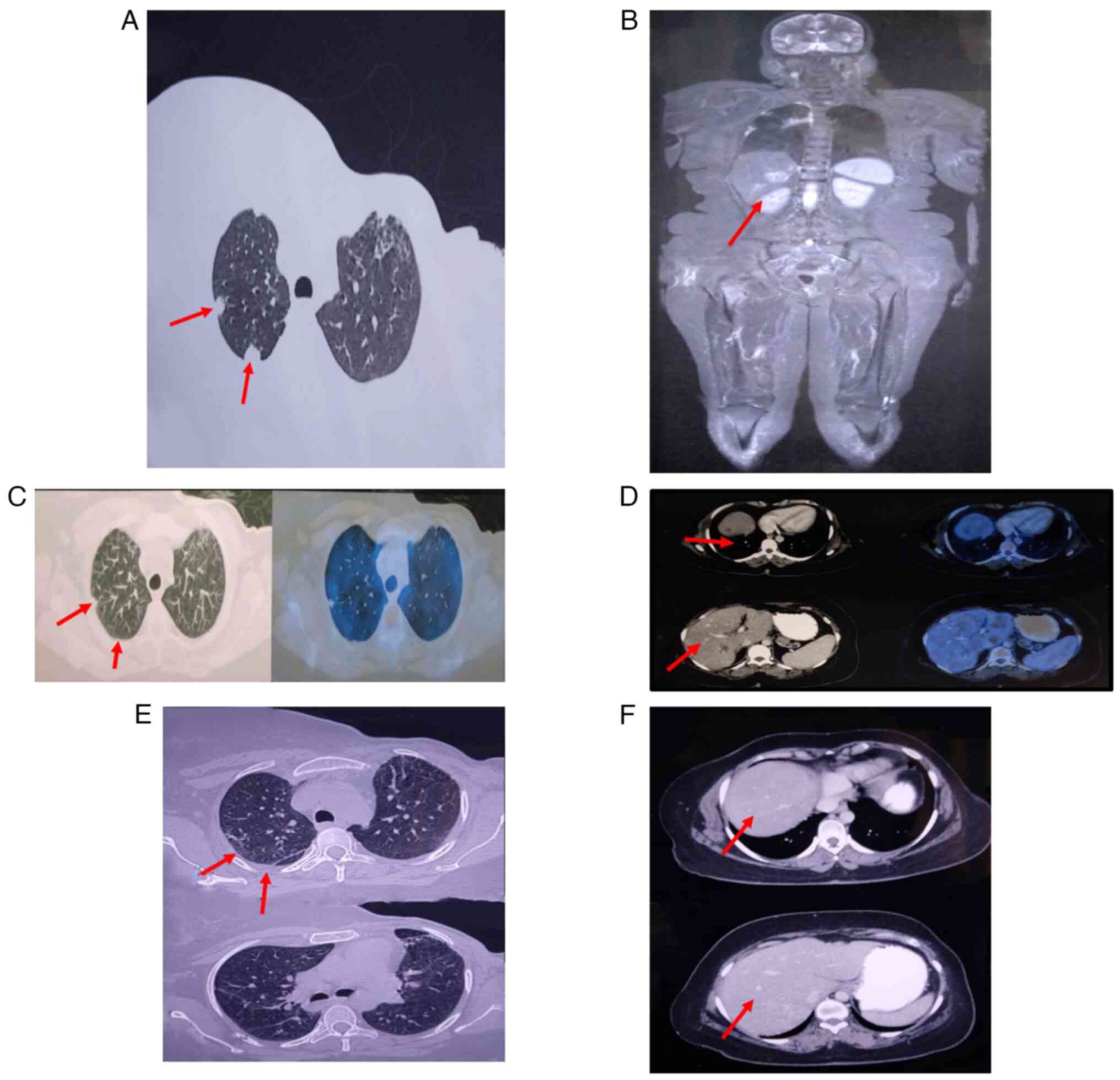
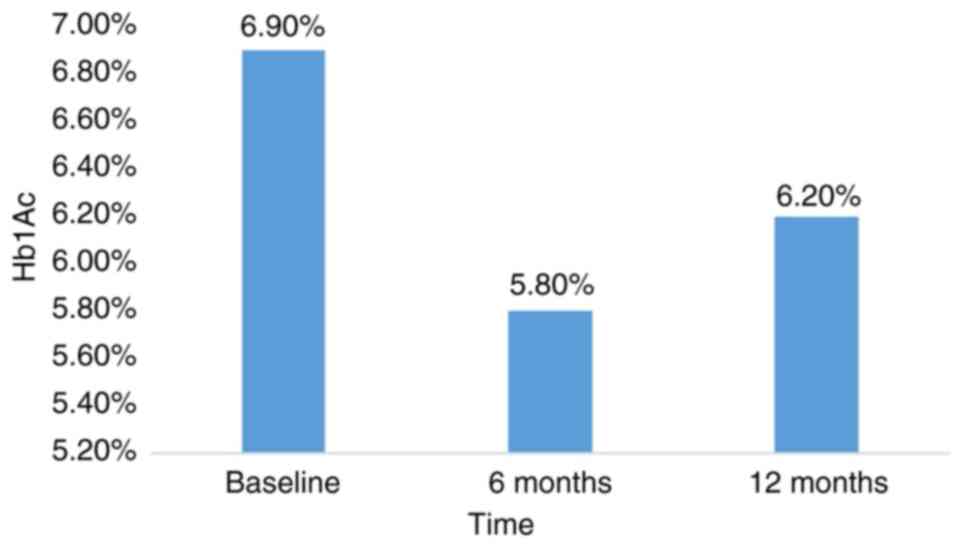
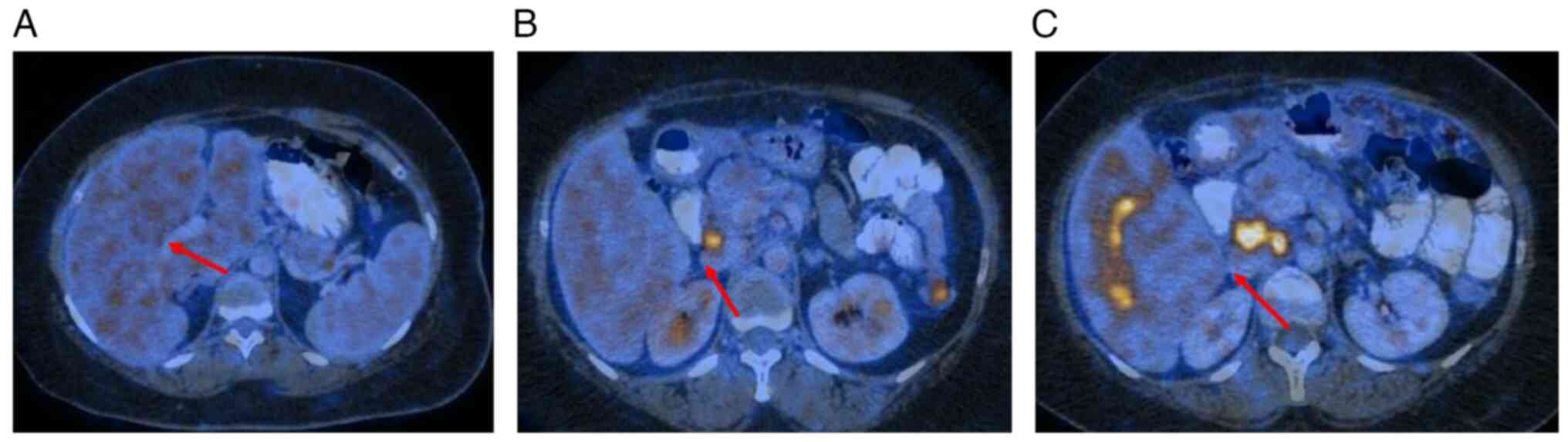
|
1
|
Li C and Li X: Advances in therapy for
hormone receptor (HR)-positive, human epidermal growth factor
receptor 2 (HER2)-negative advanced breast cancer patients who have
experienced progression after treatment with CDK4/6 inhibitors.
Onco Targets Ther. 14:2929–2939. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sharma R: Global, regional, national
burden of breast cancer in 185 countries: Evidence from GLOBOCAN
2018. Breast Cancer Res Treat. 187:557–567. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mehrotra R and Yadav K: Breast cancer in
India: Present scenario and the challenges ahead. World J Clin
Oncol. 13:209–218. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Walsh EM, Smith KL and Stearns V:
Management of hormone receptor-positive, HER2-negative early breast
cancer. Semin Oncol. 47:187–200. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Duggan C, Dvaladze A, Rositch AF, Ginsburg
O, Yip CH, Horton S, Camacho Rodriguez R, Eniu A, Mutebi M, Bourque
JM, et al: The breast health global initiative 2018 global summit
on improving breast healthcare through resource-stratified phased
implementation: Methods and overview. Cancer. 126 (Suppl
10):S2339–S2352. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Walter V, Fischer C, Deutsch TM, Ersing C,
Nees J, Schütz F, Fremd C, Grischke EM, Sinn P, Brucker SY, et al:
Estrogen, progesterone, and human epidermal growth factor receptor
2 discordance between primary and metastatic breast cancer. Breast
Cancer Res Treat. 183:137–144. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Afifi N and Barrero CA: Understanding
breast cancer aggressiveness and its implications in diagnosis and
treatment. J Clin Med. 12(1375)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
von Minckwitz G, Martin M, Wilson G, Alba
E, Schmidt M, Biganzoli L and Awada A: Optimizing taxane use in MBC
in the emerging era of targeted chemotherapy. Crit Rev Oncol
Hematol. 85:315–331. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
McKeage K: Nanosomal docetaxel lipid
suspension: A guide to its use in cancer. Clin Drug Investig.
37:405–410. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ahmad A, Sheikh S, Taran R, Srivastav SP,
Prasad K, Rajappa SJ, Kumar V, Gopichand M, Paithankar M, Sharma M,
et al: Therapeutic efficacy of a novel nanosomal docetaxel lipid
suspension compared with taxotere in locally advanced or metastatic
breast cancer patients. Clin Breast Cancer. 14:177–181.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Samar A, Tiwari S, Subramanian S, Joshi N,
Sejpal J, Khan MA and Ahmad I: A multicentric, retrospective
efficacy and safety study of nanosomal docetaxel lipid suspension
in metastatic castration-resistant prostate cancer. Prostate
Cancer. 2020(4242989)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Badiginchala R, Dattatreya PS, Suresh AVS,
Nirni SS, Andra VV, Bunger D and Chaturvedi A: Efficacy and safety
of nanosomal docetaxel lipid suspension (NDLS) versus conventional
docetaxel as neoadjuvant and adjuvant therapy for primary operable
breast cancer. Onco Targets Ther. 16:215–225. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Subramanian S, Prasanna R, Biswas G, Das
Majumdar SK, Joshi N, Bunger D, Khan MA and Ahmad I: Nanosomal
docetaxel lipid suspension-based chemotherapy in breast cancer:
Results from a multicenter retrospective study. Breast Cancer (Dove
Med Press). 12:77–85. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cardoso F, Paluch-Shimon S,
Schumacher-Wulf E, Matos L, Gelmon K, Aapro MS, Bajpai J, Barrios
CH, Bergh J, Bergsten-Nordström E, et al: 6th and 7th International
consensus guidelines for the management of advanced breast cancer
(ABC guidelines 6 and 7). Breast. 76(103756)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Patel R, Klein P, Tiersten A and Sparano
JA: An emerging generation of endocrine therapies in breast cancer:
A clinical perspective. NPJ Breast Cancer. 9(20)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Palumbo R, Torrisi R, Sottotetti F, Presti
D, Rita Gambaro A, Collovà E, Ferzi A, Agostinetto E, Maria Teragni
C, Saltalamacchia G, et al: Patterns of treatment and outcome of
palbociclib plus endocrine therapy in hormone
receptor-positive/HER2 receptor-negative metastatic breast cancer:
A real-world multicentre Italian study. Ther Adv Med Oncol.
13(1758835920987651)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ho MY and Mackey JR: Presentation and
management of docetaxel-related adverse effects in patients with
breast cancer. Cancer Manag Res. 6:253–259. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
U.S Food and Drug: Taxotere® (docetaxel)
Injection Concentrate III. Initial U.S. approval on 1996 and
Revised on 11/2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020449s063lbl.pdf.
Accessed February 02, 2025.
|
|
20
|
Bravo Gonzalez RC, Huwyler J, Boess F,
Walter I and Bittner B: In vitro investi-gation on the impact of
the surface-active excipients Cremophor EL, Tween 80 and SOlutol HS
15 on the metabo-lism of midazolam. Biopharm Drug Dispos. 25:37–49.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Marre F, Sanderink GJ, de Sousa G,
Gaillard C, Martinet M and Rahmani R: Hepatic biotransformation of
docetaxel (Taxotere) in vitro: involvement of the CYP3A subfamily
in humans. Cancer Res. 56:1296–1302. 1996.PubMed/NCBI
|
|
22
|
Fulton B and Spencer CM: Docetaxel. A
review of its pharmacodynamic and pharmacokinetic properties and
therapeutic efficacy in the management of metastatic breast cancer.
1996. Drugs. 51:1075–1092. 1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dieras V, Limentani S, Romieu G,
Tubiana-Hulin M, Lortholary A, Kaufman P, Girre V, Besenval M and
Valero V: Phase II multicenter study of larotaxel (XRP9881), a
novel taxoid, in patients with metastatic breast cancer who
previously received taxane-based therapy. Ann Oncol. 19:1255–1260.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Clarke SJ and Rivory LP: Clinical
pharmacokinetics of docetaxel. Clin Pharmacokinet. 36:99–114.
1999.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Royer I, Monsarrat B, Sonnier M, Wright M
and Cresteil T: Metabolism of docetaxel by human cytochromes P450:
Interactions with paclitaxel and other antineoplastic drugs. Cancer
Res. 56:58–65. 1996.PubMed/NCBI
|
|
26
|
Baker SD, Sparreboom A and Verweij J:
Clinical pharmacokinetics of docetaxel : Recent developments. Clin
Pharmacokinet. 45:235–252. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yamamoto N, Tamura T, Murakami H,
Shimoyama T, Nokihara H, Ueda Y, Sekine I, Kunitoh H, Ohe Y, Kodama
T, et al: Randomized pharmacokinetic and pharmacodynamic study of
docetaxel: dosing based on body-surface area compared with
individualized dosing based on cytochrome P450 activity estimated
using a urinary metabolite of exogenous cortisol. J Clin Oncol.
23:1061–1069. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Alexandre J, Rey E and Dieras V:
Prospective study of predictive factors of docetaxel (DCX)-induced
febrile neutro-penia (FN): Relevance of in vivo cytochrome 3A
(CYP3A) phenotyping [abstract no. 2046]. J Clin Oncol. 23 (16
Suppl. Pt 1)(146S)2005.
|
|
29
|
Hendrikx JJ, Lagas JS, Song JY, Rosing H,
Schellens JH, Beijnen JH, Rottenberg S and Schinkel AH: Ritonavir
inhibits intratumoral docetaxel metabolism and enhances docetaxel
antitumor activity in an immunocompetent mouse breast cancer model.
Int J Cancer. 138:758–769. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
International Diabetes Federation (IDF).
India Diabetes Report (2000-2045). 10th Edition. IDF, Brussels,
20021. https://diabetesatlas.org/data/en/country/93/in.html.
Accessed on July, 2024.
|
|
31
|
Eketunde AO: Diabetes as a risk factor for
breast cancer. Cureus. 12(e8010)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yoo KE, Kang RY, Lee JY, Lee YJ, Suh SY,
Kim KS, Kim HS, Lee SH and Lee BK: Awareness of the adverse effects
associated with prophylactic corticosteroid use during docetaxel
therapy. Support Care Cancer. 23:1969–1977. 2015.PubMed/NCBI View Article : Google Scholar
|