Introduction
Lung cancer remains the leading cause of
cancer-related mortality worldwide, with non-small cell lung cancer
(NSCLC) accounting for 85% of cases (1,2).
Early diagnosis of NSCLC involves the detection of tumor markers,
CT scans and biopsies. Although biopsy is the ‘gold standard’, it
is invasive and may cause pain and infection. In recent years,
liquid biopsy has proven to be a non-invasive method for early
screening in which circulating tumor cells, circulating tumor DNA
and exosomes in the blood are detected (3). The treatment methods for NSCLC
include surgery, radiotherapy, chemotherapy, molecular targeted
therapy and immunotherapy.
Driver gene mutations are frequently detected in
patients with advanced NSCLC. The epidermal growth factor receptor
(EGFR) gene is the gene with the highest mutation rate in Asian
patients with NSCLC, and EGFR signal transduction plays a notable
role in tumorigenesis. EGFR-tyrosine kinase inhibitors (TKIs) such
as gefitinib are therefore an important treatment method for
patients with advanced NSCLC harboring EGFR sensitive mutations
(4-6).
While EGFR-TKIs can improve the survival of patients with advanced
NSCLC, their use is complicated by adverse effects that range from
mild dermatological reactions to rare but fatal drug-induced
interstitial lung disease (ILD).
There is no single, absolute standard for the
diagnosis of ILD caused by EGFR-TKIs, but the following elements
form a basis: (i) New or worsening respiratory symptoms or hypoxia;
(ii) new radiographical findings via high-resolution computed
tomography (HRCT) consistent with ILD; and (iii) temporal
association with gefitinib initiation and the exclusion of other
likely causes (7,8). Gefitinib-induced ILD, with a reported
incidence of 1-5%, typically manifests within 4 weeks of treatment
initiation and carries a mortality rate exceeding 30% (9,10).
The clinical presentation and course of EGFR-TKI-induced ILD can
vary widely. Early diagnosis and intervention are critical.
However, clinical overlap with tumor progression or infection
complicates the management of this disease. In the present study, a
case of gefitinib-induced ILD was presented. Unlike typical
EGFR-TKI-induced ILD, the patient manifested acute high fever as
the initial symptom-a rare feature not commonly reported in the
literature. Furthermore, rapid recurrence of severe ILD within 24 h
of gefitinib rechallenge provides unequivocal evidence of causality
and highlights the peril of re-exposure. These characteristics
amplify the novelty of the current report and emphasize the
diagnostic challenges, therapeutic strategies and considerations
for rechallenging EGFR-TKIs.
Case presentation
A 68-year-old male patient with a 15-year smoking
history and hypertension presented with a paroxysmal cough in
August 2021. The patient had no other chronic comorbidities,
including chronic obstructive pulmonary disease, autoimmune
disorders or other conditions affecting respiratory function or
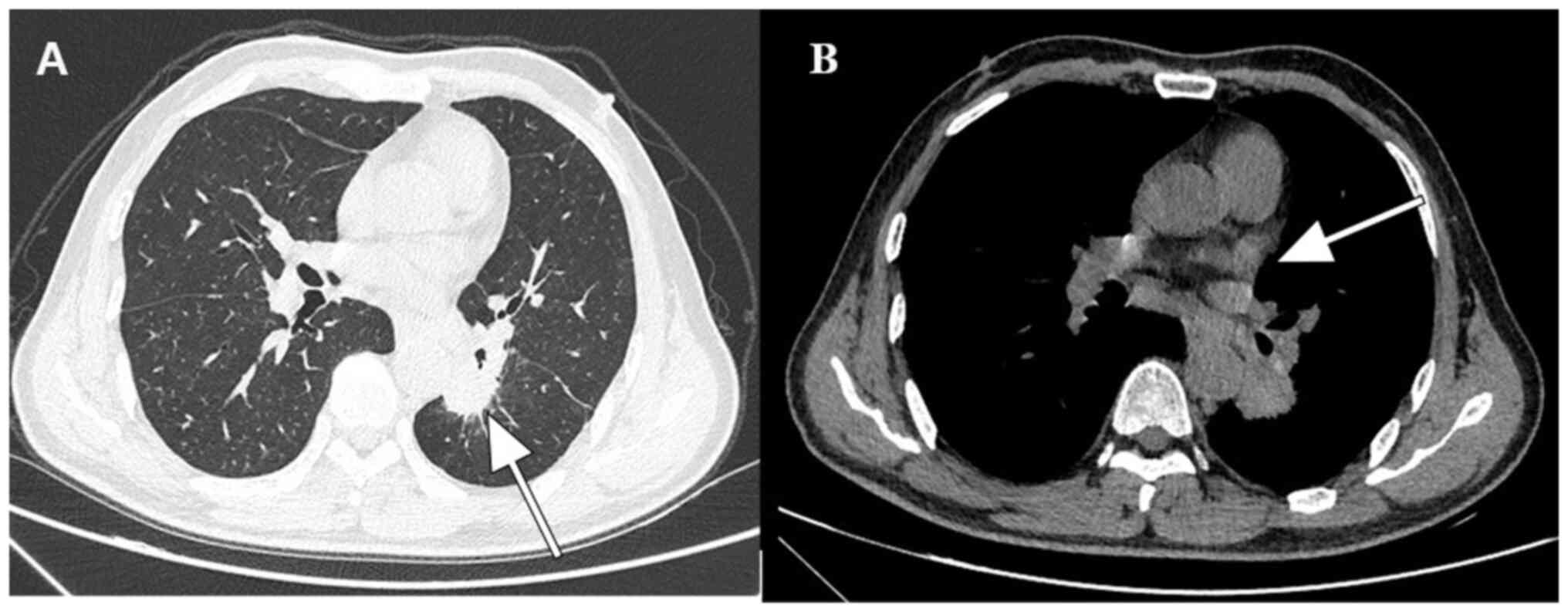
drug metabolism. Chest CT revealed a left lower lobe mass and
mediastinal lymphadenopathy (Fig.
1A and B). A subsequent biopsy
indicated lung adenocarcinoma. Whole-body CT examination further
revealed mediastinal and left hilar lymph node metastasis.
Therefore, lung adenocarcinoma (cT2N3M0, stage IIIB) was diagnosed.
Considering the difficulty of complete surgical resection, the
patient was finally diagnosed with unresectable locally advanced
NSCLC. Subsequent pathological gene detection indicated an EGFR
exon 19 deletion. After one cycle of chemotherapy with pemetrexed
(500 mg/m2, with a total dose of 900 mg) and carboplatin
(AUC=5, with a dose of 600 mg), gefitinib (250 mg/day) was
initiated in late September 2021 due to severe bone marrow
suppression, which necessitated the discontinuation of
chemotherapy.
A total of 20 days post-gefitinib initiation, the
patient developed a fever (41˚C) and progressive dyspnea. On
October 17, the patient further developed wheezing, palpitations
and shortness of breath, with a blood oxygen saturation of only
80%. Arterial blood gas analysis showed a PaO2 of 43
mmHg and a PaCO2 of 28 mmHg. Physical examination
revealed tachypnea with bilateral fine crackles audible over the
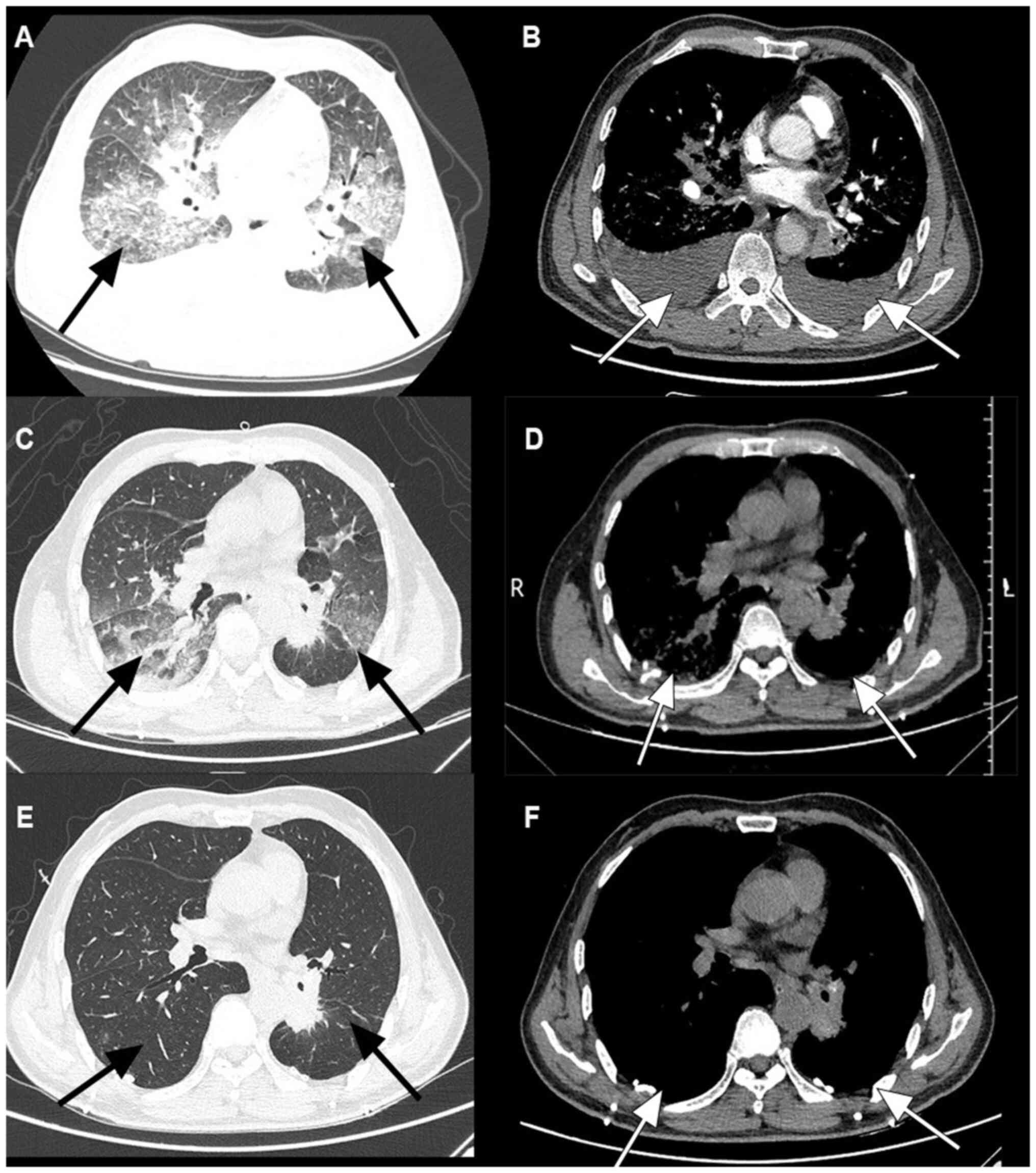
lower lung fields. A repeat chest CT showed extensive diffuse
disease in both lungs, with interstitial disease and bilateral
pleural effusion (Fig. 2A and
B). Since the pulmonary
inflammation had progressed rapidly and the patient had developed
type I respiratory failure, the patient was transferred to the ICU
for further treatment. The patient underwent a series of tests for
infection, including blood, urine and sputum cultures as well as
respiratory virus, fungal and tuberculosis testing. The results of
the laboratory tests and virus indicators were negative, suggesting
a low possibility of bacterial or viral pneumonia. In addition, a
cardiac cause was ruled out following cardiac ultrasonography.
Therefore, ILD caused by gefitinib was highly suspected. Immediate
methylprednisolone therapy was commenced, gefitinib administration
was halted, an oxygen mask (oxygen flow rate of 5 l/min) was used,
and thoracentesis and catheter drainage were performed. The patient
was treated with intravenous methylprednisolone 80 mg daily for 1
week, followed by 40 mg daily for 1 week, which was then
transitioned to oral prednisone and tapered as follows: 30 mg daily
for 1 week, 20 mg daily for 1 week, 10 mg daily for 1 week and
finally 5 mg daily for 2 weeks before discontinuation. No
additional tapering strategies were used. The total duration of
corticosteroid therapy was 7 weeks. After 6 days of treatment, the
condition of the patient notably improved, spontaneous breathing
was stable, PaO2 increased to 98% and reexamination by
chest CT showed that the inflammation was relieved (Fig. 2C and D). The patient was considered stable,
discharged and the steroid administration was tapered weekly as
described. The patient returned to the hospital after 4 weeks and
follow-up chest CT showed that the inflammation had been controlled
(Fig. 2E and F).
After steroid withdrawal for 1 month, gefitinib was
restarted at 250 mg/day due to disease progression. Within 2 weeks,
ILD had recurred, prompting the permanent discontinuation of
gefitinib.
Discussion
Gefitinib-induced ILD poses significant diagnostic
challenges due to its non-specific clinical presentation, which
often overlaps with infectious pneumonia, tumor progression or
cardiogenic pulmonary edema (1,11).
In the present case, the patient's acute onset of fever, hypoxemia
and bilateral ground-glass opacities on CT raised the suspicion of
ILD, but rigorous exclusion of alternative etiologies was required.
Notably, while dyspnea and cough are the most common presenting
symptoms, often accompanied by hypoxemia and radiographical
evidence of diffuse lung injury, the initial manifestation of a
high-grade fever observed in the present patient is uncommon.
Large-scale studies and literature reviews of EGFR-TKI-related ILD
consistently report that high fever over 40˚C is an uncommon
symptom compared with respiratory symptoms (12,13).
When fever does occur, it is often low-grade or moderate. However,
in the present case, the absence of microbial pathogens in the
cultures and the indication of normal cardiac function by
echocardiography strengthened the diagnosis of drug-induced injury.
The absence of specific early symptoms transforms ILD into a
‘silent threat’, necessitating proactive surveillance. HRCT remains
the cornerstone for evaluating ILD as it reliably highlights
characteristic patterns such as diffuse alveolar damage and
interstitial thickening (14).
Periodic HRCT in high-risk populations is essential for early
detection, enabling timely intervention before irreversible
fibrosis occurs. While balancing risks and costs, HRCT use can
significantly improve diagnostic accuracy and patient outcomes.
Future advances in biomarker detection may refine this approach,
but HRCT remains indispensable today.
While ILD is a recognized adverse effect of
EGFR-TKIs, its incidence and severity vary significantly between
generations (15).
First-generation TKIs such as gefitinib carry a substantially
higher risk of ILD compared with third-generation TKIs such as
osimertinib. This difference is attributed to the greater
selectivity of osimertinib for mutant EGFR over wild-type EGFR,
potentially reducing off-target effects in the lung parenchyma
(16). Additionally, the severity
and incidence of ILD induced by gefitinib may differ from the ILD
induced by other EGFR-TKIs. Among the reported cases of ILD caused
by gefitinib, the majority occurred within 4 weeks of
administration (17). In the
POLARSTAR study, 58.5% of the ILD cases also developed within 4
weeks of erlotinib treatment, while osimertinib-induced ILD is more
likely to be delayed (median time: 3-6 months) (13,18,19).
The pathogenesis of EGFR-TKI-related ILD remains
incompletely understood, but emerging evidence implicates both
direct epithelial toxicity and immune-mediated mechanisms. For
instance, gefitinib inhibits heat shock protein 70 (HSP70), a
molecular chaperone critical for mitigating oxidative stress and
repairing alveolar epithelial damage (20). Preclinical studies have
demonstrated that HSP70 downregulation exacerbates pulmonary
fibrosis, a finding consistent with the rapid progression of ILD
observed in the present patient (21,22).
Additionally, gefitinib upregulates interleukin-6, a
pro-inflammatory cytokine linked to acute lung injury (23). The temporal association between
drug initiation and symptom onset (20 days) in the present case
aligns with the proposed immunological mechanisms, where cytokine
storms precipitate diffuse alveolar damage (24). These findings highlight the dual
role of EGFR-TKIs in targeting oncogenic signaling pathways while
inadvertently disrupting pulmonary homeostasis.
The immediate discontinuation of gefitinib and the
initiation of glucocorticoids are pivotal to reversing ILD
progression (15,25,26).
In the present study, the patient received methylprednisolone at 80
mg/day, a moderate-dose regimen that achieved rapid clinical
improvement. This aligns with studies advocating for early,
aggressive steroid therapy to suppress immune hyperactivation
(13,27). However, the optimal dosing remains
a contentious topic; severe cases may require pulse therapy (such
as methylprednisolone 1 g/day for 3 days) to control fulminant
inflammation (26,28). The marked radiological resolution
in the present case underscores the importance of timely
intervention. Nonetheless, steroid tapering must be gradual to
prevent relapse as abrupt withdrawal may exacerbate subclinical
inflammation (29).
Reintroducing EGFR-TKIs after ILD recovery remains a
clinical dilemma (30). While
EGFR-TKIs can often be successfully reintroduced at the same or
reduced dose after the resolution of mild/moderate ILD with
appropriate management, same-dose gefitinib rechallenge carries a
significant and well-documented risk of rapid, frequently fatal,
ILD recurrence. A study suggests that switching to third-generation
agents (such as osimertinib) or dose reduction may mitigate
recurrence risk (31). In the
present study, after re-administering gefitinib in response to
strong patient demand, ILD rapidly recurred, highlighting the
unpredictability of this approach. Notably, pre-existing risk
factors, such as smoking history and male sex, likely heightened
the susceptibility to recurrent injury (32). Current guidelines recommend
permanent discontinuation of the culprit EGFR-TKI, with alternative
therapies considered for disease control (33). In select cases, cautious
rechallenge combined with steroid or with acetylcysteine
prophylaxis may be attempted, but this requires meticulous
monitoring (13).
In conclusion, gefitinib-induced ILD is rare but
with high mortality, and the exact pathogenesis remains unclear,
which needs further exploration in the future. Patients with NSCLC
with high risk factors for ILD should be monitored. Once ILD
occurs, it is necessary to stop the EGFR-TKI in time and give
effective treatment. Further research is needed to elucidate
biomarkers predictive of ILD risk, such as serum IL-6 levels or
HSP70 expression, which could guide personalized therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JL conceptualized the study, developed methodology
and wrote the original draft. BQ and ZPW contributed to data
acquisition and analysis, and were involved in writing, reviewing
and editing the manuscript. JL and BQ confirm the authenticity of
all the raw data. YPS visualized data and supervised the study. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
publication, which included the acquisition of clinical data and
associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Balata H, Fong KM, Hendriks LE, Lam S,
Ostroff JS, Peled N, Wu N and Aggarwal C: Prevention and early
detection for NSCLC: Advances in thoracic oncology 2018. J Thorac
Oncol. 14:1513–1527. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hendriks LEL, Remon J, Faivre-Finn C,
Garassino MC, Heymach JV, Kerr KM, Tan DSW, Veronesi G and Reck M:
Non-small-cell lung cancer. Nat Rev Dis Primers.
10(71)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shan L, Qiao Y, Ma L, Zhang X, Chen C, Xu
X, Li D, Qiu S, Xue X, Yu Y, et al: AuNPs/CNC nanocomposite with A
‘Dual Dispersion’ Effect for LDI-TOF MS analysis of intact proteins
in NSCLC serum exosomes. Adv Sci (Weinh).
11(e2307360)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shi Y, Wu L, Ji Y, Chen G, Li B, Bi M,
Yang R, Miao L, Zhang G, Gao H, et al: Efficacy and safety of
limertinib versus gefitinib as first-line treatment for locally
advanced or metastatic non-small-cell lung cancer with
EGFR-sensitising mutation: A randomised, double-blind,
double-dummy, phase 3 trial. Lancet Respir Med. 13:677–686.
2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gibson AJW, D'Silva A, Elegbede AA, Tudor
RA, Dean ML, Bebb DG and Hao D: Impact of Asian ethnicity on
outcome in metastatic EGFR-mutant non-small cell lung cancer. Asia
Pac J Clin Oncol. 15:343–352. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Velimirovic M, Brignola M, Chheng E, Smith
M and Hassan KA: Management of pulmonary toxicities associated with
systemic therapy in non-small cell lung cancer. Curr Treat Options
Oncol. 25:1297–1311. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Conte P, Ascierto PA, Patelli G, Danesi R,
Vanzulli A, Sandomenico F, Tarsia P, Cattelan A, Comes A, De
Laurentiis M, et al: Drug-induced interstitial lung disease during
cancer therapies: Expert opinion on diagnosis and treatment. ESMO
Open. 7(100404)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hotta K, Kiura K, Takigawa N, Yoshioka H,
Harita S, Kuyama S, Yonei T, Fujiwara K, Maeda T, Aoe K, et al:
Comparison of the incidence and pattern of interstitial lung
disease during erlotinib and gefitinib treatment in Japanese
Patients with non-small cell lung cancer: The Okayama lung cancer
study group experience. J Thorac Oncol. 5:179–184. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Riely GJ, Wood DE, Ettinger DS, Aisner DL,
Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, et
al: Non-small cell lung cancer, version 4.2024, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
22:249–274. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Beom SH, Kim DW, Sim SH, Keam B, Park JH,
Lee JO, Kim TM, Lee SH and Heo DS: Gefitinib-induced interstitial
lung disease in Korean lung cancer patients. Cancer Res Treat.
48:88–97. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Matsuno O: Drug-induced interstitial lung
disease: Mechanisms and best diagnostic approaches. Respir Res.
13(39)2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ohmori T, Yamaoka T, Ando K, Kusumoto S,
Kishino Y, Manabe R and Sagara H: Molecular and clinical features
of EGFR-TKI-associated lung injury. Int J Mol Sci.
22(792)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Park SC, Tan J, Wang X, Lederman D, Leader
JK, Kim SH and Zheng B: Computer-aided detection of early
interstitial lung diseases using low-dose CT images. Phys Med Biol.
56:1139–1153. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miyagahara T, Fujimori N, Ueda K,
Takamatsu Y, Matsumoto K, Teramatsu K, Takaoka T, Suehiro Y,
Shimokawa Y, Omori K, et al: Incidence and appropriate management
of drug-induced interstitial lung disease in Japanese patients with
unresectable pancreatic cancer: A multicenter retrospective study.
Asia Pac J Clin Oncol. 19:533–541. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Le T and Gerber DE: Newer-generation EGFR
inhibitors in lung cancer: How are they best used? Cancers (Basel).
11(336)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kawata T, Higashimori M, Itoh Y, Tomkinson
H, Johnson MG, Tang W, Nyberg F, Jiang H and Tanigawara Y:
Gefitinib exposure and occurrence of interstitial lung disease in
Japanese patients with non-small-cell lung cancer. Cancer Chemother
Pharmacol. 83:849–858. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Taronna G, Leonetti A, Gustavo Dall'Olio
F, Rizzo A, Parisi C, Buti S, Bordi P, Brocchi S, Golfieri R,
Ardizzoni A, et al: Transient asymptomatic pulmonary opacities and
interstitial lung disease in EGFR-mutated non-small cell lung
cancer treated with osimertinib. Tumori. 108:592–599.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gemma A, Kudoh S, Ando M, Ohe Y, Nakagawa
K, Johkoh T, Yamazaki N, Arakawa H, Inoue Y, Ebina M, et al: Final
safety and efficacy of erlotinib in the phase 4 POLARSTAR
surveillance study of 10708 Japanese patients with non-small-cell
lung cancer. Cancer Sci. 105:1584–1590. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang X, Zhang X, Huang W and Ge X: The
role of heat shock proteins in the regulation of fibrotic diseases.
Biomed Pharmacother. 135(111067)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Namba T, Tanaka K, Hoshino T, Azuma A and
Mizushima T: Suppression of expression of heat shock protein 70 by
gefitinib and its contribution to pulmonary fibrosis. PLoS One.
6(e27296)2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tanguy J, Pommerolle L, Garrido C, Kolb M,
Bonniaud P, Goirand F and Bellaye PS: Extracellular heat shock
proteins as therapeutic targets and biomarkers in fibrosing
interstitial lung diseases. Int J Mol Sci. 22(9316)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ishiguro Y, Ishiguro H and Miyamoto H:
Epidermal growth factor receptor tyrosine kinase inhibition
up-regulates interleukin-6 in cancer cells and induces subsequent
development of interstitial pneumonia. Oncotarget. 4:550–559.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fujita K, Hirose T, Kusumoto S, Sugiyama
T, Shirai T, Nakashima M, Akiyama Y and Sasaki Y: High exposure to
erlotinib and severe drug-induced interstitial lung disease in
patients with non-small-cell lung cancer. Lung Cancer. 86:113–114.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Akamatsu H, Inoue A, Mitsudomi T,
Kobayashi K, Nakagawa K, Mori K, Nukiwa T, Nakanishi Y and Yamamoto
N: Interstitial lung disease associated with gefitinib in Japanese
patients with EGFR-mutated non-small-cell lung cancer: Combined
analysis of two Phase III trials (NEJ 002 and WJTOG 3405). Jpn J
Clin Oncol. 43:664–668. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fontes ESM, Campainha S, Marques ID, Dinis
R, Inácio JR, Mendes JJ, Luís R, Ferreira AM, Racha-Pacheco R, Rolo
R, et al: Diagnosis and management of drug-induced interstitial
lung disease in the context of anti-cancer therapy: A
multidisciplinary viewpoint by portuguese experts. Clin Drug
Investig. 44:801–810. 2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kuo LC, Lin PC, Wang KF, Yuan MK and Chang
SC: Successful treatment of gefitinib-induced acute interstitial
pneumonitis with high-dose corticosteroid: A case report and
literature review. Med Oncol. 28:79–82. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin X, Guo H, Zhao W, Li M, Lin G, Chu Q,
Chen E, Chen L, Chen R, Chu T, et al: Expert consensus on cancer
treatment-related lung injury. J Thorac Dis. 17:1844–1875.
2025.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang X, Li H, Zhu M and Zhang Y:
Re-administration of gefitinib following diffuse interstitial lung
disease in a patient with advanced lung adenocarcinoma: A case
report and review of the literature. Oncol Lett. 9:2419–2421.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nishioka N, Imai H, Endo M, Notsu A,
Doshita K, Igawa S, Yokouchi H, Ninomiya T, Tokito T, Soda S, et
al: Real-world data on subsequent therapy for first-line
osimertinib-induced pneumonitis: Safety of EGFR-TKI rechallenge
(Osi-risk Study TORG-TG2101). Target Oncol. 19:423–433.
2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kiriu T, Tamura D, Tachihara M, Sekiya R,
Hazama D, Katsurada M, Nakata K, Nagano T, Yamamoto M, Kamiryo H,
et al: Successful osimertinib rechallenge with steroid therapy
after osimertinib-induced interstitial lung disease. Intern Med.
57:91–95. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shibaki R, Ozawa Y, Noguchi S, Murakami Y,
Takase E, Azuma Y, Maebeya M, Sugimoto T, Hayata A, Hayakawa T, et
al: Impact of pre-existing interstitial lung abnormal shadow on
lung injury development and severity in patients of non-small cell
lung cancer treated with osimertinib. Cancer Med. 11:3743–3750.
2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Endo M, Johkoh T, Kimura K and Yamamoto N:
Imaging of gefitinib-related interstitial lung disease:
Multi-institutional analysis by the West Japan thoracic oncology
group. Lung Cancer. 52:135–140. 2006.PubMed/NCBI View Article : Google Scholar
|