Introduction
Breast cancer (BC) is the most common malignancy
among female patients worldwide (1-3).
In India, the incidence and mortality of breast cancer have
increased over the past two decades (2). In 2020, GLOBOCAN reported 178,361
cases of BC in India, representing 13.5% of all cancer cases and
10.6% of all cancer-associated deaths (3). Hormone receptor (HR)-positive, human
epidermal growth factor receptor 2 (HER2)-negative BC,
characterized by estrogen or progesterone receptors and the absence
of HER2, constitutes 60-70% of all BC cases (4,5).
While this subtype is more common in postmenopausal patients, it
can also affect premenopausal patients, with risk increasing with
age (6).
In metastatic BC (MBC), the primary goals are
disease control and maintaining quality of life. Endocrine therapy
is the first choice for HR-positive, HER2-negative MBC. When
hormonal agents fail or metastases progress, chemotherapy (either
single-agent or combination) is recommended based on disease
aggressiveness, metastatic sites and patient factors such as age,
comorbidities and performance status. In palliative settings,
chemotherapy remains central (7).
Anthracyclines and taxanes are recommended for HR-positive and
HER2-negative MBC. Traditional taxanes such as docetaxel and
paclitaxel have notable toxicity profiles, leading to treatment
interruption and discontinuations. Although premedication with
corticosteroids, antihistamines and other agents can reduce some
toxicities, they do not eliminate the long-term challenges
(8).
The conventional formulation of docetaxel uses
polysorbate 80 and ethanol, which are associated with
hypersensitivity reaction, fluid retention, peripheral neuropathy
and other adverse effects (9,10).
To address these issues, the nanosomal docetaxel lipid suspension
(NDLS; Intas Pharmaceuticals Ltd., Ahmedabad, India) formulation
was developed using lipids generally recognized as safe by the
United States Food and Drug Administration (FDA) and is free of
polysorbate 80 and ethanol and the related toxicities. NDLS has
been approved by the Drugs Controller General of India for several
types of cancer, including hormone-resistant prostate, advanced
breast and non-small cell lung cancer (10). NDLS has been developed, using the
‘NanoAqualip’ technology, in which the drug is encapsulated in a
lipid core and avoids the use of organic solvents or detergents at
any stage of the manufacturing process. NDLS enhances systemic
availability of docetaxel through enhanced permeability and
retention effect, thereby potentially improving therapeutic
outcomes (11). To evaluate the
efficacy and safety of NDLS in treating BC, an open-label,
randomized, multiple-dose, parallel-group study was conducted; this
study compared NDLS with polysorbate-based docetaxel in patients
with locally advanced or MBC who had previously failed
chemotherapy. Overall therapeutic response (complete + partial)
rate in patients with metastatic BC treated with NDLS and Taxotere
were 35.5 and 26.3%, respectively, indicating a better response in
patients treated with NDLS. Patients in the NDLS group were not
premedicated with steroid premedication but the safety results of
NDLS were found to be comparable with Taxotere (10). The safety and efficacy of NDLS has
also been demonstrated in real-world settings (11-13).
The present study reports the case of a premenopausal patient with
HR-positive MBC with metastases to the liver, lung and bones and
managed with NDLS.
Case report
In July 2017, a 40-year-old female patient presented
to Cancure Cancer Centre at Tiruchirappalli, India and was
diagnosed with HR-positive, HER2-negative left breast carcinoma.
The patient underwent a modified radical mastectomy and received
adjuvant chemotherapy with 5-fluorouracil 500 mg/m2
i.v., doxorubicin 50 mg/m2 i.v. and cyclophosphamide 500
mg/m2 i.v., every 3 weeks for 4 cycles, followed by four
cycles of paclitaxel (175 mg/m2 i.v every three weeks)
and 50 Gy/25 fractions of post-mastectomy radiation therapy.
Tamoxifen (20 mg orally once daily) was administered starting
January 2018.
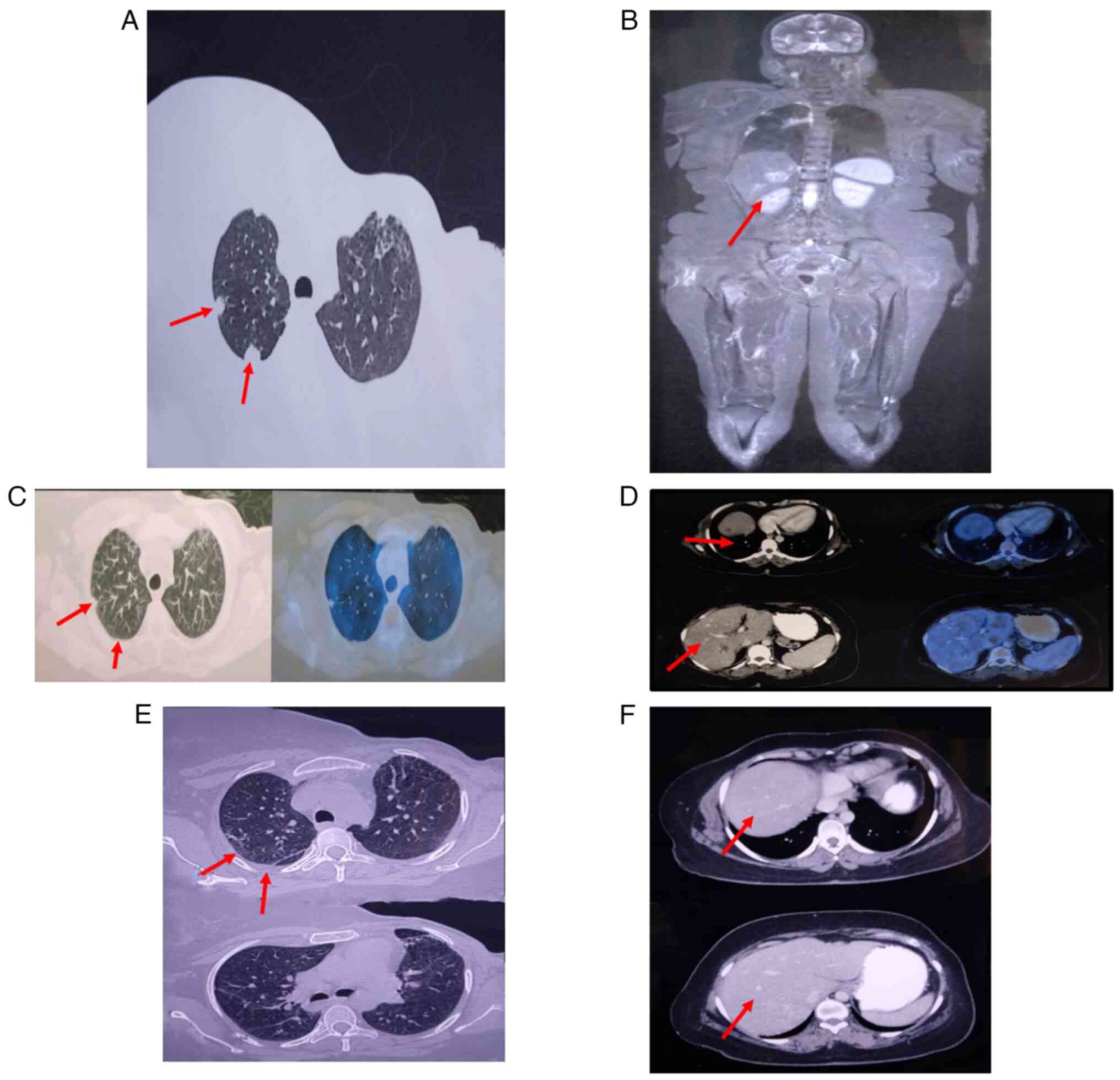
In March 2021, a routine follow-up showed a solitary
liver nodule. By October 2021, the patient had developed bilateral
lung nodules (Fig. 1A) and
multiple liver metastases (Fig.
1B), as well as metastases to the spine, sacral region and
other bones. In December 2021, ultrasonography (USG) guided
histopathological examination was performed. The tissue was
collected from liver secondary lesion with the largest sample
measuring 0.6x0.1x0.1 cm3. Samples underwent fixation
with buffered formalin for 5 h at room temperature (10 mm
thickness) and rinsing with phosphate buffered saline. The
dehydration was done with increasing concentrations of ethanol.
Xylene was used as clearing agent to displace ethanol and remove
fat from tissue and it was embedded in molten paraffin wax.
Further, the formalin-fixed paraffin-embedded (FFPE) tissue block
was stored at room temperature, was oriented in the center of
block, and sectioning (5-10 um slice) was done with microtome. The
tissue section was stained using hematoxylin and eosin (H&E),
it was deparaffinized and rehydrated and stained in Mayers
hematoxylin for 1 min followed by washing with tap water. The
tissue section was counterstained with alcoholic eosin for 1 min
and dehydrated with ethanol and cleared with xylene for 1 min. The
tissue section was stained at 120˚C for 3 min and was further
mounted in slide with cover slip for microscopic study using light
microscope (Olympus CX23-10x, 40x) for morphological evaluation.
TissueQuant Software (Version 4.0, Manipal School of Information
Sciences, Manipal, India) was used for image analysis.
The histopathological examination (HPE) confirmed
metastatic adenocarcinoma with high Ki-67 of 30% (normal range ≤5%:
low; ≥30%: high), and an elevated CA15-3 of 74.2 U/ml (normal
range: ≤30 U/ml). Due to secondary endocrine resistance and
visceral crisis, palliative chemotherapy with NDLS (75
mg/m2 i.v., every three weeks) was planned. The patient
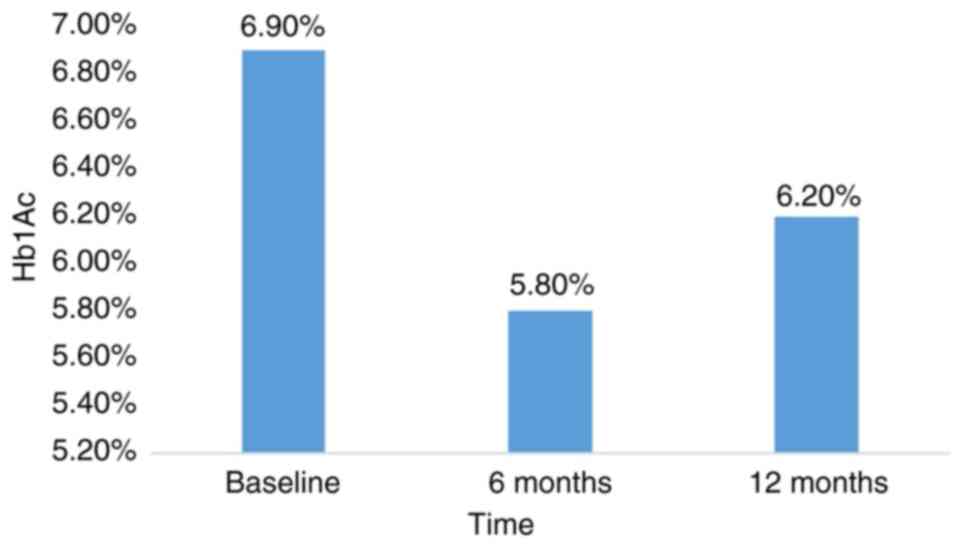
was diagnosed with type 2 diabetes in September 2021; HbA1c level
at diagnosis was 7.2% (data not shown). Treatment was initiated
with oral teneligliptin (20 mg) and metformin (500 mg) twice daily
along with proper diabetic diet and physical exercise. The HbA1c
level was 6.9% before the initiation of NDLS therapy (Fig. 1). To avoid
hypersensitivity/infusion-associated reactions with conventional
docetaxel, and corticosteroid premedication-associated
hyperglycemia, NDLS 120 mg (75 mg/m2 every three weeks)
was administered. The patient received six cycles of NDLS and
monthly zoledronic acid from January to June 2022 with no serious
adverse events. Following 6 and 12 cycles of NDLS, the HbA1c level
was 5.8 and 6.2% respectively (Fig.
2).
In June 2022, positron emission tomography-computed
tomography (PET-CT) scan (Siemens 64 slice Hybrid) showed a partial
response with decreased size of lung (Fig. 1C) and liver lesions (Fig. 1D), though residual osseous
metastases remained. By December 2022, PET-CT revealed inactive
lung (Fig. 1E) and hepatic
(Fig. 1F) metastases with
persistent but less active skeletal metastases, indicating a
continued partial response. Maintenance hormonal therapy with
leuprolide (11.25 mg intramuscular once every three months), oral
letrozole and denosumab (120 mg subcutaneously every four weeks)
was started and oral palbociclib (at a daily dose of 125 mg, with a
regimen of 21 days on medication followed by 7 days off, every 28
days) was added in January 2023. Follow-up PET-CT scans in April
2023 showed stable lytic and sclerotic lesions with decreased
metabolic activity in femoral lesions (data not shown). However, a
PET-CT scan in November 2023 indicated disease progression,
evidenced by an increased in the size of lesions in both lobes of
the liver, predominantly in the left lobe. Subsequently, the
patient was initiated on palliative hormonal therapy with
fulvestrant (500 mg i.m) administered once a month for five months,
until March 2024. A follow-up PET-CT in March 2024 revealed further
disease progression, with enlargement of liver lesions, peritoneal
nodules, and pelvic nodes (Fig.
3). An USG-guided liver biopsy and IHC analysis confirmed the
presence of ER+/PR-/HER2-positive disease with a Ki67 index of 60%.
Subsequently, the patient was commenced on palliative anti-HER2
therapy with trastuzumab emtansine (3.6 mg/kg, i.v, every 3 weeks).
However, the patient succumbed to the disease in July 2024 leading
to a progression-free survival (PFS) of approximately two years and
an overall survival of two years and six months with NDLS and
palliative maintenance therapy.
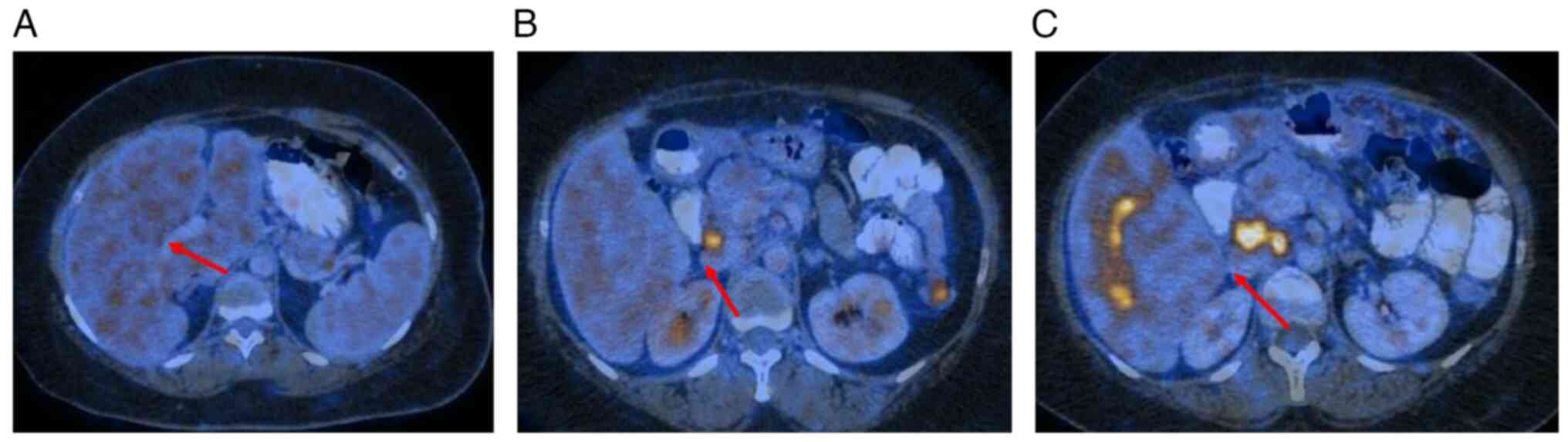 | Figure 3Positron emission tomography-computed
tomography-based follow-up responses. (A) In April 2023, the stable
multiple ill-defined FDG non-avid hypodense lesions were seen in
segments III, V, VI and VIII of liver, the largest one measuring
~17x15 mm2 in segment VIII. (B) In November 2023, there
was an increase in the size of hypodense lesions seen in both lobes
of liver, predominantly in the left lobe largest measuring ~48x43
mm2 in segment III (previously measured ~17x15
mm2 in segment VIII) with appearance of metabolic
activity (SUV max=7.4): Active hepatic metastases (C) I n March
2024, there was a further increase in size, number and metabolic
activity of hypodense lesions seen in both lobes of the liver,
predominantly in the left lobe measuring ~56x51 mm2
(previously measured ~48x43 mm2) in segment III (SUV
max=10) NDLS, nanosomal Docetaxel Lipid Suspension. Red arrows
indicate the site of metastatic lesions. |
Discussion
BC is the most common malignancy in the world
(14). The
HR-positive/HER2-negative subtype, accounting for ~70% of new BC
cases (14,15), is typically treated with endocrine
therapy (ET), including selective estrogen receptor (ER) modulators
and down regulators and aromatase inhibitors. ET remains the
preferred treatment unless a visceral crisis is present. Visceral
crisis, marked by severe organ dysfunction due to rapid disease
progression, affects 10-15% of patients with advanced BC and
necessitates more aggressive treatment. In cases of visceral
crisis, cyclin-dependent kinase 4/6 inhibitors (CDK4/6is) combined
with ET have shown better survival outcomes compared with ET alone
(16). Palbociclib, the first
FDA-approved CDK4/6i, has shown positive real-world outcomes in
combination with ET (16,17). The present patient, who was treated
with palbociclib following NDLS chemotherapy, experienced
improvement in condition.
Despite generally good prognoses for early
ER-positive, HER2-negative BC, some patients face recurrences years
after initial treatment (15).
Docetaxel is commonly used for MBC, especially following
anthracycline-based therapy failure. Docetaxel is a potent
antineoplastic agent commonly used in the treatment of various
types of cancer, including BC. Its mechanism of action involves the
disruption of the microtubular network, which serves a crucial role
in both mitotic and interphase cellular functions. It binds to free
tubulin and promotes its assembly into stable microtubules while
simultaneously inhibiting their disassembly (18) This leads to the formation of
abnormally stable microtubule bundles that no longer perform their
key functions in cell division. Consequently, the drug induces cell
cycle arrest, primarily in the G2/M phase, thereby preventing
proper mitotic progression and leading to cell death. Unlike many
spindle poisons currently used in clinical practice, docetaxel
binding to microtubules does not alter the number of protofilaments
in the microtubules. This suggests that docetaxel may function
through a unique mechanism compared with other drugs that affect
microtubule dynamics by altering protofilament numbers (19). Docetaxel is primarily metabolized
by the cytochrome P450 enzyme CYP3A4, making it a substrate for
this enzyme. In vitro studies have demonstrated that the
metabolism of docetaxel is influenced by the concomitant
administration of drugs that either induce or inhibit CYP3A4 or are
metabolized by this enzyme (20-24).
In vivo studies have shown a 2.2-fold increase in docetaxel
exposure when co-administered with ketoconazole, a potent CYP3A4
inhibitor. Protease inhibitors, especially ritonavir, increase
docetaxel exposure due to their inhibitory effects on CYP3A4
(25-28).
Therefore, concomitant use of docetaxel with drugs that inhibit
CYP3A4 should be approached with caution, as this may increase the
risk of docetaxel-associated toxicity. In clinical practice, if the
use of a potent CYP3A4 inhibitor is unavoidable, close monitoring
for signs of toxicity is recommended (29). A dose reduction of docetaxel may be
considered in such cases to minimize the risk of adverse effects
(19).
NDLS, a lipid-based docetaxel formulation free from
polysorbate 80 and ethanol, has demonstrated higher response rates
and better tolerability compared with conventional docetaxel
(11). Intravenous NDLS, at 75
mg/m² every 3 weeks for six cycles, offers improved outcomes
compared with docetaxel with no severe hypersensitivity reactions
(10). In real-world settings,
NDLS has shown an objective response rate of 64.7% and a disease
control rate of 70.6%, with a median OS of 30.4 months (13).
In India, diabetes is a comorbid condition prevalent
among patients with BC. Up to 10 to 20% of patients with breast
cancer have type 2 diabetes mellitus. The key risk factors for type
2 diabetes are old age and obesity, which are also risk factors for
breast cancer (30,31). The present diabetic patient with
HR-positive MBC received 12 cycles of NDLS, achieving a partial
response with no notable safety issues. The HbA1c level of the
patient was 7.2% at the time of diagnosis. After 12 cycles of NDLS
treatment, HbA1c level was 6.2%. With conventional docetaxel,
corticosteroids are administered as premedication to minimize the
infusion-associated toxicity responsible for hyperglycemia
(32). With NDLS, the
corticosteroid premedication is avoided, which helps avoid
hyperglycemia and contributes to the overall glycemic control of
the patient. A retrospective study by Subramanian et al
(13) in patients with BC (n=91)
who were treated with NDLS formulation and had a median follow-up
duration of 21 months, suggested that NDLS-based treatment was well
tolerated without notable safety concerns; 33% of the patients with
MBC were diabetic at baseline (14). This implies NDLS formulation avoids
the need for steroid premedication, which could facilitate better
glycemic control. The present case report highlighted the
successful use of 12 cycles of NDLS in a diabetic patient with
HR-positive MBC, demonstrating its efficacy and tolerability.
However, the present case report did not include biopsies or
molecular analyses, particularly for markers of resistance to
establish the molecular or cellular pathways of NDLS.
The present study reports the successful management
of a diabetic patient with HR-positive, HER2-negative MBC using an
extended 12-cycle regimen of NDLS combined with maintenance
hormonal therapy. The patient achieved a PFS of ~2 years and an OS
of two years and six months. Further studies with larger sample
sizes and a longer follow-up are needed to confirm efficacy and
safety.
Acknowledgements
The authors would like to thank Ms Sakshi Srivastava
and Dr Mehul R. Chorawala (both Intas Pharmaceuticals Limited,
Ahmedabad, India) for providing scientific writing assistance.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available due to institutional restrictions but may be
requested from the corresponding author.
Authors' contributions
AC, IA and PR analyzed data and wrote the
manuscript. IA, PR, LP and DB conceived and designed the study and
wrote and revised the manuscript. AC, DP, LP performed the
literature review and constructed figures. LP and DB analyzed and
interpreted data. DB, AC and IA edited the manuscript. All authors
have read and approved the final manuscript. AC and IA confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The study was conducted in accordance with the
ethical principles of International Conference on Harmonization E6
(R2) guideline on Good Clinical Practice and Declaration of
Helsinki (Fortaleza, Brazil, October 2013). Informed consent was
obtained from the patient.
Patient cconsent for publication
The patient provided written consent for publication
of the case report and accompanying images.
Competing interests
LP, DB and AC are affiliated with Intas
Pharmaceuticals Limited, who supplied NDLS, which was developed
based on patented technology. The remaining authors declare that
they have no competing interests.
References
|
1
|
Li C and Li X: Advances in therapy for
hormone receptor (HR)-positive, human epidermal growth factor
receptor 2 (HER2)-negative advanced breast cancer patients who have
experienced progression after treatment with CDK4/6 inhibitors.
Onco Targets Ther. 14:2929–2939. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sharma R: Global, regional, national
burden of breast cancer in 185 countries: Evidence from GLOBOCAN
2018. Breast Cancer Res Treat. 187:557–567. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mehrotra R and Yadav K: Breast cancer in
India: Present scenario and the challenges ahead. World J Clin
Oncol. 13:209–218. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Walsh EM, Smith KL and Stearns V:
Management of hormone receptor-positive, HER2-negative early breast
cancer. Semin Oncol. 47:187–200. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Duggan C, Dvaladze A, Rositch AF, Ginsburg
O, Yip CH, Horton S, Camacho Rodriguez R, Eniu A, Mutebi M, Bourque
JM, et al: The breast health global initiative 2018 global summit
on improving breast healthcare through resource-stratified phased
implementation: Methods and overview. Cancer. 126 (Suppl
10):S2339–S2352. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Walter V, Fischer C, Deutsch TM, Ersing C,
Nees J, Schütz F, Fremd C, Grischke EM, Sinn P, Brucker SY, et al:
Estrogen, progesterone, and human epidermal growth factor receptor
2 discordance between primary and metastatic breast cancer. Breast
Cancer Res Treat. 183:137–144. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Afifi N and Barrero CA: Understanding
breast cancer aggressiveness and its implications in diagnosis and
treatment. J Clin Med. 12(1375)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
von Minckwitz G, Martin M, Wilson G, Alba
E, Schmidt M, Biganzoli L and Awada A: Optimizing taxane use in MBC
in the emerging era of targeted chemotherapy. Crit Rev Oncol
Hematol. 85:315–331. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
McKeage K: Nanosomal docetaxel lipid
suspension: A guide to its use in cancer. Clin Drug Investig.
37:405–410. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ahmad A, Sheikh S, Taran R, Srivastav SP,
Prasad K, Rajappa SJ, Kumar V, Gopichand M, Paithankar M, Sharma M,
et al: Therapeutic efficacy of a novel nanosomal docetaxel lipid
suspension compared with taxotere in locally advanced or metastatic
breast cancer patients. Clin Breast Cancer. 14:177–181.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Samar A, Tiwari S, Subramanian S, Joshi N,
Sejpal J, Khan MA and Ahmad I: A multicentric, retrospective
efficacy and safety study of nanosomal docetaxel lipid suspension
in metastatic castration-resistant prostate cancer. Prostate
Cancer. 2020(4242989)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Badiginchala R, Dattatreya PS, Suresh AVS,
Nirni SS, Andra VV, Bunger D and Chaturvedi A: Efficacy and safety
of nanosomal docetaxel lipid suspension (NDLS) versus conventional
docetaxel as neoadjuvant and adjuvant therapy for primary operable
breast cancer. Onco Targets Ther. 16:215–225. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Subramanian S, Prasanna R, Biswas G, Das
Majumdar SK, Joshi N, Bunger D, Khan MA and Ahmad I: Nanosomal
docetaxel lipid suspension-based chemotherapy in breast cancer:
Results from a multicenter retrospective study. Breast Cancer (Dove
Med Press). 12:77–85. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cardoso F, Paluch-Shimon S,
Schumacher-Wulf E, Matos L, Gelmon K, Aapro MS, Bajpai J, Barrios
CH, Bergh J, Bergsten-Nordström E, et al: 6th and 7th International
consensus guidelines for the management of advanced breast cancer
(ABC guidelines 6 and 7). Breast. 76(103756)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Patel R, Klein P, Tiersten A and Sparano
JA: An emerging generation of endocrine therapies in breast cancer:
A clinical perspective. NPJ Breast Cancer. 9(20)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Palumbo R, Torrisi R, Sottotetti F, Presti
D, Rita Gambaro A, Collovà E, Ferzi A, Agostinetto E, Maria Teragni
C, Saltalamacchia G, et al: Patterns of treatment and outcome of
palbociclib plus endocrine therapy in hormone
receptor-positive/HER2 receptor-negative metastatic breast cancer:
A real-world multicentre Italian study. Ther Adv Med Oncol.
13(1758835920987651)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ho MY and Mackey JR: Presentation and
management of docetaxel-related adverse effects in patients with
breast cancer. Cancer Manag Res. 6:253–259. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
U.S Food and Drug: Taxotere® (docetaxel)
Injection Concentrate III. Initial U.S. approval on 1996 and
Revised on 11/2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020449s063lbl.pdf.
Accessed February 02, 2025.
|
|
20
|
Bravo Gonzalez RC, Huwyler J, Boess F,
Walter I and Bittner B: In vitro investi-gation on the impact of
the surface-active excipients Cremophor EL, Tween 80 and SOlutol HS
15 on the metabo-lism of midazolam. Biopharm Drug Dispos. 25:37–49.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Marre F, Sanderink GJ, de Sousa G,
Gaillard C, Martinet M and Rahmani R: Hepatic biotransformation of
docetaxel (Taxotere) in vitro: involvement of the CYP3A subfamily
in humans. Cancer Res. 56:1296–1302. 1996.PubMed/NCBI
|
|
22
|
Fulton B and Spencer CM: Docetaxel. A
review of its pharmacodynamic and pharmacokinetic properties and
therapeutic efficacy in the management of metastatic breast cancer.
1996. Drugs. 51:1075–1092. 1996.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dieras V, Limentani S, Romieu G,
Tubiana-Hulin M, Lortholary A, Kaufman P, Girre V, Besenval M and
Valero V: Phase II multicenter study of larotaxel (XRP9881), a
novel taxoid, in patients with metastatic breast cancer who
previously received taxane-based therapy. Ann Oncol. 19:1255–1260.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Clarke SJ and Rivory LP: Clinical
pharmacokinetics of docetaxel. Clin Pharmacokinet. 36:99–114.
1999.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Royer I, Monsarrat B, Sonnier M, Wright M
and Cresteil T: Metabolism of docetaxel by human cytochromes P450:
Interactions with paclitaxel and other antineoplastic drugs. Cancer
Res. 56:58–65. 1996.PubMed/NCBI
|
|
26
|
Baker SD, Sparreboom A and Verweij J:
Clinical pharmacokinetics of docetaxel : Recent developments. Clin
Pharmacokinet. 45:235–252. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yamamoto N, Tamura T, Murakami H,
Shimoyama T, Nokihara H, Ueda Y, Sekine I, Kunitoh H, Ohe Y, Kodama
T, et al: Randomized pharmacokinetic and pharmacodynamic study of
docetaxel: dosing based on body-surface area compared with
individualized dosing based on cytochrome P450 activity estimated
using a urinary metabolite of exogenous cortisol. J Clin Oncol.
23:1061–1069. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Alexandre J, Rey E and Dieras V:
Prospective study of predictive factors of docetaxel (DCX)-induced
febrile neutro-penia (FN): Relevance of in vivo cytochrome 3A
(CYP3A) phenotyping [abstract no. 2046]. J Clin Oncol. 23 (16
Suppl. Pt 1)(146S)2005.
|
|
29
|
Hendrikx JJ, Lagas JS, Song JY, Rosing H,
Schellens JH, Beijnen JH, Rottenberg S and Schinkel AH: Ritonavir
inhibits intratumoral docetaxel metabolism and enhances docetaxel
antitumor activity in an immunocompetent mouse breast cancer model.
Int J Cancer. 138:758–769. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
International Diabetes Federation (IDF).
India Diabetes Report (2000-2045). 10th Edition. IDF, Brussels,
20021. https://diabetesatlas.org/data/en/country/93/in.html.
Accessed on July, 2024.
|
|
31
|
Eketunde AO: Diabetes as a risk factor for
breast cancer. Cureus. 12(e8010)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yoo KE, Kang RY, Lee JY, Lee YJ, Suh SY,
Kim KS, Kim HS, Lee SH and Lee BK: Awareness of the adverse effects
associated with prophylactic corticosteroid use during docetaxel
therapy. Support Care Cancer. 23:1969–1977. 2015.PubMed/NCBI View Article : Google Scholar
|