Secondary lymphedema is a chronic progressive
disease characterized by the abnormal accumulation of interstitial
fluid and tissue swelling (1). Its
core pathological mechanism is due to the structural or functional
damage of the lymphatic drainage system. Such damage is often
caused by tumor treatments (such as axillary lymph node dissection
and radiotherapy for breast cancer), parasitic infections (such as
filariasis) or trauma (2). The
clinical manifestations include asymmetric swelling of the limbs,
fibrosis and recurrent infections. In severe cases, it can progress
to lymphangiosarcoma or limb dysfunction, notably impairing the
quality of life of patients (3).
Among them, breast cancer-related lymphedema (BCRL) accounts for
20-65% of secondary cases (4). Its
risk factors include the use of taxane, obesity and radiotherapy to
regional lymph nodes at doses exceeding 50 Gy (5-7).
A global cancer statistic shows that breast cancer ranks first in
the incidence of female malignant tumors (accounting for 24.5% of
newly diagnosed cancer cases) (8,9).
Reported incidence rates range from 6.7-62.5% depending on surgical
and radiotherapy protocols (10,11).
However, modern techniques such as sentinel lymph node biopsy and
immediate lymphatic reconstruction have reduced the risk to 5-10
and 7.0%, respectively (12,13).
BCRL is still a problem that should be solved in the field of
cancer rehabilitation.
Currently, the management of BCRL faces a twofold
challenge: i) An absence of standardized assessment tools; and ii)
limitations of treatment methods. Although the International
Society of Lymphology has proposed a staging system based on
clinical symptoms (such as the subclinical stage or lymphostatic
elephantiasis), it is subjective and unable to quantify the degree
of fibrosis (3). While
bioimpedance spectroscopy and 3D imaging techniques [such as
near-infrared fluorescence lymphatic imaging with 3D reconstruction
(14,15), high-resolution volumetric analysis
(15) and multiparametric
MRI-based radiomics (16,17)] can enhance the sensitivity of early
detection, standardized imaging protocols are lacking and are
device-dependent, which make it difficult to popularize (3,9). In
terms of treatment, conservative therapies (such as compression
therapy and manual lymphatic drainage) can relieve symptoms, but
their efficacy is limited for advanced fibrosis (18,19).
Surgical interventions (such as lymphatic-venous anastomosis and
liposuction) can improve the appearance, but they have risks such
as damage to residual lymphatic vessels, aesthetic deformities and
the formation of unstable scars (20-22).
In this context, mesenchymal stem cell (MSC) therapy is a focus of
research due to its unique dual mechanisms of tissue repair and
immunomodulation (23). MSCs
promote lymphangiogenesis by paracrine-secreting factors such as
vascular endothelial growth factor C (VEGF-C) and stromal
cell-derived factor 1α. Additionally, they inhibit the release of
proinflammatory factors such as TNF-α and IL-6, which alleviates
fibrosis (24,25).
In the present review, the pathophysiological
mechanisms of BCRL, which are the foundation for understanding the
disease, were investigated. Subsequently, MSC therapy, with its
advantages and disadvantages as a treatment for secondary
lymphedema, in the context of BCRL in particular, was evaluated.
Finally, the present study summarized and appraised the relevant
animal and clinical experiments on various stem cell therapies for
secondary lymphedema, including those associated with BCRL. Through
this process, the present review aimed to assess the progress made
and the problems yet to be solved in this field.
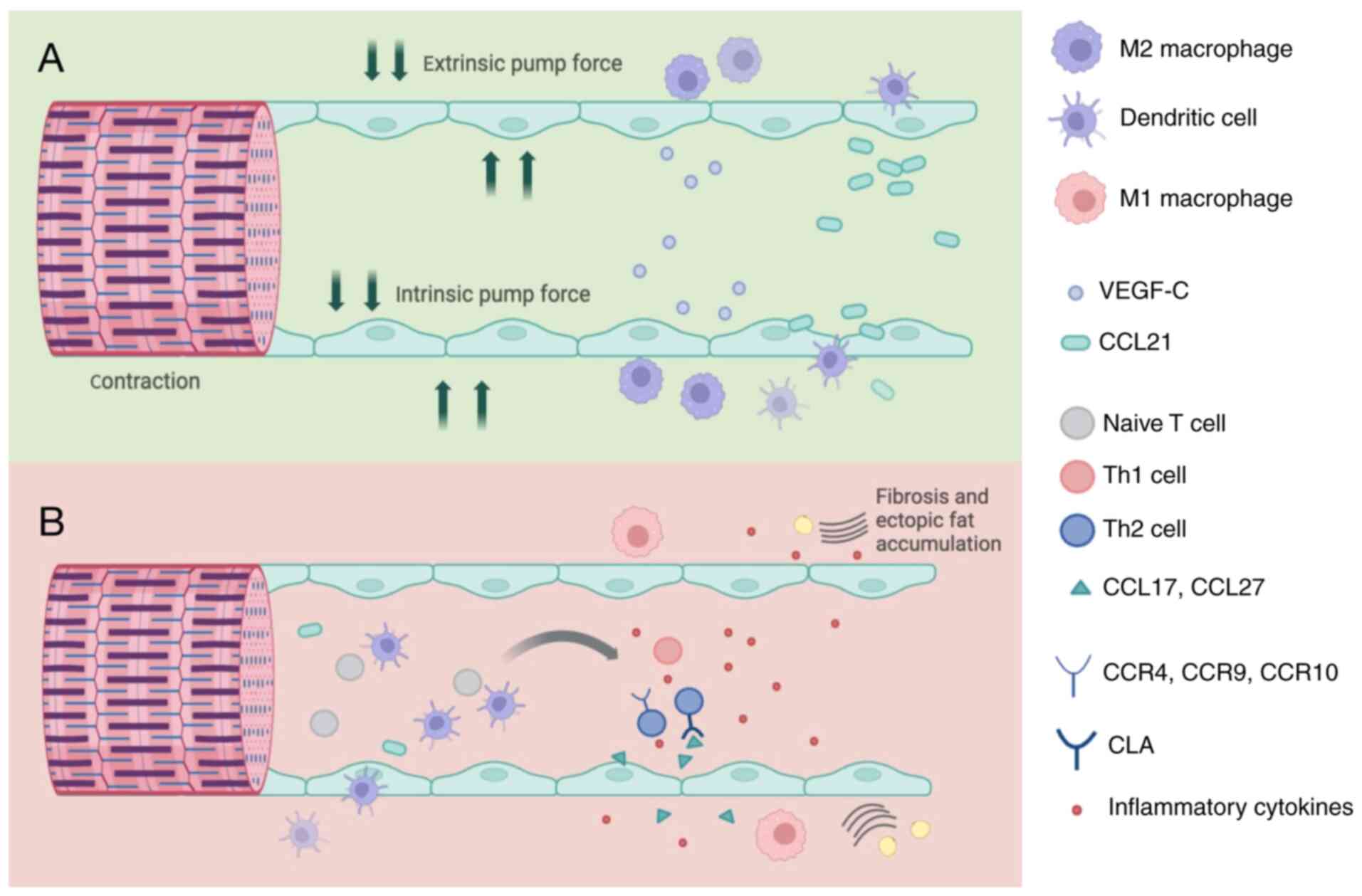
The physiological function of lymphatic vessels is
to facilitate the exchange of materials such as proteins, lipids
and water between tissue stroma and blood vessels. Its unique
structure enables the transportation of macromolecular proteins,
lipids and a notable volume of tissue fluid into lymph fluid, which
are typically challenging to transfer through the venous system
(26). A number of patients with
cancer undergoing radiotherapy and chemotherapy may experience
lymphatic vascular insufficiency and impaired drainage due to
specific therapeutic agents [for example, doxorubicin decreases the
lymphatic pumping frequency (27)]
and physical factors [for example, radiation-induces axillary
fibrosis at doses >50 Gy (28)]. Additionally, to maintain the
normal function of lymphatic vessels, the pressure within the
vessels should be kept ≤0 mm H2O, as elevated
intraluminal pressure disrupts cytoskeletal dynamics and impairs
fluid drainage. This is evidenced by studies on lymphatic
biomechanics and pathological conditions such as lymphedema
(29-31).
Surgical interventions often disrupt axillary lymphatic outflow and
cause lymphatic blockage (32),
leading to a notable increase in lymphatic pressure. This increased
pressure within the lymphatic system affects the transport of
proteins and immune cells, resulting in the accumulation of
protein-rich fluids in the extracellular matrix and triggering an
inflammatory response (Fig.
1).
Lymphography-assisted stem cell therapy represents a
novel approach for treating secondary lymphedema, offering benefits
such as quantifying the severity of lymphedema and enhancing
diagnostic accuracy. A study by Peña Quián et al (44) conducts multiple shallow and deep
stem cell injections in the lymphatic vessel trace and groin region
of 2 patients with chronic lower limb lymphedema, in order to
evaluate the anatomical structure and function of the lymphatic
system. The study demonstrates that after stem cell implantation,
imaging reveals the presence of new lymphatic branches not observed
before treatment, along with thicker radioactive columns.
Additionally, patients exhibit an increase in dermal reflux and
proximal lymph node activity, suggesting improved lymphatic
drainage.
MSCs, as pivotal tools in regenerative medicine,
vary in biological traits and therapeutic effects by tissue source.
In secondary lymphedema management, bone marrow-derived MSCs
(BMSCs) and adipose-derived MSCs (ADSCs) demonstrate distinct
clinical profiles. Regarding proliferative capacity, BMSCs show
notable donor age-dependent limitations in vitro expansion,
whereas ADSCs obtained through minimally invasive lipoaspiration
maintain superior passage stability (47). Differentiation analyses reveal that
BMSCs possess enhanced osteochondral differentiation potential
mediated by constitutive activation of the Wnt/β-catenin pathway
within their native bone marrow niche (48). By contrast, ADSCs have adipogenic
lineage predisposition while demonstrating unique therapeutic value
in ischemic tissue repair through robust secretion of angiogenic
factors, such as VEGF (49).
Safety evaluations indicate a comparable absence of severe adverse
events between both cell types; however, the immunogenic risk is
notably reduced for ADSCs in allogeneic transplantation due to
their human leukocyte antigen-DR isotype-negative phenotype
(50).
Over the past decade, there have been a number of
reports on MSC-based treatments for lymphedema in both animal
studies and clinical trials (Fig.
2; Tables I and II). In the animal studies, lymphedema
models are predominantly established using mouse posterior limbs
and tails (51-53).
Evaluating the treatment outcomes involves immunohistochemical
staining methods such as perimeter measurements, depth of skin
edema, imaging techniques, anti-CD31 staining for angiogenesis,
anti-lymphatic vessel endothelial receptor-1 (LYVE-1) staining for
lymphangiogenesis and staining for VEGF receptor expression levels.
Additionally, no notable adverse events associated with MSC
transplantation were observed in the clinical trials analyzed in
the present review.
In a randomized controlled trial involving 50
Chinese patients with lymphedema following breast cancer surgery
(54), quantitative analysis
reveals that BMSC transplantation achieves an improved long-term
cure result compared with complex decongestant therapy (CDT). At a
12-month follow-up, the BMSC group demonstrates a 78.5% reduction
in mean limb edema volume (vs. 54.5% in CDT) and an 82% decrease in
visual analog scale pain scores (vs. 60% in CDT).
Previously, a number of studies have attempted to
apply the arteriovenous ring technique to lymphatic tissue
engineering (57-59).
Robering et al (60)
combine human lymphoendothelial cells (LECs) and BMSCs into the
fibrin matrix surrounding the atrioventricular ring, and
demonstrate preliminary progress in the culture of human lymphatic
blood vessels in rats, providing a possible way to generate
transplantable lymphatic blood vessel networks.
In addition to the lymphangiogenic effects of
VEGF-C, evidence delineates platelet-mediated regenerative
pathways. In a murine tail lymphedema model, ADSCs/platelet-rich
plasma (PRP) combination treatment increases the mean surface area
of anti-LYVE-1-stained lymphatic vessels at 2 weeks by 4.1±1.0%,
nearly a 2-fold increase compared with ADSC only therapy groups
(2.5±0.3%). In addition, PRP treatment shows the best results
regarding a reduction in wound size and improving wound
epithelialization, with less of an increase in wound perfusion
(25.1±31 PU vs. 33.8±33 PU in the ADSCs only group) (63). In a subsequent study, Hayashida
et al (64) demonstrates
that the number of LYVE-1 immunoreactive lymphatic vessels in a
lymphedema mouse model undergoing ADSC transplantation notably
increases when using indocyanine green lymphatic vessel imaging.
The mechanism study by Ogino et al (65) further reveals that after 2 weeks of
ADSCs transplantation, the proliferative lymphatic vessel ratio
increases by 0.61±0.04%, recovers the orientation of type I
collagen fibers from parallel to random and increases the number of
type III collagen fibers. A study by Dai et al (66) further divides ADSCs into those
expressing podoplanin and those not expressing podoplanin. In a
mouse model of limb lymphedema, transplantation of
podoplanin-positive ADSCs resulted in a notable generation of
lymphatic vessels and remission of lymphedema, compared with both
the podoplanin-negative ADSCs and the unsorted ADSC population (the
original, heterogeneous cell mixture from which the
podoplanin-positive and -negative subpopulations were isolated).
The study reveals a large number of lymphangiogenic cytokines such
as VEGF-C and D in the podoplanin-positive supernatant, which are
absent in the podoplanin-negative and unclassified groups.
Furthermore, immunocolocalization reveals that podoplanin-positive
cells in lymphatics are LYVE-1 positive.
Recent studies in extracellular vesicle (EV)
therapeutics demonstrate the superior efficacy of ADSC-derived EVs
compared with conventional therapeutic approaches [such as the
transplantation of intact ADSCs and standard decongestive therapy
(including manual lymphatic drainage, compression bandaging and
exercise)] in promoting lymphatic regeneration and reducing edema
volume in secondary lymphedema management (68-70).
The potential oncogenic risk of EV treatment is reduced compared
with direct stem cell transplantation, as ADSC-EVs lack cellular
replication capacity and exhibit targeted delivery of therapeutic
cargo without promoting tumor proliferation or metastasis in
preclinical models (70-72).
The previous study reveals that ADSCs-EV can promote the
proliferation and migration of LECs and enhance lymphatic
formation. In murine models, EV treatment achieves a 65.1±4.5% limb
volume reduction at 4 weeks post treatment, with an increase in the
total number of LYVE-1-positive lymphatic vessels (25.3±5.2% vs.
16.1±2.8% in controls) (70).
Mechanistically, an analysis of mRNA expression levels reveals a
2-fold enrichment of lymphangiogenic markers such as VEGF-C,
prospero-related homeobox 1 (Prox1), LYVE-1 and podoplanin, in an
EV treatment group compared with a PBS treatment group (73-75).
In addition to BMSCs and ADSCs, other cell
populations associated with stem cells have also been considered
for the treatment of secondary lymphedema.
In addition to the application of LECs, BMSCs also
expand regulatory T cells (Tregs) in vitro and in
vivo studies (72,78,79),
and Treg induction may serve a positive role in stem cell
transplantation in the treatment of lymphedema (80). Furthermore, intense inflammatory
response and immune cell infiltration serve an important role in
the pathogenesis of secondary lymphedema. A study by Gousopoulos
et al (81) quantifies this
axis in murine lymphedema models and reveals that a depletion of
CD4+ T cells (shown via anti-CD4 antibodies) results in
a notable reduction of the tissue area that is covered by lymphatic
vessels. Furthermore, IL-2/anti-IL-2 complex-induced Treg expansion
achieves edema reduction with a decrease in the CD45+,
CD206+ and CD68+ infiltrates. Adoptive
transfer of splenic Tregs decreases dermal TGF-β1, reduces collagen
deposition (shown with Sirius Red staining) without altering VEGF-C
levels and increases the fluorescence intensity of the
lymphatic-specific tracer (82-84).
Additionally, other studies investigate the
utilization of autologous peripheral blood stem cell
transplantation as a treatment for primary lymphedema (85,86).
A clinical study involving 10 patients reveal that this treatment
can improve edema symptoms to an extent (85); however, further high-quality
clinical studies are required to verify its effectiveness and scope
of application.
BCRL is predominantly induced by radical surgery,
radiotherapy and cytotoxic chemotherapy, and manifests in 20% of
patients with upper extremity, thoracic or breast involvement
(87). The emerging therapeutic
potential of MSCs in degenerative, autoimmune and genetic disorders
has extended to lymphedema management. BMSCs demonstrate
therapeutic efficacy through paracrine-mediated lymphangiogenic
properties that facilitate damaged network reconstruction. However,
their clinical implementation is constrained by invasive harvesting
procedures and age-dependent proliferative decline (88-90).
By contrast, ADSCs exhibit superior proangiogenic capabilities via
elevated VEGF secretion, coupled with minimally invasive harvesting
procedures, demonstrating enhanced ischemic tissue
revascularization and lymphatic remodeling in preclinical models.
However, the adipogenic tendency of ADSCs may increase the risk of
post-transplant fat deposition (91). The present review suggests that the
selection of the MSCs source is optimized according to BCRL
heterogeneity and pathological stage (acute edema/chronic
fibrosis), and that the synergistic effects of multi-source MSCs
combination therapies is investigated.
While MSCs exhibit therapeutic potential in
autoimmune diseases and chronic inflammation through their
immunomodulatory properties and paracrine effects, their clinical
application presents a complex duality of advantages and risks that
requires examination. A prominent limitation is their unpredictable
differentiation behavior (92). As
a study by Yoon et al (93)
demonstrates, transplanting MSCs may form ectopic tissues due to
microenvironmental cues, where BMSCs without immunophenotypic
purification (for example, those that lack CD105⁺, CD90⁺ or CD73⁺)
cause myocardial calcification instead of functional repair.
Furthermore, their dual role in oncology is also concerning. While
MSCs can inhibit tumors via immune modulation (94), they may paradoxically promote tumor
progression by suppressing natural killer/CD8+ T cell
activity, polarizing Th2 responses and stimulating angiogenesis
(95). Such contradictions
highlight the need for further mechanistic studies to reconcile the
regenerative benefits of MSCs with their pathological risks.
Current animal models for BCRL successfully mimic
localized swelling but fail to recapitulate the inflammatory tumor
microenvironment, which obscures the risks associated with MSC
therapies. Cancer exerts an influence on the immune response
through the release of various factors, such as cytokines and
chemokines, and these factors have the capacity to modify the
capability of the immune system to recognize and eliminate cancer
cells (96). Limitations in animal
lifespans and experimental durations restrict long-term safety
evaluations, especially for delayed tumorigenic effects. By
contrast, clinical trials observe no MSC-triggered cancer
recurrence or metastasis in cases with BCRL. ADSCs, prioritized for
their availability, ethical compliance and trophic factor release,
show clinical potential. However, their inherent tumor tropism and
unaddressed chronic safety issues emphasize the necessity to
balance regenerative benefits against oncological hazards during
therapeutic development.
Although MSCs demonstrate therapeutic efficacy in
clinical trials through tissue regeneration and immunomodulation,
their clinical translation remains hindered by unresolved
biological risks such as uncontrolled differentiation and unclear
long-term safety (97,98). While MSC-derived exosomes show
reduced oncogenic concerns compared with whole-cell therapies,
several trials have yielded favorable clinical outcomes, showcasing
both safety and efficacy (99-101).
Previous studies highlight MSCs transplantation safety in
short-term applications, yet gaps in the understanding of chronic
inflammatory responses, genomic instability and tumor
microenvironment interactions remain. To advance MSC-based
therapies, further studies are imperative, in which direct
comparisons of their regenerative benefits against latent
pathological risks should be prioritized.
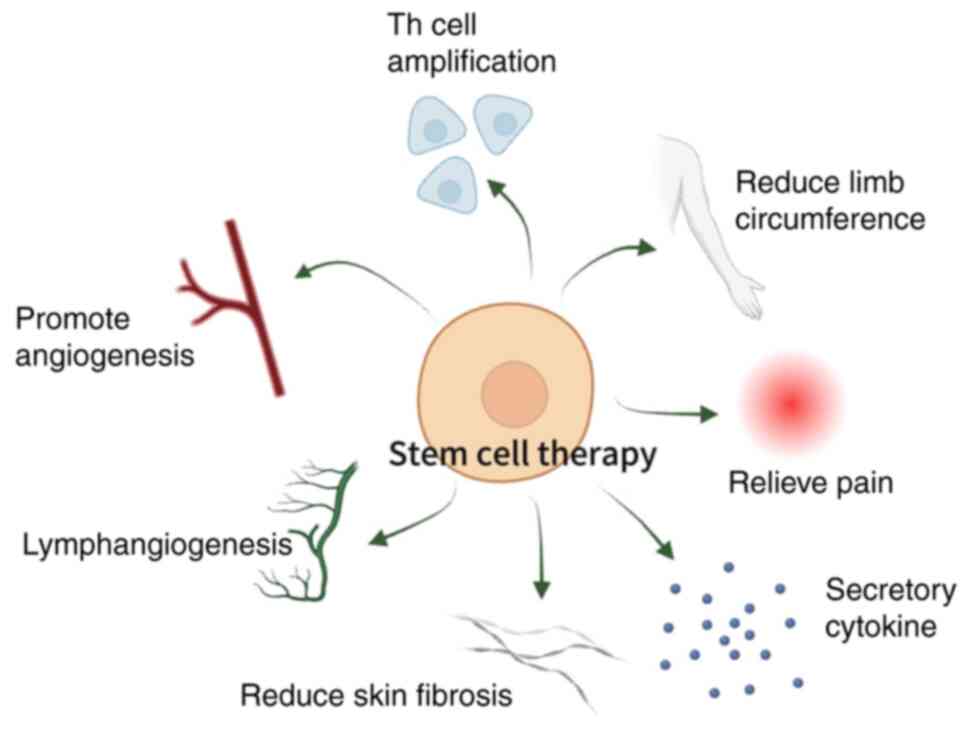
MSC therapies exhibit promise in ameliorating BCRL
by addressing edema, fostering lymphangiogenesis and mitigating
fibrosis. However, due to the absence of a universally recognized
or standardized treatment regimen for BCRL, additional clinical
studies with larger sample sizes and extended follow-up periods are
required to further investigate this prospective therapeutic
modality.
Not applicable.
Funding: No funding was received.
Not applicable.
YZ, JC and FL designed, guided and modified the
present study and manuscript. SH conducted the study, wrote the
manuscript and collected and collated the data. All authors read
and approved the final version of the manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Grada AA and Phillips TJ: Lymphedema:
Pathophysiology and clinical manifestations. J Am Acad Dermatol.
77:1009–1020. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hu LR and Pan J: Adipose-derived stem cell
therapy shows promising results for secondary lymphedema. World J
Stem Cells. 12:612–620. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vargo M, Aldrich M, Donahue P, Iker E,
Koelmeyer L, Crescenzi R and Cheville A: Current diagnostic and
quantitative techniques in the field of lymphedema management: A
critical review. Med Oncol. 41(241)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McLaughlin SA, Brunelle CL and Taghian A:
Breast cancer-related lymphedema: Risk factors, screening,
management, and the impact of locoregional treatment. J Clin Oncol.
38:2341–2350. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Aguilera-Eguía RA, Seron P,
Gutiérrez-Arias R and Zaror C: Which physical therapy intervention
is most effective in reducing secondary lymphoedema associated with
breast cancer? Protocol for a systematic review and network
meta-analysis. BMJ Open. 12(e065045)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang H, Wang L, Chen Y, Wu Q, Chen G,
Shen X, Wang Q, Yan Y, Yu Y, Zhong Y, et al: Outcomes of novel
coronavirus disease 2019 (COVID-19) infection in 107 patients with
cancer from Wuhan, China. Cancer. 126:4023–4031. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jariwala P and Kaur N: A descriptive study
on prevalence of arm/shoulder problems and its impact on quality of
life in breast cancer survivors. Indian J Cancer. 58:201–206.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hasenoehrl T, Palma S, Ramazanova D, Kölbl
H, Dorner TE, Keilani M and Crevenna R: Resistance exercise and
breast cancer-related lymphedema-a systematic review update and
meta-analysis. Support Care Cancer. 28:3593–3603. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
DiSipio T, Rye S, Newman B and Hayes S:
Incidence of unilateral arm lymphoedema after breast cancer: A
systematic review and meta-analysis. Lancet Oncol. 14:500–515.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dessources K, Aviki E and Leitao MM Jr:
Lower extremity lymphedema in patients with gynecologic
malignancies. Int J Gynecol Cancer. 30:252–260. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bruno C, Cesta CE, Hjellvik V, Ulrichsen
SP, Bjørk MH, Esen B, Gillies MB, Gissler M, Havard A, Karlstad Ø,
et al: Corrigendum to Antipsychotic use during pregnancy and risk
of specific neurodevelopmental disorders and learning difficulties
in children: A multinational cohort study [eClinicalMedicine 70
(2024) 102531/DOI: 10.1016/j.eclinm.2024.102531]. EClinicalMedicine
81: 103139, 2025.
|
|
13
|
Hassan AM, Fisher CS and Hassanein AH: ASO
author reflections: navigating the nuances of lymphedema prevention
with immediate lymphatic reconstruction. Ann Surg Oncol: Apr 20,
2025 (Epub ahead of print).
|
|
14
|
Bouhali S, Merchant F, Karni RJ, Gutierrez
C and Rasmussen JC: 3D rendering and analysis of dermal backflow as
an early indicator of cancer-acquired lymphedema using RGB-D and
near-infrared fluorescence lymphatic imaging. Proc SPIE 12930,
Medical Imaging 2024: Clinical and Biomedical Imaging, 1293008,
2024.
|
|
15
|
Frueh FS, Körbel C, Gassert L, Müller A,
Gousopoulos E, Lindenblatt N, Giovanoli P, Laschke MW and Menger
MD: High-resolution 3D volumetry versus conventional measuring
techniques for the assessment of experimental lymphedema in the
mouse hindlimb. Sci Rep. 6(34673)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang N, Liao C, Cao X, Nishimura M,
Brackenier YWE, Yurt M, Gao M, Abraham D, Alkan C, Iyer SS, et al:
Spherical echo-planar time-resolved imaging (sEPTI) for rapid 3D
quantitative T2* and susceptibility imaging.
Magn Reson Med. 93:121–137. 2025.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xie K, Jiang H, Chen X, Ning Y, Yu Q, Lv
F, Liu R, Zhou Y, Xu L, Yue Q and Peng J: Multiparameter MRI-based
model integrating radiomics and deep learning for preoperative
staging of laryngeal squamous cell carcinoma. Sci Rep.
15(16239)2025.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rogan S, Taeymans J, Luginbuehl H, Aebi M,
Mahnig S and Gebruers N: Therapy modalities to reduce lymphoedema
in female breast cancer patients: A systematic review and
meta-analysis. Breast Cancer Res Treat. 159:1–14. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gao Y, Ma T, Han M, Yu M and Wang X, Lv Y
and Wang X: Effects of acupuncture and moxibustion on breast
cancer-related lymphedema: A systematic review and meta-analysis of
randomized controlled trials. Integr Cancer Ther.
20(15347354211044107)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Keeley V: Advances in understanding and
management of lymphoedema (cancer, primary). Curr Opin Support
Palliat Care. 11:355–360. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Raju A and Chang DW: Vascularized lymph
node transfer for treatment of lymphedema: A comprehensive
literature review. Ann Surg. 261:1013–1023. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
No authors listed. Successful mesenchymal
stem cell treatment of leg ulcers complicated by Behcet disease: A
case report and literature review: Erratum. Medicine (Baltimore).
97(e0670)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Toyserkani NM, Christensen ML, Sheikh SP
and Sørensen JA: Stem cells show promising results for lymphoedema
treatment-a literature review. J Plast Surg Hand Surg. 49:65–71.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Das M, Mayilsamy K, Mohapatra SS and
Mohapatra S: Mesenchymal stem cell therapy for the treatment of
traumatic brain injury: Progress and prospects. Rev Neurosci.
30:839–855. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pittenger MF, Discher DE, Péault BM,
Phinney DG, Hare JM and Caplan AI: Mesenchymal stem cell
perspective: Cell biology to clinical progress. NPJ Regen Med.
4(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jensen MR, Simonsen L, Karlsmark T and
Bülow J: Microvascular filtration is increased in the forearms of
patients with breast cancer-related lymphedema. J Appl Physiol
(1985). 114:19–27. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Castilla DM, Liu ZJ, Tian R, Li Y,
Livingstone AS and Velazquez OC: A novel autologous cell-based
therapy to promote diabetic wound healing. Ann Surg. 256:560–572.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wariss BR, de Souza Abrahão K, de Aguiar
SS, Bergmann A and Thuler LCS: Effectiveness of four inflammatory
markers in predicting prognosis in 2374 women with breast cancer.
Maturitas. 101:51–56. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cong C, Rao C, Ma Z, Yu M, He Y, He Y, Hao
Z, Li C, Lou H and Gao D: ‘Nano-lymphatic’ photocatalytic
water-splitting for relieving tumor interstitial fluid pressure and
achieving hydrodynamic therapy†. Mater Horiz. 7:3266–3274.
2020.
|
|
30
|
Zhuang T, Lei Y, Chang JJ, Zhou YP, Li Y,
Li YX, Yang YF, Chen MH, Meng T, Fu SM, et al: A2AR-mediated
lymphangiogenesis via VEGFR2 signaling prevents salt-sensitive
hypertension. Eur Heart J. 44:2730–2742. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schoofs H, Daubel N, Schnabellehner S,
Grönloh MLB, Palacios Martínez S, Halme A, Marks AM, Jeansson M,
Barcos S, Brakebusch C, et al: Dynamic cytoskeletal regulation of
cell shape supports resilience of lymphatic endothelium. Nature.
641:465–475. 2025.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen CE, Chiang NJ, Perng CK, Ma H and Lin
CH: Review of preclinical and clinical studies of using cell-based
therapy for secondary lymphedema. J Surg Oncol. 121:109–120.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Avraham T, Zampell JC, Yan A, Elhadad S,
Weitman ES, Rockson SG, Bromberg J and Mehrara BJ: Th2
differentiation is necessary for soft tissue fibrosis and lymphatic
dysfunction resulting from lymphedema. FASEB J. 27:1114–1126.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nishioka T, Katayama KI, Kumegawa S, Isono
K, Baba T, Tsujimoto H, Yamada G, Inoue N and Asamura S: Increased
infiltration of CD4+ T cell in the complement deficient
lymphedema model. BMC Immunol. 24(42)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Uemura K, Katayama KI, Nishioka T,
Watanabe H, Yamada G, Inoue N and Asamura S: Dynamics of immune
cell infiltration and fibroblast-derived IL-33/ST2 axis induction
in a mouse model of post-surgical lymphedema. Int J Mol Sci.
26(1371)2025.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ogata F, Fujiu K, Matsumoto S, Nakayama Y,
Shibata M, Oike Y, Koshima I, Watabe T, Nagai R and Manabe I:
Excess lymphangiogenesis cooperatively induced by macrophages and
CD4(+) T cells drives the pathogenesis of lymphedema. J Invest
Dermatol. 136:706–714. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ogino R, Yokooji T, Hayashida M, Suda S,
Yamakawa S and Hayashida K: Emerging anti-inflammatory
pharmacotherapy and cell-based therapy for lymphedema. Int J Mol
Sci. 23(7614)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Higgins ET, Busse WW, Esnault S, Christian
BT, Klaus DR, Bach JC, Frye CJ and Rosenkranz MA: Fueling the fire
in the lung-brain axis: The salience network connects
allergen-provoked TH17 responses to psychological stress in asthma.
Brain Behav Immun. 128:276–288. 2025.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
39
|
De Castro V, Abdellaoui O, Dehecq B, Ndao
B, Mercier-Letondal P, Dauvé A, Garnache-Ottou F, Adotévi O, Loyon
R and Godet Y: Characterization of the aryl hydrocarbon receptor as
a potential candidate to improve cancer T cell therapies. Cancer
Immunol Immunother. 74(200)2025.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li H, Wu Y, Song XH, Li CX, Cai Y and Chen
C: Expression of Th1 and Th2 cytokines in serum of patients with
lupus nephritis. Mod Prev Med. 40 746:2013.
|
|
41
|
Zhu Q, Yang H, Altaf F, Wu N, Hu Y, Su L,
Li J, Liu J, Wang G, Igbiriki DG, et al: SOCS8 deficiency models
MAFLD-like progression in the zebrafish gut-liver axis. Water
Biology and Security. Elsevier, pp100414, 2025.
|
|
42
|
Lee SO and Kim IK: Molecular
pathophysiology of secondary lymphedema. Front Cell Dev Biol.
12(1363811)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Duhon BH, Phan TT, Taylor SL, Crescenzi RL
and Rutkowski JM: Current mechanistic understandings of lymphedema
and lipedema: Tales of fluid, fat, and fibrosis. Int J Mol Sci.
23(6621)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Peña Quián Y, Hernández Ramirez P, Batista
Cuellar JF, Perera Pintado A and Coca Pérez MA: Lymphoscintigraphy
for the assessment of autologous stem cell implantation in chronic
lymphedema. Clin Nucl Med. 40:217–219. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Toyserkani NM, Jensen CH, Tabatabaeifar S,
Jørgensen MG, Hvidsten S, Simonsen JA, Andersen DC, Sheikh SP and
Sørensen JA: Adipose-derived regenerative cells and fat grafting
for treating breast cancer-related lymphedema: Lymphoscintigraphic
evaluation with 1 year of follow-up. J Plast Reconstr Aesthet Surg.
72:71–77. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jørgensen MG, Toyserkani NM, Hansen FCG,
Thomsen JB and Sørensen JA: Prospective validation of indocyanine
green lymphangiography staging of breast cancer-related lymphedema.
Cancers (Basel). 13(1540)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kern S, Eichler H, Stoeve J, Klüter H and
Bieback K: Comparative analysis of mesenchymal stem cells from bone
marrow, umbilical cord blood, or adipose tissue. Stem Cells.
24:1294–1301. 2006.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yu SJ, Kim HJ, Lee ES, Park CG, Cho SJ and
Jeon SH: β-catenin accumulation is associated with increased
expression of nanog protein and predicts maintenance of MSC
self-renewal. Cell Transplant. 26:365–377. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zuk PA, Zhu M, Ashjian P, De Ugarte DA,
Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P and Hedrick
MH: Human adipose tissue is a source of multipotent stem cells. Mol
Biol Cell. 13:4279–4295. 2002.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Le Blanc K and Ringdén O: Immunomodulation
by mesenchymal stem cells and clinical experience. J Intern Med.
262:509–525. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hassanein AH, Sinha M, Neumann CR, Mohan
G, Khan I and Sen CK: A murine tail lymphedema model. J Vis Exp.
(10.3791/61848)2021.PubMed/NCBI View
Article : Google Scholar
|
|
52
|
Arruda G, Ariga S, de Lima TM, Souza HP
and Andrade M: A modified mouse-tail lymphedema model. Lymphology.
53:29–37. 2020.PubMed/NCBI
|
|
53
|
Yu J and Guo W: Modern medical progress of
peripheral lymphedema treated by integrated traditional Chinese and
western medicine. Adv Clin Med. 12:4228–4234. 2022.
|
|
54
|
Hou C, Wu X and Jin X: Autologous bone
marrow stromal cells transplantation for the treatment of secondary
arm lymphedema: A prospective controlled study in patients with
breast cancer related lymphedema. Jpn J Clin Oncol. 38:670–674.
2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhou H, Wang M, Hou C, Jin X and Wu X:
Exogenous VEGF-C augments the efficacy of therapeutic
lymphangiogenesis induced by allogenic bone marrow stromal cells in
a rabbit model of limb secondary lymphedema. Jpn J Clin Oncol.
41:841–846. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ismail AM, Abdou SM, Abdelnaby AY, Hamdy
MA, El Saka AA and Gawaly A: Stem cell therapy using bone
marrow-derived mononuclear cells in treatment of lower limb
lymphedema: A randomized controlled clinical trial. Lymphat Res
Biol. 16:270–277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Weigand A, Beier JP, Arkudas A, Al-Abboodi
M, Polykandriotis E, Horch RE and Boos AM: The arteriovenous (AV)
loop in a small animal model to study angiogenesis and vascularized
tissue engineering. J Vis Exp. (54676)2016.PubMed/NCBI View
Article : Google Scholar
|
|
58
|
Boos AM, Loew JS, Weigand A, Deschler G,
Klumpp D, Arkudas A, Bleiziffer O, Gulle H, Kneser U, Horch RE and
Beier JP: Engineering axially vascularized bone in the sheep
arteriovenous-loop model. J Tissue Eng Regen Med. 7:654–664.
2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Weigand A, Horch RE, Boos AM, Beier JP and
Arkudas A: The arteriovenous loop: Engineering of axially
vascularized tissue. Eur Surg Res. 59:286–299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Robering JW, Al-Abboodi M, Titzmann A,
Horn I, Beier JP, Horch RE, Kengelbach-Weigand A and Boos AM:
Tissue engineering of lymphatic vasculature in the arteriovenous
loop model of the rat. Tissue Eng Part A. 27:129–141.
2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Levi B, Glotzbach JP, Sorkin M, Hyun J,
Januszyk M, Wan DC, Li S, Nelson ER, Longaker MT and Gurtner GC:
Molecular analysis and differentiation capacity of adipose-derived
stem cells from lymphedema tissue. Plast Reconstr Surg.
132:580–589. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Dhumale P, Nielsen JV, Hansen ACS, Burton
M, Beck HC, Jørgensen MG, Toyserkani NM, Haahr MK, Hansen ST, Lund
L, et al: CD31 defines a subpopulation of human adipose-derived
regenerative cells with potent angiogenic effects. Sci Rep.
13(14401)2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ackermann M, Wettstein R, Senaldi C,
Kalbermatten DF, Konerding MA, Raffoul W and Erba P: Impact of
platelet rich plasma and adipose stem cells on lymphangiogenesis in
a murine tail lymphedema model. Microvasc Res. 102:78–85.
2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Hayashida K, Yoshida S, Yoshimoto H,
Fujioka M, Saijo H, Migita K, Kumaya M and Akita S: Adipose-derived
stem cells and vascularized lymph node transfers successfully treat
mouse hindlimb secondary lymphedema by early reconnection of the
lymphatic system and lymphangiogenesis. Plast Reconstr Surg.
139:639–651. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ogino R, Hayashida K, Yamakawa S and
Morita E: Adipose-derived stem cells promote intussusceptive
lymphangiogenesis by restricting dermal fibrosis in irradiated
tissue of mice. Int J Mol Sci. 21(3885)2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Dai T, Jiang Z, Cui C, Sun Y, Lu B, Li H,
Cao W, Chen B, Li S and Guo L: The roles of
podoplanin-positive/podoplanin-negative cells from adipose-derived
stem cells in lymphatic regeneration. Plast Reconstr Surg.
145:420–431. 2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Jørgensen MG, Toyserkani NM, Jensen CH,
Andersen DC, Sheikh SP and Sørensen JA: Adipose-derived
regenerative cells and lipotransfer in alleviating breast
cancer-related lymphedema: An open-label phase I trial with 4 years
of follow-up. Stem Cells Transl Med. 10:844–854. 2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Yang S, Sun Y and Yan C: Recent advances
in the use of extracellular vesicles from adipose-derived stem
cells for regenerative medical therapeutics. J Nanobiotechnology.
22(316)2024.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Kasseroller RG and Brenner E:
Effectiveness of manual lymphatic drainage in intensive phase I
therapy of breast cancer-related lymphedema-a retrospective
analysis. Support Care Cancer. 32(5)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Tashiro K, Yoshioka Y and Ochiya T:
Extracellular vesicles from adipose-derived stem cells relieve
extremity lymphedema in mouse models. Plast Reconstr Surg.
152:1011–1021. 2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Cheng X, Henick BS and Cheng K: Anticancer
therapy targeting cancer-derived extracellular vesicles. ACS Nano.
18:6748–6765. 2024.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Yuan Z, Zhu Z, Zhu F, Ding F, Wang Y, Wang
X, Luo X, Yang J, Liu F and Sun D: Impact of human adipose
tissue-derived stem cells on dermatofibrosarcoma protuberans cells
in an indirect co-culture: An in vitro study. Stem Cell Res Ther.
12(440)2021.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Diao X, Guo C, Zheng H, Zhao K, Luo Y, An
M, Lin Y, Chen J, Li Y, Li Y, et al: SUMOylation-triggered ALIX
activation modulates extracellular vesicles circTLCD4-RWDD3 to
promote lymphatic metastasis of non-small cell lung cancer. Signal
Transduct Target Ther. 8(426)2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Li Y, Zheng H, Luo Y, Lin Y, An M, Kong Y,
Zhao Y, Yin Y, Ai L, Huang J and Chen C: An HGF-dependent positive
feedback loop between bladder cancer cells and fibroblasts mediates
lymphangiogenesis and lymphatic metastasis. Cancer Commun (Lond).
43:1289–1311. 2023.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zhang HF, Wang YL, Tan YZ, Wang HJ, Tao P
and Zhou P: Enhancement of cardiac lymphangiogenesis by
transplantation of CD34+VEGFR-3+ endothelial
progenitor cells and sustained release of VEGF-C. Basic Res
Cardiol. 114(43)2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Kawai Y, Shiomi H, Abe H, Naka S, Kurumi Y
and Tani T: Cell transplantation therapy for a rat model of
secondary lymphedema. J Surg Res. 189:184–191. 2014.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Deng J, Dai T, Sun Y, Zhang Q, Jiang Z, Li
S and Cao W: Overexpression of Prox1 induces the differentiation of
human adipose-derived stem cells into lymphatic endothelial-like
cells in vitro. Cell Reprogram. 19:54–63. 2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Ou HX, Guo BB, Liu Q, Li YK, Yang Z, Feng
WJ and Mo ZC: Regulatory T cells as a new therapeutic target for
atherosclerosis. Acta Pharmacol Sin. 39:1249–1258. 2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Chen DB: Experimental study of bone marrow
mesenchymal stem cells (BMSCs) promoting hematopoietic
reconstruction and immune regulation POST-HSCT. Fujian Medical
University, 2022.
|
|
80
|
Salek Farrokhi A, Zarnani AH, Rezaei
Kahmini F and Moazzeni SM: Mesenchymal stem cells induce expansion
of regulatory T cells in abortion-prone mice. Reproduction.
161:477–487. 2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Gousopoulos E, Proulx ST, Bachmann SB,
Scholl J, Dionyssiou D, Demiri E, Halin C, Dieterich LC and Detmar
M: Regulatory T cell transfer ameliorates lymphedema and promotes
lymphatic vessel function. JCI Insight. 1(e89081)2016.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Choi G, Na H, Kuen DS, Kim BS and Chung Y:
Autocrine TGF-β1 maintains the stability of Foxp3+
regulatory T cells via IL-12Rβ2 downregulation. Biomolecules.
10(819)2020.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Christofi P, Pantazi C, Psatha N,
Sakellari I, Yannaki E and Papadopoulou A: Promises and pitfalls of
next-generation treg adoptive immunotherapy. Cancers (Basel).
15(5877)2023.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Mao LL, Yuan H, Wang WW, Wang YJ, Yang MF,
Sun BL, Zhang ZY and Yang XY: Adoptive regulatory T-cell therapy
attenuates perihematomal inflammation in a mouse model of
experimental intracerebral hemorrhage. Cell Mol Neurobiol.
37:919–929. 2017.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Ehyaeeghodraty V, Molavi B, Nikbakht M,
Malek Mohammadi A, Mohammadi S, Ehyaeeghodraty N, Fallahi B,
Mousavi SA, Vaezi M and Sefidbakht S: Effects of mobilized
peripheral blood stem cells on treatment of primary lower extremity
lymphedema. J Vasc Surg Venous Lymphat Disord. 8:445–451.
2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Białobrzeska M, Stępniewski J, Martyniak
A, Szuba A and Dulak J: Generation of human induced pluripotent
stem cell line from peripheral blood of patient with
lymphedema-distichiasis syndrome. Stem Cell Res.
85(103693)2025.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Ren Y, Kebede MA, Ogunleye AA, Emerson MA,
Evenson KR, Carey LA, Hayes SC and Troester MA: Burden of
lymphedema in long-term breast cancer survivors by race and age.
Cancer. 128:4119–4128. 2022.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Huang Y, Luo L, Xu Y, Li J, Wu Z, Zhao C,
Wen J, Jiang P, Zhu H, Wang L, et al: UHRF1-mediated epigenetic
reprogramming regulates glycolysis to promote progression of B-cell
acute lymphoblastic leukemia. Cell Death Dis.
16(351)2025.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Deng Y, Lin A, Lai C, He W, Li J, Zhang N,
Huang S, Tong L, Lai Y, Huo Y and Xu J: Combined inhibition of
importin-β and PBR enhances osteogenic differentiation of BMSCs by
reducing nuclear accumulation of glucocorticoid receptor and
promoting its mitochondrial translocation. J Steroid Biochem Mol
Biol. 250(106731)2025.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Liu Y, Xu W, Liu G, Ma L and Li Z:
Therapeutic efficacy of autologous bone marrow mesenchymal stem
cell transplantation in patients with spinal cord injury: A
meta-analysis. EFORT Open Rev. 10:309–315. 2025.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Xiang Q, Xu F, Li Y, Liu X, Chen Q, Huang
J, Yu N, Zeng Z, Yuan M, Zhang Q, et al: Transcriptome analysis and
functional identification of adipose-derived mesenchymal stem cells
in secondary lymphedema. Gland Surg. 9:558–574. 2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Volarevic V, Markovic BS, Gazdic M,
Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako
M and Stojkovic M: Ethical and safety issues of stem cell-based
therapy. Int J Med Sci. 15:36–45. 2018.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Yoon YS, Park JS, Tkebuchava T, Luedeman C
and Losordo DW: Unexpected severe calcification after
transplantation of bone marrow cells in acute myocardial
infarction. Circulation. 109:3154–3157. 2004.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Lan T, Luo M and Wei X: Mesenchymal
stem/stromal cells in cancer therapy. J Hematol Oncol.
14(195)2021.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Ljujic B, Milovanovic M, Volarevic V,
Murray B, Bugarski D, Przyborski S, Arsenijevic N, Lukic ML and
Stojkovic M: Human mesenchymal stem cells creating an
immunosuppressive environment and promote breast cancer in mice.
Sci Rep. 3(2298)2013.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Marcella S, Braile M, Grimaldi AM,
Soricelli A and Smaldone G: Exploring thymic stromal lymphopoietin
in the breast cancer microenvironment: A preliminary study. Oncol
Lett. 29(182)2025.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Zang L, Li Y, Hao H, Liu J, Cheng Y, Li B,
Yin Y, Zhang Q, Gao F, Wang H, et al: Efficacy and safety of
umbilical cord-derived mesenchymal stem cells in Chinese adults
with type 2 diabetes: A single-center, double-blinded, randomized,
placebo-controlled phase II trial. Stem Cell Res Ther.
13(180)2022.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Astori G, Amati E, Bambi F, Bernardi M,
Chieregato K, Schäfer R, Sella S and Rodeghiero F: Platelet lysate
as a substitute for animal serum for the ex-vivo expansion of
mesenchymal stem/stromal cells: Present and future. Stem Cell Res
Ther. 7(93)2016.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Thongsit A, Oontawee S, Siriarchavatana P,
Rodprasert W, Somparn P, Na Nan D, Osathanon T, Egusa H and
Sawangmake C: Scalable production of anti-inflammatory exosomes
from three-dimensional cultures of canine adipose-derived
mesenchymal stem cells: Production, stability, bioactivity, and
safety assessment. BMC Vet Res. 21(81)2025.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Xie X, Song Q, Dai C, Cui S, Tang R, Li S,
Chang J, Li P, Wang J, Li J, et al: Clinical safety and efficacy of
allogenic human adipose mesenchymal stromal cells-derived exosomes
in patients with mild to moderate Alzheimer's disease: A phase I/II
clinical trial. Gen Psychiatr. 36(e101143)2023.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Chu M, Wang H, Bian L, Huang J, Wu D,
Zhang R, Fei F, Chen Y and Xia J: Nebulization therapy with
umbilical cord mesenchymal stem cell-derived exosomes for COVID-19
pneumonia. Stem Cell Rev Rep. 18:2152–2163. 2022.PubMed/NCBI View Article : Google Scholar
|