During flare-ups of chronic inflammatory diseases,
humans are more susceptible to various types of cancer, including
breast cancer, colorectal cancer, and gastric mucosal cancer. Tumor
transformation caused by chronic inflammation accounts for 15% of
the total cancer cases worldwide (1). Chronic inflammation is typically
characterized by tissue damage, followed by cell proliferation and
repair at the site of injury, where inflammatory factors are
recruited. Among these factors, the significant increase in the
interleukin (IL)-6 family of cytokines has been strongly linked to
the progression and aggressiveness of tumors, particularly in
relation to their staging and ability to invade surrounding tissues
(2). Various cytokines within the
IL-6 family, including IL-6, IL-11, IL-27, and IL-31, are known to
accelerate tumor growth by influencing and modulating the tumor
microenvironment (TME) (3). These
inflammatory factors not only affect the occurrence and development
of tumors but also constitute an integral part of the tumor
microenvironment. The tumor microenvironment is a comprehensive
concept, encompassing immune cell responses, activation,
differentiation, and cytokine secretion. It can either promote or
counteract tumor development and mediate disease validation and
resistance, depending on the interference of specific cytokines
(4). Neuroendocrine tumors (NETs)
are a rare, heterogeneous disease characterized by the secretion of
growth hormones (5). In NET cells,
the signaling and activation of the STAT3/IL-6 axis are essential
in driving tumor growth, supporting its survival, and influencing
cellular differentiation (6,7).
Therefore, the expression levels of the IL-6 family may vary among
patients with NETs. This review provides an overview of the
association between the IL-6 family and NETs over the past decade,
categorizing and summarizing the role and impact of the IL-6 family
in different types of NETs, and supplements the previous
shortcomings of single tumor descriptions related to IL-6.
To understand the process and steps of tumor
development, the tissue response surrounding the tumor must be
included in the research scope, which encompasses circulation and
metastasis. A tumor consists of a complex array of tissues,
including the extracellular matrix (ECM), activated fibroblasts,
chemokines, inflammatory factors, and blood and lymphatic vessels,
all of which are collectively referred to as the TME (8). Inflammatory factors and other
substances in the TME can activate downstream proteins or genes
(9). In addition, various factors
of the TME interact and influence each other, collectively
promoting tumor progression or metastasis. Notably, the TME not
only comprises normal tissues, but also dysregulated factors, such
as microRNAs (miRNAs/miRs) in the vicinity of tumor tissues. miRNAs
of tumor cell origin are closely associated with the production of
immunologically heterogeneous TMEs and the loss of effector cells,
and they are determinants of the immune outcome of cancer (10,11).
These tumor cell-derived miRNAs can be seeded in different TME
regions, and because miRNAs subtly suppress genes and
preferentially inhibit dose-sensitive targets, they are key to
immune-mediated tumor clearance (12,13).
In the TME, miRNAs can promote gastric cancer cell immune escape by
targeting granule 2 and activating the binding of zinc finger E-box
to the homology box 1/programmed cell death ligand 1 (PD-L1) axis,
which enhances the inhibitory effect of gastric malignant cells on
T-cell activation (14). In the
lung TME, lung tumor cells secrete miR-21/29 to target
intracellular Toll-like receptor 8, which induces the secretion of
NK-κB-mediated pro-inflammatory factors TNF-α and IL-6 to achieve
tumor cell invasion (15). In
colorectal tumors, miR-27 can alter tumor antigen presentation, and
higher levels of miR-27 in colorectal cancer has been shown to
reduce T-cell infiltration, which is associated with a poor
prognosis after distant metastasis (16). It is now clear that miRNAs serve an
important role in the TME; however, it is unclear whether they have
a role in the tumor escape process. It has been shown that tumor
cells secrete miRNA-rich exocrine vesicles, which are involved in
signaling pathways, such as PTEN/AKT and suppressor of cytokine
signaling 1 (SOCS1)/STAT1, inducing high PD-L1 expression in the
TME and suppressing CD8+ T cells, which can thus promote
tumor immune escape and progression (17). Song et al (18) also demonstrated another way in
which miRNAs disrupt antigen presentation by participating in the
IFNγ-activated Janus kinase (JAK)/STAT signaling pathway in cancer
cells, while also silencing SOCS1 and promoting the tumor escape
process.
NETs are a group of heterogeneous malignant tumors
that originate from cells with neuroendocrine features, dispersed
across various organs and systems in the body (19,20).
The most common site for NETs is the gastrointestinal tract, and
the extensive cellular diversity reflects the heterogeneity of the
disease itself (21). The
pathogenic mechanisms of NETs vary depending on the original
structure of the cells (22).
These pathogenic processes may involve pathways such as
enterochromaffin-like cell hyperplasia, gene mutations or
chromosome loss, miRNA-assisted dissemination, the impact of lipids
or saturated fatty acids on oxidative stress, and cross-talk or
abnormal activation of signaling pathways (23-29).
The present review mainly focuses on the mechanisms and novel
insights into how inflammatory factors, including the IL-6 family,
form NETs through signaling pathway cross-talk or abnormal
activation.
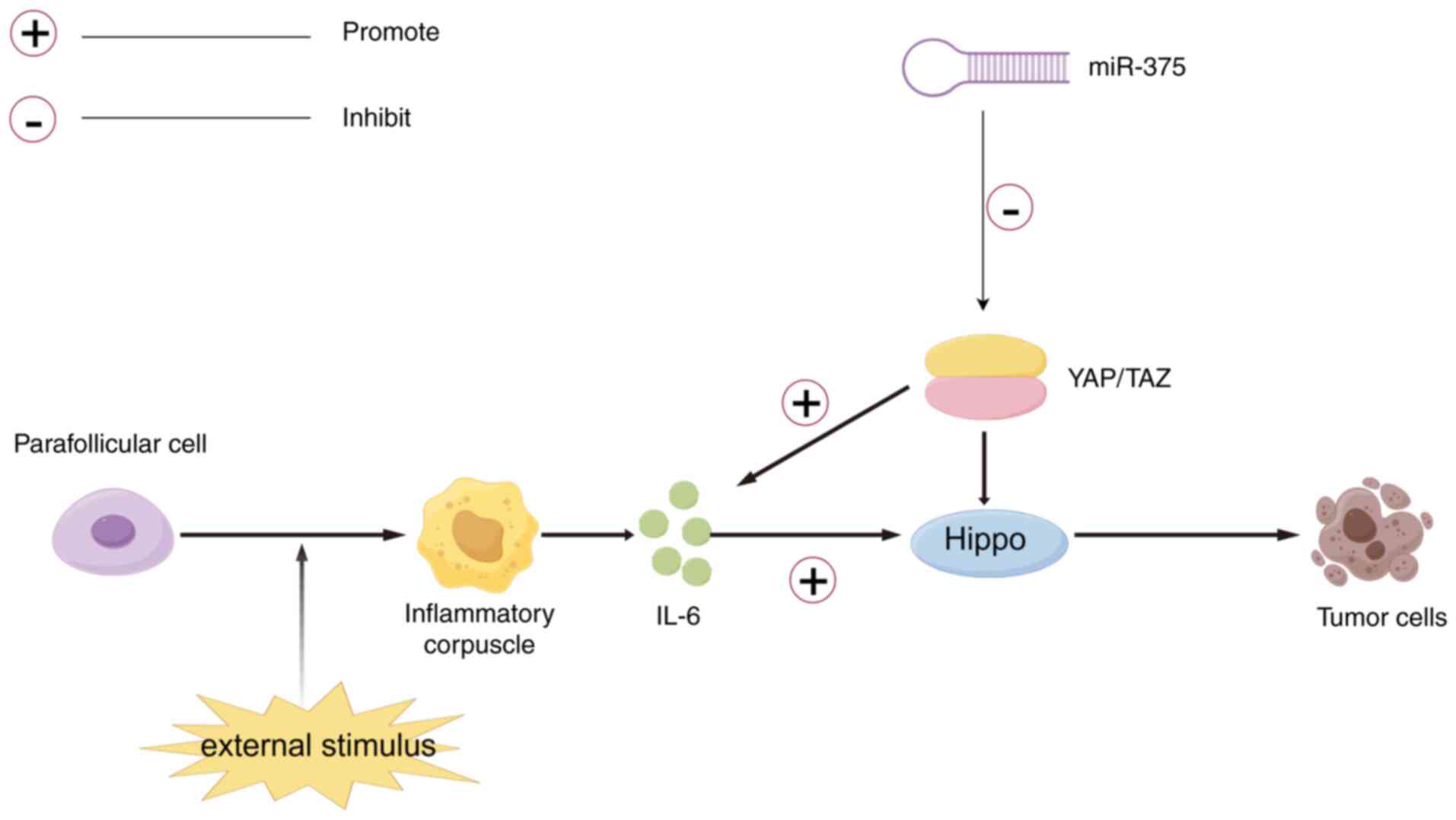
MTC primarily arises from the parafollicular cells
of the thyroid and accounts for 3-10% of global cases (30). Research has shown that
dysregulation of the Hippo pathway is associated with the
development of various tumors, including MTC (31). The Hippo pathway transmits both
intracellular and extracellular signals to control cell migration,
proliferation and differentiation (32). In mammals, two transcriptional
co-activators, Yes-associated protein (YAP) and TAZ, have been
identified; these can activate upstream phosphorylated kinases of
the Hippo pathway to exert tumor-suppressive effects on the pathway
(31-33).
When YAP is dephosphorylated in tumor cells, it promotes the
expression of YAP/TAZ, and while disrupting the Hippo pathway, YAP
can also stimulate the activation of IL-6 in cancer cells (34,35).
The abundant expression of IL-6 promotes tumor cell cycle
progression and inhibits apoptosis. Moreover, IL-6 is involved in
epithelial-mesenchymal transition (EMT) in tumor cells, a mechanism
associated with cancer cell invasion and metastasis (35). IL-6 and EMT interact with the TME
and the Hippo pathway, influencing the onset and spread of MTC
(36). Research has demonstrated
that miR-375 is significantly upregulated in MTC, whereas it is not
highly expressed in other thyroid histopathologies, and that there
is an association between miR-183 and miR-375 upregulation in MTC
and lateral cervical lymph node metastasis and mortality. Notably,
miR-375 causes YAP to be downregulated; therefore, it may be useful
to assess how miR-375 functions in MTC, and whether it is also via
the Hippo pathway (37) (Fig. 1).
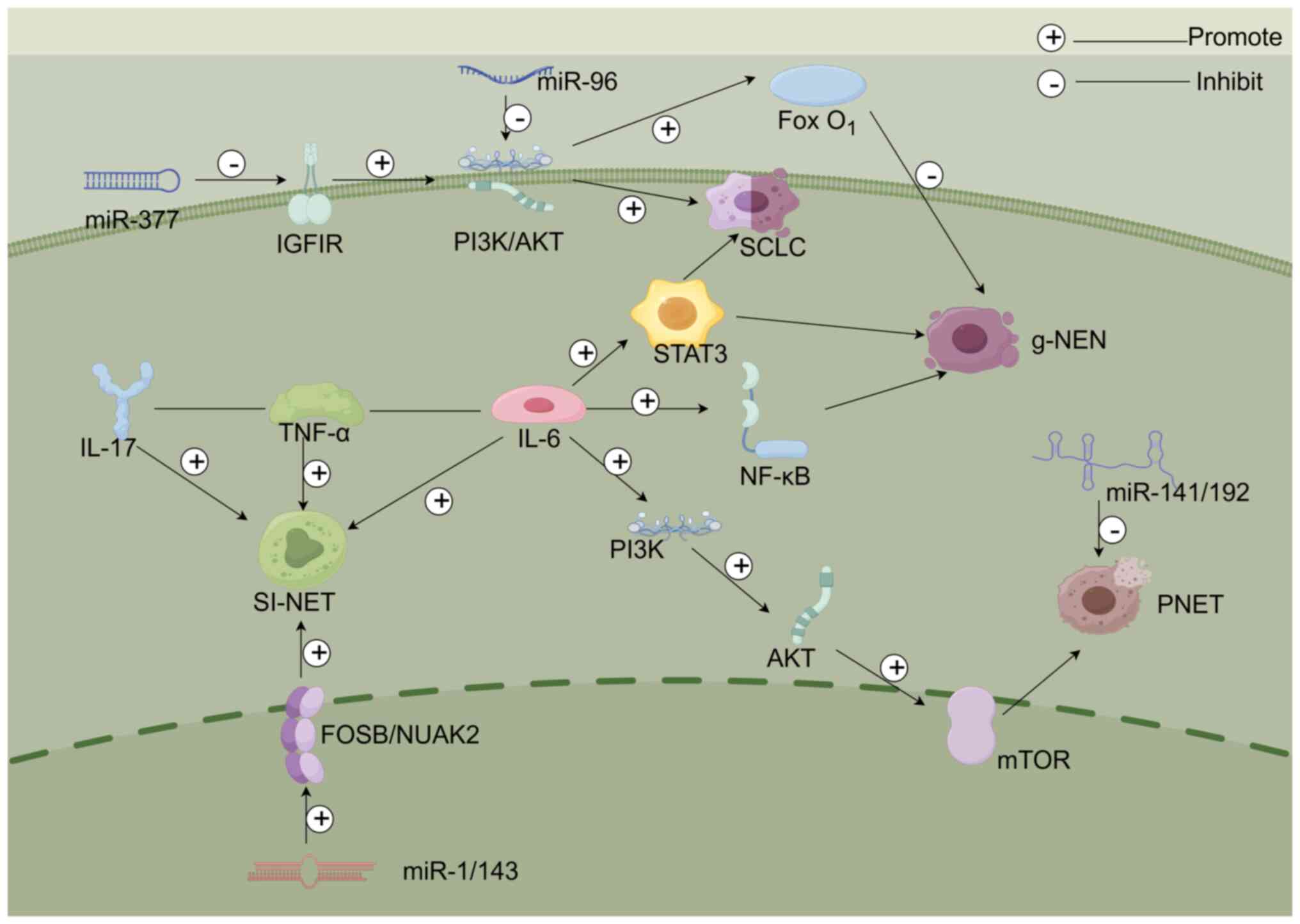
SCLC is classified as a type of pulmonary NET.
Notably, SCLC is typically diagnosed at a late stage, making both
diagnosis and treatment more challenging (38). Abnormal secretion of antidiuretic
hormone is a prominent feature of SCLC, with paraneoplastic
Cushing's syndrome being the second most common characteristic
(39). It has also been observed
that activation of STAT3 is common in SCLC cases (40). The results of a retrospective study
have indicated that macrophage-derived factors, such as IL-6, are
involved in the activation of STAT3 in SCLC cells (41). This suggests that IL-6 may
stimulate the expression of STAT3, thereby influencing the
proliferation or metastasis of SCLC. Furthermore, to investigate
the specific heterogeneity of SCLC, Lu et al (42) conducted a study and revealed that
the neuroendocrine marker expression in SCLC neuroendocrine cells
co-cultured with fibroblasts was decreased, whereas neuroendocrine
marker protein expression in SCLC cells cultured alone was
increased. Furthermore, this previous study continued to explore
the mechanism by which fibroblasts induce the reduction of
neuroendocrine marker expression and demonstrated that the
phosphorylation levels of JAK2/STAT3 in SCLC cells co-cultured with
fibroblasts were elevated (42).
Additionally, NOTCH signaling has been shown to serve a role in the
reprogramming of biomarker phenotypes in SCLC, with c-MYC as a key
upstream regulatory factor (43).
Notably, it has been shown that IL-6 from fibroblasts indeed
activates the JAK2/STAT3 pathway in SCLC cells, and phosphorylated
STAT3 binds to and upregulates MYC expression upon fibroblast
stimulation (42). In addition,
the insulin-like growth factor 1 (IGF1)/IGF1R signaling axis can
crosstalk with multiple pathways to affect the development of
malignant tumors, in which IGF1R triggers the PI3K/AKT signaling
pathway, driving the advancement of SCLC, whereas miR-377
enrichment can inhibit IGF1R and suppress SCLC metastasis (44) (Fig.
2).
NETs can progress throughout the entire digestive
tract, with the highest incidence observed in the small intestine
(53). Small intestinal NETs
originate from the enterochromaffin cells of the gastrointestinal
tract. Because these tumors grow slowly, the majority of patients
are diagnosed at later stages, with liver metastasis being the most
prevalent type of metastasis. Additionally, small intestinal NETs
can secrete excessive hormones, leading to carcinoid syndrome in
20% of patients (54-56).
Research has indicated that these tumors express TNF-α and IL-17B
cytokines, and the ECM also expresses TNF-α. Specifically, IL-17
partially activates downstream targets by triggering NF-κB and
phosphorylated STAT3. Moreover, STAT3 binds to the SYP promoter,
suggesting that the expression of IL-17 may correlate with SYP.
Furthermore, IL-17 activates TNF-α-related signaling pathways
(57-60).
In addition, the IL-17 cytokine synergizes with IL-6 and TNF-α to
activate STAT3, promoting the growth of intestinal tumors (60).
Rectal NETs are the second most frequent type of NET
after small intestinal NETs, accounting for 12-27% of all
gastrointestinal NETs (61-63).
Rectal NETs originate from neuroendocrine cells and their
malignancy status can be uncertain, with some exhibiting
neuroendocrine functions while others do not. The incidence of
rectal NETs is higher than that of colonic NETs (64). Due to the low incidence and
difficulty in detecting colorectal tumors, the mechanisms and
treatment approaches remain to be explored, which may represent a
novel direction for future discoveries.
In enteric NETs, miR-204-5p/miR-375 expression has
been shown to be increased, whereas miR-1 and miR-143 expression
has been revealed to be decreased. Furthermore, the reduced
expression of miR-1 and miR-143 has been reported to activate the
oncogenes FOSB and NUAK2, which in turn can enhance lymph node and
liver metastasis in enteric NETs (52). Finally, sustained elevation of IL-6
promotes fribroblast proliferation and alters the expression of
miRNAs that regulate various signaling pathways, contributing to
the development of intestinal fibrosis; the most notable
consequence of intestinal fibrosis is its effects on intestinal
structure, impairing nutrient absorption and destroying the
microflora, ultimately leading to malignant transformation
(65).
PNETs are rare tumors considered to arise from the
pancreatic islet cells, which normally secrete insulin and glucagon
(66). PNETs are highly
heterogeneous tumors that exhibit a wide range of pathological
features; they can be classified into different grades based on the
Ki67 index, mitotic count and morphological characteristics
(67). Although PNETs are
categorized as well-differentiated or poorly differentiated, most
of them are well-differentiated (68). While the gene mutation rate in
PNETs is relatively low, there are mutations related to
tumorigenesis and progression, such as those in the multiple
endocrine neoplasia 1 (MEN1) and α-thalassemia/mental retardation
syndrome X-linked (ATRX) genes and the mTOR pathway (69). Some studies have shown that genomic
alterations in well-differentiated PNETs include changes in RNA
expression after activation of the mTOR pathway, aneuploidy, and
loss of MEN1 and MUTYH germline genes (28,70,71).
According to previous studies, MEN1 is the most common mutation in
PNETs, and 37% of cases have altered MEN1 gene expression.
Furthermore, in PNETs, MEN1 is involved in mTOR signaling, histone
modification and DNA repair damage, which determines cell fate
(72-74).
ATRX mutations cause disorders in chromatin remodeling and mitotic
function during chromosome mitosis, and these abnormalities may
lead to chromosomal mutations ultimately contributing to the
development of PNETs (75). In
addition, a recent study has shown that loss of ATRX/death domain
associated protein and the presence of alternative lengthening of
telomeres may serve as predictors of distant metastasis, and are
associated with poorer overall survival and relapse-free survival
in non-functional PNETs measuring ≤2.0-cm (76). The association between patients
with PNETs and mutations in mTOR signaling-related genes is high,
and these mutations lead to upregulation of mTOR activity, which is
closely linked to poor prognosis and aggressive tumor behavior in
patients with PNETs (72,77).
MEN1 encodes menin, a nuclear protein that is
involved in chromatin remodeling during DNA replication,
functioning as a scaffold to regulate gene transcription. In
addition, menin is an important component of the histone
methyltransferase complex. When the MEN1 gene is mutated, DNA
replication and chromosome division are affected, which can lead to
a disrupted state of cytokinesis and differentiation. In a
validation set of 68 PNET cases, mutations in the MEN1 gene were
identified in 30 cases, suggesting MEN1 as a major driver of PNET
development. In addition, 12 of the 68 cases harbored mutations in
the ATRX gene, which encodes a protein located in the structural
domain of the helicase enzyme. Notably, the activity of the
helicase enzyme is altered when ATRX is mutated, which is also a
major factor affecting PNET (78).
Research has shown that MEN1 also serves a role in regulating mTOR
signaling. mTOR has a regulatory role in lipid peroxidation and
ferroptosis, and MEN1 overexpression has been shown to inhibit the
mTOR signaling pathway. At the same time, mTOR regulates lipid
peroxidation and ferroptosis in PNET; thus, MEN1 inhibits the mTOR
signaling pathway, as well as lipid peroxidation and ferroptosis,
in PNET. In addition, mTOR, as a signaling hub, serves an important
role in regulating amino acid, glucose, nucleotide, fatty acid and
lipid metabolism. When MEN1 is upregulated and inhibits mTOR, it
also has an effect on basic cellular metabolism and energy
conversion, which is one of the major reasons for the occurrence of
PNETs (78).
These aforementioned mutations can be targeted for
PNET treatment, particularly in cases where MEN1 mutations occur.
In low-grade, intermediate-stage PNETs, mTOR pathway inhibitors can
serve as potential therapeutic targets (78). According to Li et al
(79), IL-6 can act as an upstream
factor to enhance activation of the PI3K/AKT/mTOR pathway.
Therefore, it may be hypothesized that IL-6 or other inflammatory
factors could directly or indirectly influence mTOR, modulating the
expression of this signaling pathway and thereby achieving
therapeutic effects on PNETs. Moreover, single nucleotide
polymorphisms (SNPs) in genes encoding regulatory cytokines may
have a role in promoting or inhibiting the development and
progression of PNETs. Karakaxas et al (80) reported that an SNP in the IL-1β
gene, rs16944, appears to be associated with PNETs. In another
study, the characteristics of functional and non-functional PNETs
were revealed to be associated with differences in an IL-6 SNP,
described as rs1800795. Compared with patients with functional
PNETs, those with non-functional PNETs exhibited higher serum
levels of IL-6, and a positive correlation between IL-6 levels and
IL-6 gene polymorphisms was observed exclusively in patients with
non-functional PNETs (80,81). Thus, IL-6 not only influences tumor
development through signaling pathways or the TME, but may also
directly affect tumor cells through its own encoded nucleotide
sequence, impacting progression of the tumor.
Evidence has suggested that changes in macrophage
subtypes in the TME serve an important role in tumor progression
and metastasis. Heterogeneity of macrophage phenotypes has been
observed in tumor-associated macrophages (TAMs) of various
malignancies; however, CD163+ TAMs may be the major
population, and in PNETs, a previous study has shown that higher
CD163+ cell counts are associated with lymph node
metastasis, perineural invasion and lymphovascular invasion,
suggesting that CD163+ TAMs are an indicator of poor
prognosis. Furthermore, CD163+ macrophages are
associated with poor disease-free survival and disease-specific
survival in PNETs (82). In PNETs,
PIWI-interacting RNA-hsa-30937 can promote CD276 upregulation in
macrophages via the PTEN/AKT pathway, and CD276 suppresses T-cell
antitumor immunity; this results in fewer antitumor cells in the
TME, leading to a homeostatic bias towards pro-tumor
characteristics (83). In addition
to this, when hypoxia occurs, TAMs in the TME undergo M2-like
polarization, and the M2-polarized macrophages promote the
formation of pre-metastatic ecological niches and facilitate the
metastasis of pancreatic NENs through the release of MMP2, which is
a pro-carcinogenic alteration of the TME (84). Furthermore, miR-141 has been shown
to exert tumor-suppressive effects in various types of cancer,
including pancreatic cancer. By contrast, the role of the
miR-192/194 family in cancer remains intricate and controversial,
with findings suggesting both oncogenic and tumor-suppressive
effects. Specifically, miR-192 has been found to suppress tumor
angiogenesis, whereas miR-194 inhibits cancer cell proliferation,
migration and metastasis, and promotes apoptosis (85).
PitNETs are among the most common brain tumors,
accounting for 10-25% of all brain tumors. Most patients with these
tumors can be treated with surgery or medications (86). A notable factor influencing the
development of PitNETs is the TME, which is crucial in mediating
the interaction between the tumor and the host immune response
(87,88). PitNETs are typically characterized
by a high degree of macrophage infiltration within the pituitary
gland (68-70).
Studies have confirmed the presence of both M1 and M2 macrophages
in estrogen-induced PitNETs, with the expression levels of the M2
polarization factor IL-4 being significantly higher than normal
(89-91).
Therefore, tumor-associated macrophages in the TME may influence
tumor initiation and progression through cytokines and other
factors. In addition to tumor progression driven by the TME,
M2-like macrophages secrete C-C motif chemokine ligand 17 (CCL17),
which enhances the invasive ability of tumor cells through the
CCL17/CCR4/mTOR axis (92). IL-6
can also interact with the mTOR pathway to induce PD-L1(93). Thus, PitNETs can be broadly
categorized into two types, according to their progression: One is
driven by the interaction between the TME and the tumor, and the
other involves the participation of cytokines, such as IL-6 or
other factors, that activate or suppress related signaling
pathways, influencing tumor progression. Wang et al revealed
that miR-134 expression is reduced in PitNETs, and its target gene
vascular endothelial growth factor A (VEGFA) is upregulated,
accompanied by an increase in tumor invasiveness. Further research
has shown that miR-134 may inhibit tumor development by suppressing
VEGFA expression. Regarding the mechanism, VEGFA expression is
related to the PI3K/AKT signaling pathway and miR-134 is an
important component of this signaling pathway. Therefore,
upregulation of miR-134 may inhibit PI3K by activating downstream
VEGFA, thus resulting in tumor suppression (94).
miRNA expression profiles can be used as practical
biomarkers for the diagnosis and prognosis of NETs, providing
sufficient information for appropriate patient care and management
(95-99).
It has been reported that there is a direct link between miR-31 and
advanced disease stage and poor survival. miR-31 is also highly
expressed in patients with advanced breast and gastrointestinal
cancers, where it is correlated with poor prognosis, and miR-31
interacts with downstream target genes, thus enhancing the
likelihood of tumors to spread (100). Upregulation of miR-21 has been
shown to be significantly associated with advanced clinical stage,
lymph node involvement and survival in breast cancer. Furthermore,
reduced expression of miR-126/335 has been linked to cancer
metastasis (101).
Notably, various miRNA families have been
investigated as potential diagnostic biomarkers. Upregulation of
miR-21/200/210/182 has been associated with lung tumor progression,
and miR-30/451 has been linked with heterogeneous tumor behavior
(102). Furthermore, not only do
miRNAs serve as diagnostic biomarkers for lung cancer detection,
but they also play a role in prognosis and treatment (103). In breast cancer, miR-21
downregulation and the miR-32-5p/TOB1 axis could negatively
regulate the progression of triple-negative breast cancer cells by
inhibiting the expression of the oncogene TOB1. In osteosarcoma, it
was shown that HNF1A-AS1 binds to miR-32-5p to regulate the
expression of high mobility group box 1-induced apoptosis, and
prevents the proliferation, migration and invasion of osteosarcoma
cells. In retinoblastoma, miR-32 was revealed to be upregulated and
to competitively bind to long non-coding RNAs to regulate
retinoblastoma signaling pathways. miR-32 upregulation
significantly inhibited the proliferation, migration and invasion
of ovarian cancer cells through the regulation of the target gene B
and T lymphocyte attenuator. In colorectal and lung cancers,
downregulation of miR-32 expression was also associated with tumor
prognosis and progression (104).
However, in terms of the sensitivity and specificity of miRNAs, it
is unclear as to whether their diagnostic role in tumors is highly
specific. It may be possible to use double or multiple tracers of
numerous miRNAs in the same tumor, which could improve the
diagnostic value.
Another challenge is that the aforementioned miRNAs
identified in laboratory screenings, cannot currently be extracted
from clinical pathology samples, making it unclear how to
effectively apply miRNAs as biomarkers in clinical practice. In a
study from a clinical perspective, Maués et al (105) employed a method to detect miRNAs
in the normal human platelet small RNA-sequencing data sourced from
the GSE61856 repository. Their research identified a new set of
miRNAs (miR-486-5p, miR-92a-3p, miR-103a-3p, miR-151a-3p,
miR-181a-5p and miR-221-3p) that exhibited sensitive expression
patterns due to biological changes in platelets during storage.
These miRNAs could act as potential indicators for assessing the
quality and viability of platelet concentrates during storage
(106).
It is known that the IL family can function as
upstream factors or downstream targets of miRNAs, affecting their
differential expression, and the post-transcriptional regulatory
mechanisms of IL-6 expression have been extensively studied. The
majority of these regulatory factors have been shown to inhibit
IL-6 expression by binding to the 3' untranslated region of the
mRNA, leading to its degradation (118). However, further exploration is
required to determine whether miRNAs are upregulated when chronic
inflammation or the TME leads to IL-6 upregulation, as well as to
assess which miRNAs will be upregulated and whether these
IL-6-induced miRNAs could serve as biomarkers for NETs. After
immune activation, serum IL-6 levels rapidly rise under the
influence of cytokines, Toll-like receptor agonists, prostaglandins
and other stress signals. At this time, certain miRNAs will
regulate IL-6 gene expression (119). The present review aimed to only
address the interaction between IL-6 and miRNAs. Considering the
upstream and downstream genes or signaling pathways, it has been
shown that miRNA interference with IL-6 expression is influenced by
certain molecules and the JAK/STAT signaling pathway. It has been
established that miR-34 and miR-218 are tumor suppressors, and when
miR-34 is silenced, the incidence of colitis-associated intestinal
tumors in mice increases, with these tumors typically being larger
and characterized by increased IL-6 receptor expression and STAT3
activation (120,121). Other miRNAs have also been shown
to target STAT3(122). STAT3
activation regulates the IL-6 cytokine family, promoting tumor
development, influencing cell cycle progression and driving tumor
invasion (119). By contrast,
negative regulators of the JAK/STAT cascade are also inhibited by
miRNA-mediated suppression. miR-155 and miR-19 have been shown to
target SOCS1 and SOCS3, thus inhibiting the JAK/STAT signaling
pathway (123,124). IL-6 was shown to enhance cell
adhesion in human endothelial cells through the inhibition of
miR-126-3p (125). MiR-1254
enhanced proteasome stability in rectal tumors by activating the
IL-6/STAT3 pathway (126).
MiR-892c increased IL-6 expression and promoted macrophage
polarization (127). MiR-30c has
been shown to target IL-6 in mesenchymal stem cells, offering a
novel approach for the treatment of colon cancer (128). The IL-6/miR-30d axis may also
impact chemotherapy resistance in colorectal cancer (129). In summary, the relationship
between IL-6 and microRNAs remains to be fully elucidated.
Therefore, based on the aforementioned findings, it may be inferred
that miRNAs and IL-6 cytokine pathways regulate each other.
Whether the increase in IL-6-induced miRNAs can be
used as a tumor biomarker to assess disease requires further
research. Currently, it is clear that in chronic inflammation,
there is a close relationship between inflammatory factors, miRNAs
and signaling pathways. To study miRNAs as tumor biomarkers, it is
necessary to consider the upstream and downstream signaling
pathways, rather than evaluating them in isolation. According to
research, the levels of serum IL-6 are increased with the decrease
in survival rates for late-stage tumors. This signifies that in
patients with advanced tumors, serum IL-6 is elevated, and the
increase in IL-6 levels has been reported to be due to the
activation of upstream epithelial growth factor receptor (EGFR)
through the NF-κB pathway, with the elevation of EGFR being caused
by the amplification of the malignant tumor gene malignant T-cell
amplified sequence 1 (MCT-1) (130,131). Through the analysis of cytokine
arrays and cancer stem cells, it has been reported that miR-34a
antagonizes MCT-1, and high expression of MCT-1 reduces miR-34a.
miR-34a is a non-coding RNA that has been shown to inhibit the
progression of cancer cells. In essence, high expression of miR-34a
leads to reduced levels of MCT-1/EGRF/NF-κB/IL-6(132). Therefore, using MCT-1 antagonists
or increasing the expression of miR-34a, may represent a combined
strategy for treating malignant tumors. As to whether miR-34a
inhibits the expression of IL-6, thereby affecting tumor
progression, it is known that IL-6 can activate the signal
transduction of transcription factors and the activation of the
STAT family, with STAT3 located within the cell. Once
phosphorylated and activated, STAT3 can translocate to the nucleus
and bind to the promoter regions of target genes, including miR-21.
Researchers have verified this through in vitro experiments
in mouse cells, showing that phosphorylated STAT3 is expressed at
higher levels in cells treated with IL-6, leading to an increase in
the expression levels of miR-21. Additionally, previous CUT&RUN
sequencing experiments have confirmed that IL-6 regulates the
expression of miR-21 in ectopic mesenchymal cells through
phosphorylated STAT3 (133-135).
The aforementioned findings describe the advantages
of miRNAs as biomarkers; however, they do not address the
sensitivity and specificity of miRNAs in distinguishing between
different types of NETs. Kanavarioti et al (136) measured the copy numbers of five
miRNAs, let-7b, miR-15b, miR-21, miR-375 and miR-141, in both
healthy and cancerous samples, and demonstrated the equivalence of
serum and urine for testing these miRNAs. Among these miRNAs,
miR-141, miR-21 and miR-375 showed a specific increase in
expression in breast cancer, prostate cancer and pancreatic cancer.
By comparing the corresponding miRNA copy numbers with serum from
healthy males (cat. no. H6914; Sigma-Aldrich KGaA), the data were
divided into healthy and cancer samples, showing no overlap,
indicating that there were zero false negatives and zero false
positives, resulting in sensitivity, specificity, positive
predictive and negative predictive values all equal to 1. This
unprecedented distinction preliminarily validates each miRNA
(miR-21, miR-375 and miR-141) as biomarkers for these three types
of cancer (136).
According to relevant research, miR-375 exerts its
influence on tumors by targeting the expression of SNAIL1(137). In addition, miR-375 has been
shown to inhibit g-NENs through the Wnt/β-catenin pathway (138). miR-3614-5p targets various
components of the TGF-β signaling pathway to induce the progression
of pulmonary NETs (138).
Furthermore, miR-128-3p has been shown to have an impact on
inducing mesenchymal cell transformation via Wnt/β-catenin and
TGF-β in lung cancer (139).
Soldevilla et al (140)
revealed that the downstream targets and epigenetic regulation of
gastrointestinal PNETs are achieved through the PI3K/AKT and
TNF-α/NF-κB signaling pathways, respectively. Peng et al
(141) revealed that various
phenotypes of miRNA are involved in the progression of NETs, such
as pituitary tumors, through the Wnt/β-catenin and
SNHG6/miR-994/RAB11A axes.
NETs, a type of malignant tumor, are often difficult
to detect due to their subtle behavior. In the early stages of
NETs, changes are typically limited to alterations in endocrine
hormone levels. However, chronic inflammation is also present at
this stage, leading to elevated levels of inflammatory factors.
IL-6, one such factor, is expressed in various types of NETs.
Additionally, miRNAs, which serve as tumor markers, can degrade
elevated IL-6 levels. As a result, rising IL-6 levels may
reflexively induce the upregulation of specific miRNAs, suggesting
a potential target for further research. IL-6 expression varies
across different NETs and is known to exert its effects through the
JAK/STAT signaling pathway. Nevertheless, the precise role of the
JAK/STAT pathway in different NET subtypes remains unclear.
Therefore, the relationship between IL-6, miRNAs, signaling
pathways and NETs warrants further investigation.
Although IL-6 and miRNAs are currently major focal
points in the research of mechanisms related to tumors, how to
effectively implement their specific roles in clinical practice to
facilitate diagnosis and treatment is essential for guiding future
research directions. It is suggested not to solely rely on
pathological findings to understand the aforementioned mechanisms,
but also apply the expedited results of molecular mechanism
experiments to the advancement of clinical practice, which may
establish a pioneering area of focus. Recent evidence has suggested
that IL-6 can intervene in pancreatic cancer through the
miR-455/IGF1R axis (142). The
aforementioned study confirmed that IGF1R is the target gene of
miR-455 in pancreatic cancer cells, and that IL-6 significantly
decreases the expression of miR-455 in these cells. miR-455 was
found to inhibit pancreatic cancer progression by downregulating
IGF1R, while IL-6 downregulates miR-455 and upregulates IGF1R
(142). A proposed therapeutic
approach for pancreatic cancer involves inhibiting the high
expression of IL-6 and the overexpression of miR-455 in pancreatic
cancer cells. Additionally, miR-375 induces the downregulation of
YAP in medullary thyroid carcinoma (37). YAP, a key component of the Hippo
signaling pathway, not only contributes to tumor progression but
also promotes IL-6 expression (34,35).
This establishes a link between miR-375, IL-6, and medullary
thyroid carcinoma, offering new insights into potential treatment
approaches. The enrichment of miR-377 in SCLC has been shown to
inhibit the IGF1R pathway, thereby suppressing SCLC cell metastasis
(44). IL-6 stimulates STAT3
activation to promote SCLC cell metastasis, and crosstalk has been
observed between the STAT3 and IGF1R signaling pathways (143). Additionally, an indirect
relationship has been identified between IL-6 and miR-377, which
represents a novel research focus in SCLC. miR-96 is involved in
the PI3K/AKT pathway and plays a role in gastric NET progression,
while IL-6 activation of the STAT3 pathway promotes tumor cell
proliferation (48). Furthermore
crosstalk has also been observed between the STAT3 and PI3K/AKT
pathways (144). In
gastrointestinal NETs, it has been shown that elevated expression
of IL-6 induces changes in fibroblast activity and miRNA levels,
which regulate signaling pathways that ultimately affect intestinal
structure and lead to malignant metaplasia (65). PNETs and PitNETs share similar
mechanisms, with specific miRNAs and IL-6 acting on signaling
pathways that crosstalk with each other, collectively playing a
role in tumor development. In the future, it will be assessed which
miRNAs target downstream IL-6 through various signaling pathways or
competing endogenous RNA networks. In addition, an aim is set to
verify which signaling pathway is associated with this, as well as
to specifically determine the mechanism by which miRNAs achieve
targeting of downstream genes to affect tumorigenesis and
development. At present, there are limitations in the clinical use
of IL-6/miRNAs as biomarkers, because the changes in protein or RNA
expression levels cannot be assessed in real time in the clinic,
and the current pathological technology cannot extract and
distinguish which specific IL will be elevated in time, which leads
to a lack of specificity. Furthermore, as molecules in the TME,
IL-6 and miRNAs also influence downstream genes. In addition, as
molecules in the TME, it remains unclear whether crosstalk occurs
between their downstream or upstream effects, which may affect the
specificity of these biomarkers. Currently, the clinical
application of IL-6 and miRNAs as biomarkers for NENs faces several
technical bottlenecks. First, there are challenges with specificity
and timeliness in detection: Serum IL-6 levels can be easily
influenced by non-tumor factors such as infection and stress, while
assessing tissue-specific miRNA expression requires a biopsy, and
real-time quantitative analysis is difficult with existing
pathological techniques. Second, the complexity of the molecular
network presents challenges: The synergistic effects of IL-6 and
other cytokines (such as IL-1β and TNF-α) in the tumor
microenvironment, along with the crosstalk of miRNAs on multiple
signaling pathways, may limit the diagnostic efficacy of using a
single molecular marker. Therefore, future studies should integrate
multi-omics technologies (such as transcriptomics and proteomics)
to construct a regulatory network of ‘inflammatory
factors-miRNA-signaling pathways’ specific to NENs and verify the
therapeutic value of key molecular nodes through animal models.
Additionally, the development of liquid biopsy-based dynamic
monitoring technologies for miRNA/IL-6 (including digital PCR and
flow cytokine assays) will offer novel directions for the early
detection and individualized treatment of NENs.
Not applicable.
Funding: The present review was supported by the Natural Science
Foundation of Inner Mongolia Autonomous Region (grant no.
2022MS08010) and the Graduate Student Excellence Program (grant no.
YKDD2023ZY001).
Not applicable.
XG and SY developed the framework and drafted the
manuscript, contributing to the overall structure and
conceptualization of the review. CC and DL revised the content of
the manuscript and handled the correspondence with the editor. SY
generated the figures and performed the final review of the
manuscript. Data authentication is not applicable. All authors read
and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khandia R and Munjal A: Interplay between
inflammation and cancer. Adv Protein Chem Struct Biol. 119:199–245.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Soler MF, Abaurrea A, Azcoaga P, Araujo AM
and Caffarel MM: New perspectives in cancer immunotherapy:
Targeting IL-6 cytokine family. J Immunother Cancer.
11(e007530)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Habanjar O, Bingula R, Decombat C,
Diab-Assaf M, Caldefie-Chezet F and Delort L: Crosstalk of
inflammatory cytokines within the breast tumor microenvironment.
Int J Mol Sci. 24(4002)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Geisler L, Hellberg T, Lambrecht J, Jann
H, Knorr J, Eschrich J, Loosen SH, Wree A, Hammerich L, Krieg A, et
al: Inflammatory cytokines associated with diagnosis, tumor grade
and prognosis in patients with neuroendocrine tumors. J Clin Med.
11(6191)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cigrovski Berkovic M, Cacev T, Catela
Ivkovic T, Zjacic-Rotkvic V and Kapitanovic S: New insights into
the role of chronic inflammation and cytokines in the
etiopathogenesis of gastroenteropancreatic neuroendocrine tumors.
Neuroendocrinology. 99:75–84. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chang KT, Tsai CM, Chiou YC, Chiu CH, Jeng
KS and Huang CY: IL-6 induces neuroendocrine dedifferentiation and
cell proliferation in non-small cell lung cancer cells. Am J
Physiol Lung Cell Mol Physiol. 289:L446–L453. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dvorak HF: Tumors: Wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Le Bitoux MA and Stamenkovic I: Tumor-host
interactions: The role of inflammation. Histochem Cell Biol.
130:1079–1090. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Marzagalli M, Ebelt ND and Manuel ER:
Unraveling the crosstalk between melanoma and immune cells in the
tumor microenvironment. Semin Cancer Biol. 59:236–250.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yi M, Xu L, Jiao Y, Luo S, Li A and Wu K:
The role of cancer-derived microRNAs in cancer immune escape. J
Hematol Oncol. 13(25)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jia Z, Jia J, Yao L and Li Z: Crosstalk of
exosomal non-coding RNAs in the tumor microenvironment: Novel
frontiers. Front Immunol. 13(900155)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liang Y, Liu Y, Zhang Q, Zhang H and Du J:
Tumor-derived extracellular vesicles containing microRNA-1290
promote immune escape of cancer cells through the Grhl2/ZEB1/PD-L1
axis in gastric cancer. Transl Res. 231:102–112. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Solé C and Lawrie CH: MicroRNAs in
metastasis and the tumour microenvironment. Int J Mol Sci.
22(4859)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kousar K, Ahmad T, Abduh MS, Kanwal B,
Shah SS, Naseer F and Anjum S: miRNAs in regulation of tumor
microenvironment, chemotherapy resistance, immunotherapy modulation
and miRNA therapeutics in cancer. Int J Mol Sci.
23(13822)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yin Y, Liu B, Cao Y, Yao S, Liu Y, Jin G,
Qin Y, Chen Y, Cui K, Zhou L, et al: Colorectal cancer-derived
small extracellular vesicles promote tumor immune evasion by
upregulating PD-L1 expression in tumor-associated macrophages. Adv
Sci (Weinh). 9(2102620)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Song TY, Long M, Zhao HX, Zou MW, Fan HJ,
Liu Y, Geng CL, Song MF, Liu YF, Chen JY, et al: Tumor evolution
selectively inactivates the core microRNA machinery for immune
evasion. Nat Commun. 12(7003)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Muscogiuri G, Altieri B, Albertelli M,
Dotto A, Modica R, Barrea L, Fanciulli G, Feola T, Baldelli R,
Ruggeri RM, et al: Epidemiology of pancreatic neuroendocrine
neoplasms: A gender perspective. Endocrine. 69:441–450.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ruggeri RM, Benevento E, De Cicco F,
Fazzalari B, Guadagno E, Hasballa I, Tarsitano MG, Isidori AM,
Colao A and Faggiano A: NIKE Group. Neuroendocrine neoplasms in the
context of inherited tumor syndromes: A reappraisal focused on
targeted therapies. J Endocrinol Invest. 46:213–234.
2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vanoli A, La Rosa S, Luinetti O, Klersy C,
Manca R, Alvisi C, Rossi S, Trespi E, Zangrandi A, Sessa F, et al:
Histologic changes in type A chronic atrophic gastritis indicating
increased risk of neuroendocrine tumor development: The predictive
role of dysplastic and severely hyperplastic enterochromaffin-like
cell lesions. Hum Pathol. 44:1827–1837. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Modica R, La Salvia A, Liccardi A,
Cannavale G, Minotta R, Benevento E, Faggiano A and Colao A: Lipid
metabolism and homeostasis in patients with neuroendocrine
neoplasms: From risk factor to potential therapeutic target.
Metabolites. 12(1057)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Oberg K: Genetics and molecular pathology
of neuroendocrine gastrointestinal and pancreatic tumors
(gastroenteropancreatic neuroendocrine tumors). Curr Opin
Endocrinol Diabetes Obes. 16:72–78. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rindi G, Inzani F and Solcia E: Pathology
of gastrointestinal disorders. Endocrinol Metab Clin North Am.
39:713–727. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Banck MS, Kanwar R, Kulkarni AA, Boora GK,
Metge F, Kipp BR, Zhang L, Thorland EC, Minn KT, Tentu R, et al:
The genomic landscape of small intestine neuroendocrine tumors. J
Clin Invest. 123:2502–2508. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cunningham JL, Díaz de Ståhl T, Sjöblom T,
Westin G, Dumanski JP and Janson ET: Common pathogenetic mechanism
involving human chromosome 18 in familial and sporadic ileal
carcinoid tumors. Genes Chromosomes Cancer. 50:82–94.
2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Missiaglia E, Dalai I, Barbi S, Beghelli
S, Falconi M, della Peruta M, Piemonti L, Capurso G, Di Florio A,
delle Fave G, et al: Pancreatic endocrine tumors: Expression
profiling evidences a role for AKT-mTOR pathway. J Clin Oncol.
28:245–255. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ruebel K, Leontovich AA, Stilling GA,
Zhang S, Righi A, Jin L and Lloyd RV: MicroRNA expression in ileal
carcinoid tumors: Downregulation of microRNA-133a with tumor
progression. Mod Pathol. 23:367–375. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Huang M, Fanciulli G, Wu SQ, Zhang Z and
Zhang J: Analysis of the lower incidence of medullary thyroid
cancer in China. Chin Med J (Engl). 132:2516–2517. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dey A, Varelas X and Guan KL: Targeting
the Hippo pathway in cancer, fibrosis, wound healing and
regenerative medicine. Nat Rev Drug Discov. 19:480–494.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mohajan S, Jaiswal PK, Vatanmakarian M,
Yousefi H, Sankaralingam S, Alahari SK, Koul S and Koul HK: Hippo
pathway: Regulation, deregulation and potential therapeutic targets
in cancer. Cancer Lett. 507:112–123. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Meng Z, Moroishi T and Guan KL: Mechanisms
of Hippo pathway regulation. Genes Dev. 30:1–17. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mia MM, Cibi DM, Abdul Ghani SAB, Song W,
Tee N, Ghosh S, Mao J, Olson EN and Singh MK: YAP/TAZ deficiency
reprograms macrophage phenotype and improves infarct healing and
cardiac function after myocardial infarction. PLoS Biol.
18(e3000941)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Manfioletti G and Fedele M:
Epithelial-mesenchymal transition (EMT). Int J Mol Sci.
24(11386)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zheng D, Jin L, Chen B, Qi Y, Bhandari A,
Wen J, Lin B, Zhang X and Zhang W: The ETNK2 gene promotes
progression of papillary thyroid carcinoma through the HIPPO
pathway. J Cancer. 13:508–516. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ciarletto AM, Narick C, Malchoff CD,
Massoll NA, Labourier E, Haugh K, Mireskandari A, Finkelstein SD
and Kumar G: Analytical and clinical validation of pairwise
microRNA expression analysis to identify medullary thyroid cancer
in thyroid fine-needle aspiration samples. Cancer Cytopathol.
129:239–249. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dingemans AC, Früh M, Ardizzoni A, Besse
B, Faivre-Finn C, Hendriks LE, Lantuejoul S, Peters S, Reguart N,
Rudin CM, et al: Small-cell lung cancer: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up☆. Ann
Oncol. 32:839–853. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Soomro Z, Youssef M, Yust-Katz S, Jalali
A, Patel AJ and Mandel J: Paraneoplastic syndromes in small cell
lung cancer. J Thorac Dis. 12:6253–6263. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pfeiffer M, Hartmann TN, Leick M, Catusse
J, Schmitt-Graeff A and Burger M: Alternative implication of CXCR4
in JAK2/STAT3 activation in small cell lung cancer. Br J Cancer.
100:1949–1956. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Iriki T, Ohnishi K, Fujiwara Y, Horlad H,
Saito Y, Pan C, Ikeda K, Mori T, Suzuki M, Ichiyasu H, et al: The
cell-cell interaction between tumor-associated macrophages and
small cell lung cancer cells is involved in tumor progression via
STAT3 activation. Lung Cancer. 106:22–32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lu Y, Li H, Zhao P, Tian L, Liu Y, Sun X
and Cheng Y: Dynamic phenotypic reprogramming and chemoresistance
induced by lung fibroblasts in small cell lung cancer. Sci Rep.
14(2884)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lim JS, Ibaseta A, Fischer MM, Cancilla B,
O'Young G, Cristea S, Luca VC, Yang D, Jahchan NS, Hamard C, et al:
Intratumoural heterogeneity generated by Notch signalling promotes
small-cell lung cancer. Nature. 545:360–364. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hua J, Wang X, Ma L, Li J, Cao G, Zhang S
and Lin W: CircVAPA promotes small cell lung cancer progression by
modulating the miR-377-3p and miR-494-3p/IGF1R/AKT axis. Mol
Cancer. 21(123)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Delle Fave G, O'Toole D, Sundin A, Taal B,
Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, et al:
ENETS consensus guidelines update for gastroduodenal neuroendocrine
neoplasms. Neuroendocrinology. 103:119–124. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Gluckman CR and Metz DC: Gastric
neuroendocrine tumors (carcinoids). Curr Gastroenterol Rep.
21(13)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tsolakis AV, Ragkousi A, Vujasinovic M,
Kaltsas G and Daskalakis K: Gastric neuroendocrine neoplasms type
1: A systematic review and meta-analysis. World J Gastroenterol.
25:5376–5387. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang J, Li D, Cang H and Guo B: Crosstalk
between cancer and immune cells: Role of tumor-associated
macrophages in the tumor microenvironment. Cancer Med. 8:4709–4721.
2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu
Y, Gong Z, Zhang S, Zhou J, Cao K, et al: Role of tumor
microenvironment in tumorigenesis. J Cancer. 8:761–773.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Girardi DM, Silva ACB, Rêgo JFM, Coudry RA
and Riechelmann RP: Unraveling molecular pathways of poorly
differentiated neuroendocrine carcinomas of the
gastroenteropancreatic system: A systematic review. Cancer Treat
Rev. 56:28–35. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Duerr EM, Mizukami Y, Ng A, Xavier RJ,
Kikuchi H, Deshpande V, Warshaw AL, Glickman J, Kulke MH and Chung
DC: Defining molecular classifications and targets in
gastroenteropancreatic neuroendocrine tumors through DNA microarray
analysis. Endocr Relat Cancer. 15:243–256. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Korotaeva A, Mansorunov D, Apanovich N,
Kuzevanova A and Karpukhin A: MiRNA expression in neuroendocrine
neoplasms of frequent localizations. Noncoding RNA.
7(38)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ahmed M: Gastrointestinal neuroendocrine
tumors in 2020. World J Gastrointest Oncol. 12:791–807.
2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Stålberg P, Westin G and Thirlwell C:
Genetics and epigenetics in small intestinal neuroendocrine
tumours. J Intern Med. 280:584–594. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ito T, Lee L and Jensen RT:
Carcinoid-syndrome: Recent advances, current status and
controversies. Curr Opin Endocrinol Diabetes Obes. 25:22–35.
2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Di Domenico A, Wiedmer T, Marinoni I and
Perren A: Genetic and epigenetic drivers of neuroendocrine tumours
(NET). Endocr Relat Cancer. 24:R315–R334. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wei ZZ, Yu SP, Lee JH, Chen D, Taylor TM,
Deveau TC, Yu AC and Wei L: Regulatory role of the JNK-STAT1/3
signaling in neuronal differentiation of cultured mouse embryonic
stem cells. Cell Mol Neurobiol. 34:881–893. 2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Walker CD, Long H, Williams S and Richard
D: Long-lasting effects of elevated neonatal leptin on rat
hippocampal function, synaptic proteins and NMDA receptor subunits.
J Neurosci Res. 85:816–828. 2007.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Tang QP, Shen Q, Wu LX, Feng XL, Liu H, Wu
B, Huang XS, Wang GQ, Li ZH and Liu ZJ: STAT3 signal that mediates
the neural plasticity is involved in willed-movement training in
focal ischemic rats. J Zhejiang Univ Sci B. 17:493–502.
2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
De Simone V, Franzè E, Ronchetti G,
Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald
TT, Pallone F, et al: Th17-type cytokines, IL-6 and TNF-α
synergistically activate STAT3 and NF-kB to promote colorectal
cancer cell growth. Oncogene. 34:3493–3503. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wang XY, Chai NL, Linghu EQ, Li HK, Zhai
YQ, Feng XX, Zhang WG, Zou JL, Li LS and Xiang JY: Efficacy and
safety of hybrid endoscopic submucosal dissection compared with
endoscopic submucosal dissection for rectal neuroendocrine tumors
and risk factors associated with incomplete endoscopic resection.
Ann Transl Med. 8(368)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Osagiede O, Habermann E, Day C, Gabriel E,
Merchea A, Lemini R, Jabbal IS and Colibaseanu DT: Factors
associated with worse outcomes for colorectal neuroendocrine tumors
in radical versus local resections. J Gastrointest Oncol.
11:836–846. 2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Dasari A, Shen C, Halperin D, Zhao B, Zhou
S, Xu Y, Shih T and Yao JC: Trends in the incidence, prevalence,
and survival outcomes in patients with neuroendocrine tumors in the
United States. JAMA Oncol. 3:1335–1342. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Zou J, Chen S, Lian G, Li R, Li Y, Huang K
and Chen Y: Prognostic and metastasis-related factors in colorectal
neuroendocrine tumors: A cross-sectional study based on the
surveillance, epidemiology and end results. Oncol Lett.
18:5129–5138. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Tran MT: Identification of TIMP1-induced
dysregulation of epithelial-mesenchymal transition as a key pathway
in inflammatory bowel disease and small intestinal neuroendocrine
tumors shared pathogenesis. Front Genet. 15(1376123)2024.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330.
2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Inzani F, Petrone G and Rindi G: The new
World Health Organization classification for pancreatic
neuroendocrine neoplasia. Endocrinol Metab Clin North Am.
47:463–470. 2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Yachida S, Vakiani E, White CM, Zhong Y,
Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM,
et al: Small cell and large cell neuroendocrine carcinomas of the
pancreas are genetically similar and distinct from
well-differentiated pancreatic neuroendocrine tumors. Am J Surg
Pathol. 36:173–184. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Cives M, Partelli S, Palmirotta R, Lovero
D, Mandriani B, Quaresmini D, Pelle E, Andreasi V, Castelli P,
Strosberg J, et al: DAXX mutations as potential genomic markers of
malignant evolution in small nonfunctioning pancreatic
neuroendocrine tumors. Sci Rep. 9(18614)2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Mafficini A and Scarpa A: Genetics and
epigenetics of gastroenteropancreatic neuroendocrine neoplasms.
Endocr Rev. 40:506–536. 2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Conemans EB, Lodewijk L, Moelans CB,
Offerhaus GJA, Pieterman CRC, Morsink FH, Dekkers OM, de Herder WW,
Hermus AR, van der Horst-Schrivers AN, et al: DNA methylation
profiling in MEN1-related pancreatic neuroendocrine tumors reveals
a potential epigenetic target for treatment. Eur J Endocrinol.
179:153–160. 2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Scarpa A, Chang DK, Nones K, Corbo V,
Patch AM, Bailey P, Lawlor RT, Johns AL, Miller DK, Mafficini A, et
al: Whole-genome landscape of pancreatic neuroendocrine tumours.
Nature. 543:65–71. 2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Liu GY and Sabatini DM: mTOR at the nexus
of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol.
21:183–203. 2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kim J and Guan KL: mTOR as a central hub
of nutrient signalling and cell growth. Nat Cell Biol. 21:63–71.
2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Elsässer SJ, Allis CD and Lewis PW:
Cancer. New epigenetic drivers of cancers. Science. 331:1145–1146.
2011.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Hackeng WM, Brosens LAA, Kim JY,
O'Sullivan R, Sung YN, Liu TC, Cao D, Heayn M, Brosnan-Cashman J,
An S, et al: Non-functional pancreatic neuroendocrine tumours:
ATRX/DAXX and alternative lengthening of telomeres (ALT) are
prognostically independent from ARX/PDX1 expression and tumour
size. Gut. 71:961–973. 2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Kidd M, Modlin I and Öberg K: Towards a
new classification of gastroenteropancreatic neuroendocrine
neoplasms. Nat Rev Clin Oncol. 13:691–705. 2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Jiao Y, Shi C, Edil BH, de Wilde RF,
Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA,
et al: DAXX/ATRX, MEN1, and mTOR pathway genes are frequently
altered in pancreatic neuroendocrine tumors. Science.
331:1199–1203. 2011.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Li H, Wang X, Hu C, Cui J, Li H, Luo X and
Hao Y: IL-6 enhances the activation of PI3K-AKT/mTOR-GSK-3β by
upregulating GRPR in hippocampal neurons of autistic mice. J
Neuroimmune Pharmacol. 19(12)2024.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Karakaxas D, Sioziou A, Aravantinos G,
Coker A, Papanikolaou IS, Liakakos T, Dervenis C and Gazouli M:
Genetic polymorphisms of interleukin 1β gene and sporadic
pancreatic neuroendocrine tumors susceptibility. World J
Gastrointest Oncol. 8:520–525. 2016.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Berković MC, Jokić M, Marout J, Radosević
S, Zjacić-Rotkvić V and Kapitanović S: IL-6-174 C/G polymorphism in
the gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Exp
Mol Pathol. 83:474–479. 2007.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Imam R, Chang Q, Black M, Yu C and Cao W:
CD47 expression and CD163+ macrophages correlated with
prognosis of pancreatic neuroendocrine tumor. BMC Cancer.
21(320)2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Zhong Y, Tian Y, Wang Y, Bai J, Long Q,
Yan L, Gong Z, Gao W and Tang Q: Small extracellular vesicle
piR-hsa-30937 derived from pancreatic neuroendocrine neoplasms
upregulates CD276 in macrophages to promote immune evasion. Cancer
Immunol Res. 12:840–853. 2024.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Lu F, Ye M, Shen Y, Xu Y, Hu C, Chen J, Yu
P, Xue B, Gu D, Xu L, et al: Hypoxic tumor-derived exosomal
miR-4488 induces macrophage M2 polarization to promote liver
metastasis of pancreatic neuroendocrine neoplasm through RTN3/FABP5
mediated fatty acid oxidation. Int J Biol Sci. 20:3201–3218.
2024.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Enguita JM, Díaz I, García D, Cubiella T,
Chiara MD and Valdés N: Visual analytics identifies key miRNAs for
differentiating peripancreatic paraganglioma and pancreatic
neuroendocrine tumors. Front Endocrinol (Lausanne).
14(1162725)2023.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Molitch ME: Diagnosis and treatment of
pituitary adenomas: A review. JAMA. 317:516–524. 2017.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Mohme M, Riethdorf S and Pantel K:
Circulating and disseminated tumour cells-mechanisms of immune
surveillance and escape. Nat Rev Clin Oncol. 14:155–167.
2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Barnes TA and Amir E: HYPE or HOPE: The
prognostic value of infiltrating immune cells in cancer. Br J
Cancer. 117:451–460. 2017.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Yagnik G, Rutowski MJ, Shah SS and Aghi
MK: Stratifying nonfunctional pituitary adenomas into two groups
distinguished by macrophage subtypes. Oncotarget. 10:2212–2223.
2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Marques P, Barry S, Carlsen E, Collier D,
Ronaldson A, Awad S, Dorward N, Grieve J, Mendoza N, Muquit S, et
al: Chemokines modulate the tumour microenvironment in pituitary
neuroendocrine tumours. Acta Neuropathol Commun.
7(172)2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Fujiwara K, Yatabe M, Tofrizal A, Jindatip
D, Yashiro T and Nagai R: Identification of M2 macrophages in
anterior pituitary glands of normal rats and rats with
estrogen-induced prolactinoma. Cell Tissue Res. 368:371–378.
2017.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zhang A, Xu Y, Xu H, Ren J, Meng T, Ni Y,
Zhu Q, Zhang WB, Pan YB, Jin J, et al: Lactate-induced M2
polarization of tumor-associated macrophages promotes the invasion
of pituitary adenoma by secreting CCL17. Theranostics.
11:3839–3852. 2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Zhou M, Na R, Lai S, Guo Y, Shi J, Nie J,
Zhang S, Wang Y and Zheng T: The present roles and future
perspectives of interleukin-6 in biliary tract cancer. Cytokine.
169(156271)2023.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Wang X, Fang Y, Zhou Y, Guo X, Xu K, Li C,
Zhang J and Hong Y: SDF-1α/MicroRNA-134 axis regulates
nonfunctioning pituitary neuroendocrine tumor growth via targeting
VEGFA. Front Endocrinol (Lausanne). 11(566761)2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Zimmermann N, Knief J, Kacprowski T,
Lazar-Karsten P, Keck T, Billmann F, Schmid S, Luley K, Lehnert H,
Brabant G and Thorns C: MicroRNA analysis of gastroenteropancreatic
neuroendocrine tumors and metastases. Oncotarget. 9:28379–28390.
2018.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Zatelli MC, Grossrubatscher EM, Guadagno
E, Sciammarella C, Faggiano A and Colao A: Circulating tumor cells
and miRNAs as prognostic markers in neuroendocrine neoplasms.
Endocr Relat Cancer. 24:R223–R237. 2017.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Yoshimoto T, Motoi N, Yamamoto N, Nagano
H, Ushijima M, Matsuura M, Okumura S, Yamaguchi T, Fukayama M and
Ishikawa Y: Pulmonary carcinoids and low-grade gastrointestinal
neuroendocrine tumors show common MicroRNA expression profiles,
different from adenocarcinomas and small cell carcinomas.
Neuroendocrinology. 106:47–57. 2018.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Cavalcanti E, Galleggiante V, Coletta S,
Stasi E, Chieppa M, Armentano R and Serino G: Altered miRNAs
expression correlates with gastroenteropancreatic neuroendocrine
tumors grades. Front Oncol. 10(1187)2020.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Butz H and Patócs A: MicroRNAs in
endocrine tumors. EJIFCC. 30:146–164. 2019.PubMed/NCBI
|
|
100
|
Nosho K, Igarashi H, Nojima M, Ito M,
Maruyama R, Yoshii S, Naito T, Sukawa Y, Mikami M, Sumioka W, et
al: Association of microRNA-31 with BRAF mutation, colorectal
cancer survival and serrated pathway. Carcinogenesis. 35:776–783.
2014.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Yan LX, Huang XF, Shao Q, Huang MY, Deng
L, Wu QL, Zeng YX and Shao JY: MicroRNA miR-21 overexpression in
human breast cancer is associated with advanced clinical stage,
lymph node metastasis and patient poor prognosis. RNA.
14:2348–2360. 2008.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Du X, Zhang J, Wang J, Lin X and Ding F:
Role of miRNA in lung cancer-potential biomarkers and therapies.
Curr Pharm Des. 23:5997–6010. 2018.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Lobera ES, Varela MA, Jimenez RL and
Moreno RB: miRNA as biomarker in lung cancer. Mol Biol Rep.
50:9521–9527. 2023.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Zeng ZL, Zhu Q, Zhao Z, Zu X and Liu J:
Magic and mystery of microRNA-32. J Cell Mol Med. 25:8588–8601.
2021.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Maués JHDS, Moreira-Nunes CDFA and Burbano
RMR: Computational identification and characterization of new
microRNAs in human platelets stored in a blood bank. Biomolecules.
10(1173)2020.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Wang H and Chen YH: microRNA biomarkers in
clinical study. Biomolecules. 11(1810)2021.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Duran-Sanchon S, Vila-Navarro E, Marcuello
M, Lozano JJ, Muñoz J, Cubiella J, Diez MS, Bujanda L, Lanas A,
Jover R, et al: Validation of miR-1228-3p as housekeeping for
MicroRNA analysis in liquid biopsies from colorectal cancer
patients. Biomolecules. 10(16)2019.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Al-Eitan LN, Alghamdi MA, Tarkhan AH and
Al-Qarqaz FA: Gene expression profiling of MicroRNAs in HPV-induced
warts and normal skin. Biomolecules. 9(757)2019.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Fahim SA, Abdullah MS, Espinoza-Sánchez
NA, Hassan H, Ibrahim AM, Ahmed SH, Shakir G, Badawy MA, Zakhary
NI, Greve B, et al: Inflammatory breast carcinoma: Elevated
microRNA miR-181b-5p and reduced miR-200b-3p, miR-200c-3p, and
miR-203a-3p expression as potential biomarkers with diagnostic
value. Biomolecules. 10(1059)2020.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Malczewska A, Kidd M, Matar S, Kos-Kudla B
and Modlin IM: A comprehensive assessment of the role of miRNAs as
biomarkers in gastroenteropancreatic neuroendocrine tumors.
Neuroendocrinology. 107:73–90. 2018.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Li A, Yu J, Kim H, Wolfgang CL, Canto MI,
Hruban RH and Goggins M: MicroRNA array analysis finds elevated
serum miR-1290 accurately distinguishes patients with low-stage
pancreatic cancer from healthy and disease controls. Clin Cancer
Res. 19:3600–3610. 2013.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Thorns C, Schurmann C, Gebauer N,
Wallaschofski H, Kümpers C, Bernard V, Feller AC, Keck T, Habermann
JK, Begum N, et al: Global microRNA profiling of pancreatic
neuroendocrine neoplasias. Anticancer Res. 34:2249–2254.
2014.PubMed/NCBI
|
|
113
|
Roldo C, Missiaglia E, Hagan JP, Falconi
M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A and
Croce CM: MicroRNA expression abnormalities in pancreatic endocrine
and acinar tumors are associated with distinctive pathologic
features and clinical behavior. J Clin Oncol. 24:4677–4684.
2006.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Wang M, Xia X, Chu W, Xia L, Meng T, Liu L
and Liu Y: Roles of miR-186 and PTTG1 in colorectal neuroendocrine
tumors. Int J Clin Exp Med. 8:22149–22157. 2015.PubMed/NCBI
|
|
115
|
Mitsuhashi K, Yamamoto I, Kurihara H,
Kanno S, Ito M, Igarashi H, Ishigami K, Sukawa Y, Tachibana M,
Takahashi H, et al: Analysis of the molecular features of rectal
carcinoid tumors to identify new biomarkers that predict biological
malignancy. Oncotarget. 6:22114–22125. 2015.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Lloyd KA, Moore AR, Parsons BN, O'Hara A,
Boyce M, Dockray GJ, Varro A and Pritchard DM: Gastrin-induced
miR-222 promotes gastric tumor development by suppressing p27kip1.
Oncotarget. 7:45462–45478. 2016.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Bowden M, Zhou CW, Zhang S, Brais L, Rossi
A, Naudin L, Thiagalingam A, Sicinska E and Kulke MH: Profiling of
metastatic small intestine neuroendocrine tumors reveals
characteristic miRNAs detectable in plasma. Oncotarget.
8:54331–54344. 2017.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Tanaka T, Narazaki M, Masuda K and
Kishimoto T: Regulation of IL-6 in immunity and diseases. Adv Exp
Med Biol. 941:79–88. 2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Jones SA and Jenkins BJ: Recent insights
into targeting the IL-6 cytokine family in inflammatory diseases
and cancer. Nat Rev Immunol. 18:773–789. 2018.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Yang Y, Ding L, Hu Q, Xia J, Sun J, Wang
X, Xiong H, Gurbani D, Li L, Liu Y and Liu A: MicroRNA-218
functions as a tumor suppressor in lung cancer by targeting
IL-6/STAT3 and negatively correlates with poor prognosis. Mol
Cancer. 16(141)2017.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Cai Z, Li J, Zhuang Q, Zhang X, Yuan A,
Shen L, Kang K, Qu B, Tang Y, Pu J, et al: MiR-125a-5p ameliorates
monocrotaline-induced pulmonary arterial hypertension by targeting
the TGF-β1 and IL-6/STAT3 signaling pathways. Exp Mol Med. 50:1–11.
2018.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Wang P, Hou J, Lin L, Wang C, Liu X, Li D,
Ma F, Wang Z and Cao X: Inducible microRNA-155 feedback promotes
type I IFN signaling in antiviral innate immunity by targeting
suppressor of cytokine signaling 1. J Immunol. 185:6226–6233.
2010.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Collins AS, McCoy CE, Lloyd AT, O'Farrelly
C and Stevenson NJ: miR-19a: An effective regulator of SOCS3 and
enhancer of JAK-STAT signalling. PLoS One. 8(e69090)2013.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Ohta M, Kihara T, Toriuchi K, Aoki H,
Iwaki S, Kakita H, Yamada Y and Aoyama M: IL-6 promotes cell
adhesion in human endothelial cells via microRNA-126-3p
suppression. Exp Cell Res. 393(112094)2020.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Ren W, Zhang X, Li Q, Pu C and Zhang D:
Activating IL-6/STAT3 enhances protein stability of proteasome 20S
α+ β in colorectal cancer by miR-1254. Biomed Res Int.
2022(4250013)2022.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Peng Y, Wu XJ, Ji XJ, Huang GX, Wu T, Liu
X, Yang R, Pi J, Shen HB, Wang FF and Xu JF: Circular RNA
circTRAPPC6B enhances IL-6 and IL-1β expression and repolarizes
mycobacteria induced macrophages from M2- to M1-like phenotype by
targeting miR-892c-3p. J Interferon Cytokine Res. 43:269–279.
2023.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Mahjoor M, Afkhami H, Najafi M, Nasr A and
Khorrami S: The role of microRNA-30c in targeting interleukin 6, as
an inflammatory cytokine, in the mesenchymal stem cell: A
therapeutic approach in colorectal cancer. J Cancer Res Clin Oncol.
149:3149–3160. 2023.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Lv Q, Xia Q, Li A and Wang Z:
circRNA_101277 influences cisplatin resistance of colorectal cancer
cells by modulating the miR-370/IL-6 axis. Genet Res (Camb).
2022(4237327)2022.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Tseng HY, Chen YA, Jen J, Shen PC, Chen
LM, Lin TD, Wang YC and Hsu HL: Oncogenic MCT-1 activation promotes
YY1-EGFR-MnSOD signaling and tumor progression. Oncogenesis.
6(e313)2017.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Fisher DT, Appenheimer MM and Evans SS:
The two faces of IL-6 in the tumor microenvironment. Semin Immunol.
26:38–47. 2014.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Weng YS, Tseng HY, Chen YA, Shen PC, Al
Haq AT, Chen LM, Tung YC and Hsu HL: MCT-1/miR-34a/IL-6/IL-6R
signaling axis promotes EMT progression, cancer stemness and M2
macrophage polarization in triple-negative breast cancer. Mol
Cancer. 18(42)2019.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Wang Y, van Boxel-Dezaire AHH, Cheon H,
Yang J and Stark GR: STAT3 activation in response to IL-6 is
prolonged by the binding of IL-6 receptor to EGF receptor. Proc
Natl Acad Sci USA. 110:16975–16980. 2013.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Levy DE and Darnell JE Jr: Stats:
Transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
135
|
Ochoa Bernal MA, Song Y, Joshi N, Burns
GW, Paul EN, Vegter E, Hrbek S, Sempere LF and Fazleabas AT: The
regulation of MicroRNA-21 by interleukin-6 and its role in the
development of fibrosis in endometriotic lesions. Int J Mol Sci.
25(8994)2024.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Kanavarioti A, Rehman MH, Qureshi S, Rafiq
A and Sultan M: High sensitivity and specificity platform to
validate MicroRNA biomarkers in cancer and human diseases.
Noncoding RNA. 10(42)2024.PubMed/NCBI View Article : Google Scholar
|
|
137
|
De A, Powers B, De A, Zhou J, Sharma S,
Van Veldhuizen P, Bansal A, Sharma R and Sharma M: Emblica
officinalis extract downregulates pro-angiogenic molecules via
upregulation of cellular and exosomal miR-375 in human ovarian
cancer cells. Oncotarget. 7:31484–31500. 2016.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Guo F, Gao Y, Sui G, Jiao D, Sun L, Fu Q
and Jin C: miR-375-3p/YWHAZ/β-catenin axis regulates migration,
invasion, EMT in gastric cancer cells. Clin Exp Pharmacol Physiol.
46:144–152. 2019.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Li F, Yang H, Kong T, Chen S, Li P, Chen
L, Cheng J, Cui G and Zhang G: PGAM1, regulated by miR-3614-5p,
functions as an oncogene by activating transforming growth factor-β
(TGF-β) signaling in the progression of non-small cell lung
carcinoma. Cell Death Dis. 11(710)2020.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Soldevilla B, Lens-Pardo A,
Espinosa-Olarte P, Carretero-Puche C, Molina-Pinelo S, Robles C,
Benavent M, Gomez-Izquierdo L, Fierro-Fernández M, Morales-Burgo P,
et al: MicroRNA signature and integrative omics analyses define
prognostic clusters and key pathways driving prognosis in patients
with neuroendocrine neoplasms. Mol Oncol. 17:582–597.
2023.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Peng Y, Zhang X, Feng X, Fan X and Jin Z:
The crosstalk between microRNAs and the Wnt/β-catenin signaling
pathway in cancer. Oncotarget. 8:14089–14106. 2017.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Zhang Y, He H, He L and Shi B: IL-6
accelerates the proliferation and metastasis of pancreatic cancer
cells via the miR-455-5p/IGF-1R axis. Cancer Biother Radiopharm.
39:255–263. 2024.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Yang S and Li D: Role of microRNAs in
triple-negative breast cancer and new therapeutic concepts
(review). Oncol Lett. 28(431)2024.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Poon JS, Eves R and Mak AS: Both lipid-
and protein-phosphatase activities of PTEN contribute to the
p53-PTEN anti-invasion pathway. Cell Cycle. 9:4450–4454.
2010.PubMed/NCBI View Article : Google Scholar
|