LGSOC (RR=3.228; 95% CI: 1.188-8.776) with the effect size,
calculated using Cohen's D, is 0.745. These findings suggest that
the aggressive clinical course of HGSOC may be partly driven by
elevated levels of AEG-1 expression.
In conclusion, the present study demonstrated a
significant difference in AEG-1 expression between HGSOC and LGSOC,
with higher levels observed more frequently in HGSOC. These
findings contribute to an improved understanding of the molecular
distinctions between these two subtypes of EOC and may serve as
foundational data for future research. Ultimately, such insights
can support more tailored therapeutic approaches and improve
patient outcomes and quality of life.
Acknowledgements
The authors are grateful to Dr Sri Ratna Dwiningsih,
the supervisor of the study program at the Department of Obstetrics
and Gynecology at Airlangga University, for her support in
conducting this research. Mr Ary Wahyudiono, a laboratory
technician at the Department of Pathology at Universitas Airlangga
in Surabaya, assisted in the preparation of paraffin blocks for
this investigation. Ms Renata Alya Ulhaq, a medical writer,
contributed to the manuscript's substance by ensuring its
comprehensiveness.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BI and BAT were responsible for the design of the
present study, the analysis, and the composition of the manuscript.
BI and BU conducted the data extraction and performed an analysis
of the results. GA performed the histological examination and image
analysis. WS, IY, and PM contributed essential revisions to the
document. BI, BAT and BU confirm the authenticity of all the raw
data. All authors contributed to the interpretation of data and the
revision of the manuscript, read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board (IRB) of Dr Soetomo Hospital Surabaya (approval no.
1641/LOE/301.4/2/IV/2024; Surabaya, Indonesia). Data management
adhered carefully to patient confidentiality and privacy
regulations. Anonymized and de-identified data were used to uphold
participant rights and privacy. The IRB approved the use of
anonymised patient data after careful evaluation of the limited
associated risks. Acquiring written informed consent was waived by
the IRB of Dr. Soetomo Hospital Surabaya.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang J, Chan WC, Ngai CH, Lok V, Zhang L,
Lucero-Prisno DE III, Xu W, Zheng ZJ, Elcarte E, Withers M, et al:
Worldwide burden, risk factors, and temporal trends of ovarian
cancer: A global study. Cancers. 14(2230)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Andreou M, Kyprianidou M, Cortas C,
Polycarpou I, Papamichael D, Kountourakis P and Giannakou K:
Prognostic factors influencing survival in ovarian cancer patients:
A 10-year retrospective study. Cancers (Basel).
15(5710)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
International Agency for Research on
Cancer (IARC): Absolute numbers, Incidence and Mortality, Females,
in 2022. IARC, Lyon, 2022.
|
|
4
|
Akter S, Rahman MA, Hasan MN, Akhter H,
Noor P, Islam R, Shin Y, Rahman MDH, Gazi MS, Huda MN, et al:
Recent advances in ovarian cancer: Therapeutic strategies,
potential biomarkers, and technological improvements. Cells.
11(650)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Momenimovahed Z, Tiznobaik A, Taheri S and
Salehiniya H: Ovarian cancer in the world: Epidemiology and risk
factors. Int J Womens Health. 11:287–299. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kurnit KC, Fleming GF and Lengyel E:
Updates and new options in advanced epithelial ovarian cancer
treatment. Obstet Gynecol. 137:108–121. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Menon U, Gentry-Maharaj A, Burnell M,
Singh N, Ryan A, Karpinskyj C, Carlino G, Taylor J, Massingham SK,
Raikou M, et al: Ovarian cancer population screening and mortality
after long-term follow-up in the UK Collaborative trial of ovarian
cancer screening (UKCTOCS): A randomised controlled trial. Lancet.
397:2182–2193. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li Y, Cen Y, Tu M, Xiang Z, Tang S, Lu W,
Zhang H and Xu J: Nanoengineered gallium ion incorporated
formulation for safe and efficient reversal of PARP inhibition and
platinum resistance in ovarian cancer. Research (Wash D C).
6(0070)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Goulding EA, Simcock B, McLachlan J, van
der Griend R and Sykes P: Low-grade serous ovarian carcinoma: A
comprehensive literature review. Aust N Z J Obstet Gynaecol.
60:27–33. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Darelius A, Kristjansdottir B, Dahm-Kähler
P and Strandell A: Risk of epithelial ovarian cancer Type I and II
after hysterectomy, salpingectomy and tubal ligation-A nationwide
case-control study. Int J Cancer. 149:1544–1552. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pavlik EJ, Smith C, Dennis TS, Harvey E,
Huang B, Chen Q, Piecoro DW, Burgess BT, McDowell A, Gorski J, et
al: Disease-specific survival of type I and II epithelial ovarian
cancers-stage challenges categorical assignments of indolence &
aggressiveness. Diagnostics. 10(56)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu N, Zhang X, Fang C, Zhu M, Wang Z, Jian
L, Tan W, Wang Y, Li H, Xu X, et al: Progesterone enhances
niraparib efficacy in ovarian cancer by promoting
palmitoleic-acid-mediated ferroptosis. Research (Wash D C).
7(0371)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Grisham RN, Slomovitz BM, Andrews N,
Banerjee S, Brown J, Carey MS, Chui H, Coleman RL, Fader AN,
Gaillard S, et al: Low-grade serous ovarian cancer: Expert
consensus report on the state of the science. Int J Gynecol Cancer.
33:1331–1344. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Matsuo K, Machida H, Matsuzaki S, Grubbs
BH, Klar M, Roman LD, Sood AK, Gershenson DM and Wright JD:
Evolving population-based statistics for rare epithelial ovarian
cancers. Gynecol Oncol. 157:3–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dey P, Nakayama K, Razia S, Ishikawa M,
Ishibashi T, Yamashita H, Kanno K, Sato S, Kiyono T and Kyo S:
Development of low-grade serous ovarian carcinoma from benign
ovarian serous cystadenoma cells. Cancers. 14(1506)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Di Lorenzo P, Conteduca V, Scarpi E,
Adorni M, Multinu F, Garbi A, Betella I, Grassi T, Bianchi T, Di
Martino G, et al: Advanced low grade serous ovarian cancer: A
retrospective analysis of surgical and chemotherapeutic management
in two high volume oncological centers. Front Oncol.
12(970918)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
De Leo A, Santini D, Ceccarelli C,
Santandrea G, Palicelli A, Acquaviva G, Chiarucci F, Rosini F,
Ravegnini G, Pession A, et al: What is new on ovarian carcinoma:
Integrated morphologic and molecular analysis following the new
2020 world health organization classification of female genital
tumors. Diagnostics (Basel). 11(697)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hollis RL, Thomson JP, van Baal J,
Ilenkovan N, Churchman M, van de Vijver K, Dijk F, Meynert AM,
Bartos C, Rye T, et al: Distinct histopathological features are
associated with molecular subtypes and outcome in low grade serous
ovarian carcinoma. Sci Rep. 13(7681)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Romero I, Leskelä S, Mies BP, Velasco AP
and Palacios J: Morphological and molecular heterogeneity of
epithelial ovarian cancer: Therapeutic implications. EJC. 15:1–15.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yu H, Wang J, Wu B, li J and Chen R:
Prognostic significance and risk factors for pelvic and para-aortic
lymph node metastasis in type I and II ovarian cancer: A large
population-based database analysis. J Ovarian Res.
16(28)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Timofeeva AV, Asaturova AV, Sannikova MV,
Khabas GN, Chagovets VV, Fedorov IS, Frankevich VE and Sukhikh GT:
Search for new participants in the pathogenesis of high-grade
serous ovarian cancer with the potential to be used as diagnostic
molecules. Life (Basel). 12(2017)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang Y, Duval AJ, Adli M and Matei D:
Biology-driven therapy advances in high-grade serous ovarian
cancer. J Clin Invest. 134(e174013)2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang S, Dolgalev I, Zhang T, Ran H,
Levine DA and Neel BG: Both fallopian tube and ovarian surface
epithelium are cells-of-origin for high-grade serous ovarian
carcinoma. Nat Commun. 10(5367)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bischof K, Knappskog S, Hjelle SM,
Stefansson I, Woie K, Salvesen HB, Gjertsen BT and Bjorge L:
Influence of p53 isoform expression on survival in high-grade
serous ovarian cancers. Sci Rep. 9(5244)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim YM, Lee SW, Lee YJ, Lee HY, Lee JE and
Choi EK: Prospective study of the efficacy and utility of TP53
mutations in circulating tumor DNA as a non-invasive biomarker of
treatment response monitoring in patients with high-grade serous
ovarian carcinoma. J Gynecol Oncol. 30(e32)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tuna M, Ju Z, Yoshihara K, Amos CI, Tanyi
JL and Mills GB: Clinical relevance of TP53 hotspot mutations in
high-grade serous ovarian cancers. Br J Cancer. 122:405–412.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vitale SR, Groenendijk FH, van Marion R,
Beaufort CM, Helmijr JC, Dubbink HJ, Dinjens WNM, Ewing-Graham PC,
Smolders R, van Doorn HC, et al: TP53 mutations in serum
circulating cell-free tumor DNA as longitudinal biomarker for
high-grade serous ovarian cancer. Biomolecules.
10(415)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sriramulu S, Sun XF, Malayaperumal S,
Ganesan H, Zhang H, Ramachandran M, Banerjee A and Pathak S:
Emerging role and clinicopathological significance of aeg-1 in
different cancer types: A concise review. Cells.
10(1497)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yao L, Liu L, Xu W, Xi H, Lin S, Piao G,
Liu Y, Guo J and Wang X: mRNA-seq-based analysis predicts: AEG-1 is
a therapeutic target and immunotherapy biomarker for pan-cancer,
including OSCC. Front Immunol. 15(1484226)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Khan M and Sarkar D: The scope of
astrocyte elevated gene-1/metadherin (AEG-1/MTDH) in cancer
clinicopathology: A review. Genes (Basel). 12(308)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ghafar MT and Soliman NA: Chapter
six-metadherin (AEG-1/MTDH/LYRIC) expression: Significance in
malignancy and crucial role in colorectal cancer. Adv Clin Chem.
106:235–280. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen Y, Huang S, Guo R and Chen D:
Metadherin-mediated mechanisms in human malignancies. Biomark Med.
15:1769–1783. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu J, Jiao X and Gao Q: Neoadjuvant
chemotherapy-related platinum resistance in ovarian cancer. Drug
Discov Today. 25:1232–1238. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Manna D and Sarkar D: Multifunctional role
of astrocyte elevated gene-1 (AEG-1) in cancer: Focus on drug
resistance. Cancers (Basel). 13(1792)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ding Q, Chen Y, Dong S, Xu X, Liu J, Song
P, Yu C and Ma Z: Astrocyte elevated gene-1 is overexpressed in
non-small-cell lung cancer and associated with increased tumour
angiogenesis. Interact Cardiovasc Thorac Surg. 26:395–401.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li C, Liu J, Lu R, Yu G, Wang X, Zhao Y,
Song H, Lin P, Sun X, Yu X, et al: AEG -1 Overexpression: A novel
indicator for peritoneal dissemination and lymph node metastasis in
epithelial ovarian cancers. Int J Gynecol Cancer. 21:602–608.
2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nowak M and Klink M: The role of
tumor-associated macrophages in the progression and chemoresistance
of ovarian cancer. Cells. 9(1299)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Torkildsen CF, Thomsen LCV, Sande RK,
Krakstad C, Stefansson I, Lamark EK, Knappskog S and Bjørge L:
Molecular and phenotypic characteristics influencing the degree of
cytoreduction in high-grade serous ovarian carcinomas. Cancer Med.
12:14183–14195. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Millstein J, Budden T, Goode EL, Anglesio
MS, Talhouk A, Intermaggio MP, Leong HS, Chen S, Elatre W, Gilks B,
et al: Prognostic gene expression signature for high-grade serous
ovarian cancer. Ann Oncol. 31:1240–1250. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Voutsadakis IA: Low-grade serous ovarian
carcinoma: An evolution toward targeted therapy. Int J Gynecol
Cancer. 30:1619–1626. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
De Decker K, Wenzel HHB, Bart J, van der
Aa MA, Kruitwagen RFPM, Nijman HW and Kruse AJ: Stage, treatment
and survival of low-grade serous ovarian carcinoma in the
Netherlands: A nationwide study. Acta Obstet Gynecol Scand.
102:246–256. 2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Webb PM and Jordan SJ: Global epidemiology
of epithelial ovarian cancer. Nat Rev Clin Oncol. 21:389–400.
2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang Q, Cao SH, Li YY, Zhang JB, Yang XH
and Zhang B: Advances in precision therapy of low-grade serous
ovarian cancer: A review. Medicine (Baltimore).
103(e34306)2024.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Huang T, Townsend MK, Wentzensen N,
Trabert B, White E, Arslan AA, Weiderpass E, Buring JE, Clendenen
TV, Giles GG, et al: Reproductive and hormonal factors and risk of
ovarian cancer by tumor dominance: Results from the ovarian cancer
cohort consortium (OC3). Cancer Epidemiol Biomarkers Prev.
29:200–207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gaitskell K, Green J, Pirie K, Barnes I,
Hermon C, Reeves GK and Beral V: Histological subtypes of ovarian
cancer associated with parity and breastfeeding in the prospective
million women study. Int J Cancer. 142:281–289. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sung S, Hong Y, Kim BG, Choi JY, Kim JW,
Park SY, Kim JH, Kim YM, Lee JM, Kim TJ and Park SK: Stratifying
the risk of ovarian cancer incidence by histologic subtypes in the
korean epithelial ovarian cancer study (Ko-EVE). Cancer Med.
12:8742–8753. 2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Flaum N, Crosbie EJ, Edmondson RJ, Smith
MJ and Evans DG: Epithelial ovarian cancer risk: A review of the
current genetic landscape. Clin Genet. 97:54–63. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wilczyński J, Paradowska E and Wilczyński
M: High-grade serous ovarian cancer-a risk factor puzzle and
screening fugitive. Biomedicines. 12(229)2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen W, Liu H, Huang X, Qian L, Chen L,
Zhou Y, Liu Y, Liu Y, Wang Y, Zhang T, et al: A single-cell
landscape of pre- and post-menopausal high-grade serous ovarian
cancer ascites. iScience. 26(107712)2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Charkhchi P, Cybulski C, Gronwald J, Wong
FO, Narod SA and Akbari MR: Ca125 and ovarian cancer: A
comprehensive review. Cancers. 12:1–29. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Salminen L, Nadeem N, Jain S, Grènman S,
Carpén O, Hietanen S, Oksa S, Lamminmäki U, Pettersson K, Gidwani
K, et al: A longitudinal analysis of CA125 glycoforms in the
monitoring and follow up of high grade serous ovarian cancer.
Gynecol Oncol. 156:689–694. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ciucci A, Zannoni GF, Buttarelli M,
Martinelli E, Mascilini F, Petrillo M, Ferrandina G, Scambia G and
Gallo D: Ovarian low and high grade serous carcinomas: Hidden
divergent features in the tumor microenvironment. Oncotarget.
7:68033–68043. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Porter JM, McFarlane I, Bartos C,
Churchman M, May J, Herrington CS, Connolly KC, Ryan NAJ and Hollis
RL: The survival benefit associated with complete macroscopic
resection in epithelial ovarian cancer is histotype specific. JNCI
Cancer Spectr. 8(pkae049)2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Irodi A, Rye T, Herbert K, Churchman M,
Bartos C, Mackean M, Nussey F, Herrington CS, Gourley C and Hollis
RL: Patterns of clinicopathological features and outcome in
epithelial ovarian cancer patients: 35 years of prospectively
collected data. BJOG. 127:1409–1420. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chibaya L, Karim B, Zhang H and Jones SN:
Mdm2 phosphorylation by Akt regulates the p53 response to oxidative
stress to promote cell proliferation and tumorigenesis. Proc Natl
Acad Sci USA. 118(e2003193118)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wei C, Du J, Shen Y, Wang Z, Lin Q, Chen
J, Zhang F, Lin W, Wang Z, Yang Z and Ma W: Anticancer effect of
involucrasin A on colorectal cancer cells by modulating the
Akt/MDM2/p53 pathway. Oncol Lett. 25(218)2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Umapathy D, Karthikeyan MC, Ponnuchamy K,
Kannan MK, Ganeshan M and Arockiam AJV: The absence of cellular
glucose triggers oncogene AEG-1 that instigates VEGFC in HCC: A
possible genetic root cause of angiogenesis. Gene.
826(146446)2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhao T, Zhao C, Zhou Y, Zheng J, Gao S and
Lu Y: HIF-1α binding to AEG-1 promoter induced upregulated AEG-1
expression associated with metastasis in ovarian cancer. Cancer
Med. 6:1072–1081. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Rajesh Y, Reghupaty SC, Mendoza RG, Manna
D, Banerjee I, Subler MA, Weldon K, Lai Z, Giashuddin S, Fisher PB,
et al: Dissecting the balance between metabolic and oncogenic
functions of astrocyte-elevated gene-1/metadherin. Hepatol Commun.
6:561–575. 2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Rong C, Shi Y, Huang J, Wang X, Shimizu R,
Mori Y, Murai A and Liang J: The effect of metadherin on NF-κB
activation and downstream genes in ovarian cancer. Cell Transpl.
29(0963689720905506)2020.PubMed/NCBI View Article : Google Scholar
|