Introduction
Anaplastic carcinoma of the pancreas (ACP), also
known as undifferentiated carcinoma, is an extremely rare
pancreatic neoplasm. Previous reports revealed the frequency of ACP
at 0.1-0.9% of all pancreatic cancers (1,2). The
Japan Pancreas Society categorized ACP into pleomorphic type,
spindle cell type, and osteoclast-like giant cell (OCGC) type
(3). On the other hand, WHO
classification distinguished undifferentiated carcinoma with OCGC
from undifferentiated carcinoma, and categorized undifferentiated
carcinoma into anaplastic undifferentiated carcinoma, sarcomatoid
undifferentiated carcinoma, and carcinosarcoma (4). ACP is frequently observed as a large
tumor with distant metastases upon diagnosis because of the rapid
progression and high invasiveness. The prognosis is significantly
worse in patients with ACP than in those with pancreatic ductal
adenocarcinoma (PDAC) (5).
Conversely, a previous report that analyzed only surgical resection
cases revealed compatible overall survival (OS) of resected ACP
cases to that of resected PDAC cases (6). Surgical resection may be the only
curative treatment strategy for ACP, but there was no consensus
about surgical indication for patients with ACP. Furthermore, the
significance of multidisciplinary therapy for ACP has not been
determined.
The role and the optimal candidates of surgical
resection for ACP require clarification. ACP exhibits
undifferentiated carcinoma characterized by positive staining for
vimentin and Snail, and negative staining for E-cadherin in
pathological examination, indicating that the tumor cells of ACP
undergo epithelial-mesenchymal transition (7-9).
Accordingly, previous case reports revealed that ACP commonly had
poor prognosis even after radical pancreatic resection (10,11).
On the other hand, surgical resection provided prolonged survival
without disease recurrence for some cases (1,12-14).
Indeed, undifferentiated carcinoma with OCGC is a subtype that
exhibited a preferable prognosis after surgery (15,16).
The effect of surgical resection on survival may be different
according to the tumor characteristics and its progression.
Selecting the patients with survival benefits from surgery is
important for deciding the optimal treatment strategy for
pancreatic cancer. However, only a few case studies reported
surgical outcomes for ACP due to the rarity of the disease, and
prognostic factors affecting survival after surgery for ACP remain
unknown. Therefore, clinicopathological features of ACP and
prognosis after surgery warrant further investigation. We conducted
pancreatectomy for 12 patients with ACP in our department. The
current study aimed to determine the oncological outcome of surgery
for ACP and clinicopathological factors that were associated with
survival after surgery.
Materials and methods
Patients
This study retrospectively analyzed the medical
records of 12 patients with ACP who underwent pancreatic resection
at the Department of General Surgery in Chiba University (Chiba,
Japan) from 2003 to 2021. All patients underwent multidetector-row
computed tomography (MDCT), magnetic resonance imaging (MRI), and
positron emission tomography upon diagnosis to assess the clinical
stage and resectability status as presented in the National
Comprehensive Cancer Network guideline for pancreatic
adenocarcinoma version 2 (2021). Endoscopic ultrasonography-tissue
acquisition (EUS-TA) was conducted for preoperative diagnosis in
some cases. Some recent cases received neoadjuvant chemotherapy.
Surgical resection of pancreatic tumors was performed with the
standard method and technique, as described in previous studies
(17). The patients underwent
routine adjuvant chemotherapy for 6 months. Adjuvant chemotherapy
regimens include gemcitabine (GEM) monotherapy or S-1 monotherapy.
Regarding follow-up, patients underwent blood tests, including
tumor markers, every 3 months, and MDCT every 6 months. Systemic
chemotherapy was administrated once they were deemed medically fit
for it when patients had tumor recurrence postoperatively. Only
patients who underwent macroscopic curative pancreatic resection
were included in the survival analyses. In some analyses, we
compared patients with ACP who underwent curative pancreatic
resection to those with resectable PDAC who received curative
surgery at our institution between 2008 and 2021. The inclusion
criteria of resectable PDAC were as follows: i) histologically
confirmed PDAC, ii) no evidence of distant metastases, except for
para-aortic lymph node metastases, at the time of surgery. The
exclusion criteria included: i) patients diagnosed with
unresectable or metastatic disease based on preoperative
examinations, ii) patients who received only palliative surgery,
and iii) patients with insufficient follow-up data. The PDAC cohort
included 305 patients, comprising 170 males and 135 females. The
median age was 63.5 years (range: 31-89 years). The committee on
Human Research of Chiba University School of Medicine approved the
study protocol (approval code: #3302). Informed consent was
obtained using an opt-out method, as approved by the institutional
ethics committee.
Preoperative parameters
All patients underwent blood tests before treatment
to assess preoperative parameters including tumor markers,
inflammatory markers, and nutritional parameters. Carcinoembryonic
antigen (CEA), carbohydrate antigen 19-9 (CA19-9), duke pancreatic
monoclonal antigen type 2 (DUPAN-2), and s-pancreas-1 antigen
(Span-1) were measured as tumor markers. Upper normal limits of CEA
(≤5.0 ng/ml), CA19-9 (≤37.0 U/ml), DUPAN-2 (≤150 U/ml), and Span-1
(≤30 U/ml) in our institution were used as cutoff values of these
tumor markers. The neutrophil-to-lymphocyte ratio (NLR) and
prognostic nutritional index (PNI) were calculated as previously
presented regarding inflammatory and nutritional parameters
(18).
Pathological examination
Tissue samples were fixed in 10% neutral buffered
formalin for 24 to 48 h at room temperature. Following fixation,
the specimens were dehydrated through ethanol, cleared in xylene,
and embedded in paraffin. Sections (4 µm thick) were cut, mounted
on glass slides, deparaffinized, and stained with hematoxylin and
eosin (H&E) using standard protocols. Histological diagnoses
were made using H&E staining, and tumor subtypes were
classified according to the WHO Classification of Pancreatic
Tumors, 5th Edition (4) (Fig. S1). TNM classification was examined
based on the Union for International Cancer Control (UICC)
classification. Tumor necrosis and intratumoral hemorrhage were
evaluated by H&E staining (Fig.
S2A). The resection margin status was microscopically assessed
and resection margin positive (R1) was defined as tumors with
cancer cells at the transection line. Tumor cell proliferation was
investigated by Ki-67 immunohistochemical staining (Fig. S2B). For immunohistochemical
staining, ACP tissue specimens were sliced from FFPE blocks. After
microwave antigen retrieval and non-specific protein binding, the
sections were incubated overnight with Ki-67 primary antibody
(Proteintech; 27309-1-AP, dilution 1:2,000) at 4˚C. Subsequently,
the goat anti-rabbit IgG secondary antibody (Invitrogen; 65-6120,
dilution 1:500) were applied for 30 min at room temperature, and
then stained with a peroxidase DAB kit (Nacalai Tesque, Inc.).
Tumor Ki-67 immunostaining evaluation was performed based on the
percentage of positive nuclei of tumor cells from a positive field
at high power (x200). Based on the median percentage of Ki-67
positive tumor cells in the cohort, a high Ki-67 proliferative
index was defined as ≥10% of positive Ki-67 tumor cells.
Statistical analysis
Postoperative disease-free survival (DFS) and OS
were analyzed with the Kaplan-Meier curve, and statistical
significance was investigated with log-rank test. The association
between groups stratified by pathological T factor was evaluated
using Fisher's exact test and Wilcoxon rank sum test because the
data were not normally distributed and the sample size was small.
All tests were two-tailed, and p-values of <0.05 indicated a
statistically significant difference. JMP software (SAS Institute)
was used for data analysis.
Results
Patient characteristics
Table I shows
clinical and preoperative information of the patients who underwent
pancreatectomy for ACP. Of the 12 patients who underwent pancreatic
resection, 9 were male and 3 were female, with a median age of 65
years (range: 45-81). Of the 12 patients, 7 experienced
tumor-related symptoms upon diagnosis, including epigastralgia
(n=4) and back pain (n=3). Intratumoral or gastrointestinal
bleeding which required surgical intervention was observed in three
cases. The tumors were located in the pancreatic head, pancreatic
body and pancreatic tail in 6, 4 and 2 cases, respectively. The
tumor size in MDCT upon diagnosis ranged from 12 to 108 mm, with a
median of 30.5 mm. The pancreatic tumor of seven cases demonstrated
a hypodense lesion with a peripheral contrast enhancement in MDCT.
Radiological investigation revealed that 11 patients were
resectable state and 1 patient was unresectable state with distant
metastases. EUS-TA was performed for 5 cases, among which 3 were
preoperatively diagnosed as ACP.
 | Table IPerioperative characteristics of the
patients with anaplastic carcinoma of the pancreas. |
Table I
Perioperative characteristics of the
patients with anaplastic carcinoma of the pancreas.
| | MDCT findings upon
diagnosis | |
|---|
| Case | Age, years | Sex | Symptoms | Tumor location | Tumor size, mm | Hypodense lesions
with peripheral enhancement | Resectability | Diagnosis by
EUS-TA | Neoadjuvant
therapy | Operative method | Adjuvant therapy |
|---|
| 1 | 45 | M | Epigastralgia; tumor
bleeding | Tail | 70 | + | Unresectable | - | None | DP | None |
| 2 | 81 | M | Epigastralgia | Tail | 108 | + | Resectable | - | None | DP | None |
| 3 | 66 | M | Epigastralgia;
gastrointestinal bleeding | Head | 60 | + | Resectable | - | None | PD + PVR | None |
| 4 | 61 | M | Back pain | Body | 75 | + | Resectable | - | None | DP | GEM |
| 5 | 79 | M | None | Body | 12 | - | Resectable | Atypical cells | None | DP | S-1 |
| 6 | 64 | M | Back pain | Head | 31 | + | Resectable | - | None | PD | GEM |
| 7 | 60 | M | None | Body | 19 | - | Resectable | Undifferentiated
carcinoma | None | DP | S-1 |
| 8 | 68 | M | None | Head | 26 | - | Resectable | - | GS | PD | S-1 |
| 9 | 83 | F | Back pain;
gastrointestinal bleeding | Head | 33 | + | Resectable | Anaplastic
carcinoma | None | PD | GEM |
| 10 | 53 | F | Epigastralgia | Head | 30 | + | Resectable | - | None | PD | GEM |
| 11 | 65 | M | None | Head | 20 | - | Resectable | Anaplastic
carcinoma | GS | PD | S-1 |
| 12 | 65 | F | None | Body | 16 | - | Resectable | Adenocarcinoma | GS | DP | S-1 |
Treatment strategy for ACP and its
outcome
Table I also shows
treatment for the patients with ACP. Of 12 patients, 3 received 2
cycles of neoadjuvant chemotherapy by gemcitabine plus S-1 based on
a previous study (19). According
to the RECIST criteria, all 3 cases were classified as stable
disease, and all patients underwent surgical resection because no
tumor progression including distant metastases was detected during
preoperative chemotherapy. Curative pancreatic resection and
palliative pancreatic resection due to bleeding from the tumor were
performed on 11 patients and 1 patient, respectively. Surgical
procedures included pancreaticoduodenectomy for 6 patients and
distal pancreatectomy for 6 patients. One case required combined
portal vein resection. There was no mortality after surgery.
Adjuvant chemotherapy was performed for 9 cases (gemcitabine
monotherapy: 4 cases, S-1 monotherapy: 5 cases). Table II presents pathological results of
the patients. All of the cases were diagnosed as ACP. The subtypes
included 7 cases of anaplastic undifferentiated carcinoma, 1 case
of sarcomatoid undifferentiated carcinoma, 2 cases of
carcinosarcoma, and 2 cases of undifferentiated carcinoma with
OCGC. Lymph node metastases were detected in 11 cases, and R0
resection was achieved in 8 (66.7%) cases. We then analyzed the
prognosis of patients with ACP who underwent macroscopic curative
pancreatic resection using the Kaplan-Meier survival analyses. The
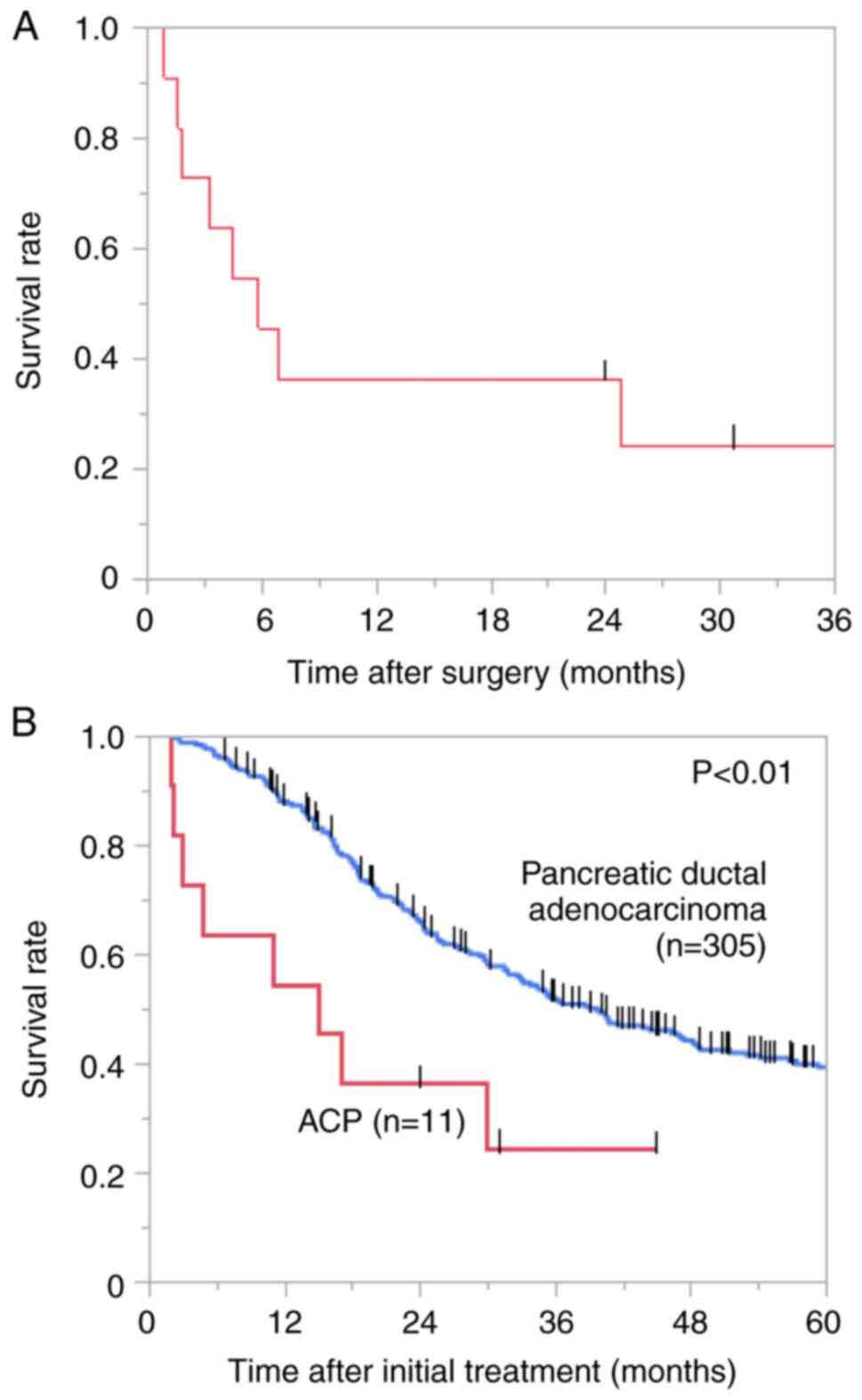
DFS curve demonstrated that the median DFS time was 5.8 months
(Fig. 1A). Table II also presents the prognosis of
each patient. The initial recurrence site after resection included
liver metastases (n=3), local recurrence (n=2), distant lymph node
metastases (n=2), and bone metastases (n=1). Seven (63.6%) cases
had early recurrence within 6 months after surgery, whereas 4 cases
were alive ≥2 years after surgery with no evidence of recurrence.
We next compared the OS of patients with ACP who underwent
macroscopic curative resection to that of patients with resectable
PDAC. Clinicopathological factors are summarized in Table SI. Even though the resectability
of all ACP cases was assessed as resectable preoperatively, the
proportion of patients with M1 disease was significantly higher in
the ACP group than in the PDAC group. Patients with ACP had
significantly shorter OS compared to those with PDAC (median OS:
15.0 vs. 39.7 months, χ²=8.60, P<0.01; Fig. 1B). The 2-year OS rate of the
patients with ACP was 36.4% (Fig.
1B).
 | Table IIPathological findings and prognosis
of the patients with anaplastic carcinoma of the pancreas. |
Table II
Pathological findings and prognosis
of the patients with anaplastic carcinoma of the pancreas.
| | Pathological
findings | |
|---|
| Case | Histology | pStage | Tumor necrosis | Intratumoral
hemorrhage | Ki-67, % | Curability | Recurrence | Overall
survival |
|---|
| 1 | Anaplastic
undifferentiated carcinoma | T3N1M1(PER) | + | + | 20.9 | R2 | - | 0.5 M; DD |
| 2 | Sarcomatoid
undifferentiated carcinoma | T3N2M0 | + | + | 11.1 | R0 | 0.9 M; liver | 2.0 M; DD |
| 3 | Anaplastic
undifferentiated carcinoma | T3N2M0 | + | + | 28.7 | R1 | 1.8 M; local | 2.2 M; DD |
| 4 | Anaplastic
undifferentiated carcinoma | T3N2M1(ADR) | + | - | 15.0 | R0 | 1.6 M; local | 3.0 M; DD |
| 5 | Anaplastic
undifferentiated carcinoma | T1N1M0 | + | - | 2.1 | R0 | 3.3 M; LYM | 4.7 M; DD |
| 6 | Anaplastic
undifferentiated carcinoma | T2N1M1(LYM) | + | - | 34.0 | R1 | 5.8 M; LYM | 11 M; DD |
| 7 | Anaplastic
undifferentiated carcinoma | T1N2M0 | - | - | 23.0 | R0 | 4.4 M; bone | 15 M; DD |
| 8 | Carcinosarcoma | T2N2M1(LYM) | - | - | 1.0 | Rx | 4.9 M; liver | 17 M; DD |
| 9 | Undifferentiated
carcinoma with OCGC | T2N2M0 | + | + | 1.0 | R0 | 24 M; no
recurrence | 24 M; AW |
| 10 | Undifferentiated
carcinoma with OCGC | T2N1M0 | - | - | 0.7 | R0 | 25 M; liver | 30 M; DD |
| 11 | Anaplastic
undifferentiated carcinoma | T1N1M0 | - | - | 1.7 | R0 | 31 M; no
recurrence | 31 M; AW |
| 12 | Carcinosarcoma | T1N0M0 | - | - | 1.0 | R0 | 45 M; no
recurrence | 45 M; AW |
Clinicopathological factors associated
with survival postoperatively for ACP
To determine pathological factors of the patients
which influence their prognosis, the association between
pathological findings and OS after surgery was investigated
(Table II). Regarding the subtype
of ACP, both cases with undifferentiated carcinoma with OCGC
experienced long survival postoperatively. On the other hand, among
ten cases diagnosed with undifferentiated carcinoma, only two cases
survived over 2 years without recurrence. With respect to the
pathological features of these two cases, pathological tumor
diameter was ≤2 cm (pT1), and tumor necrosis or intratumoral
hemorrhage was not observed in H&E staining. Furthermore, Ki67
immunohistochemical staining revealed low proliferative potential
of these two cases. Regarding local factor and lymph node
metastases, Kaplan-Meier survival analyses revealed that the median
OS time of the patients with pT3 was significantly shorter than
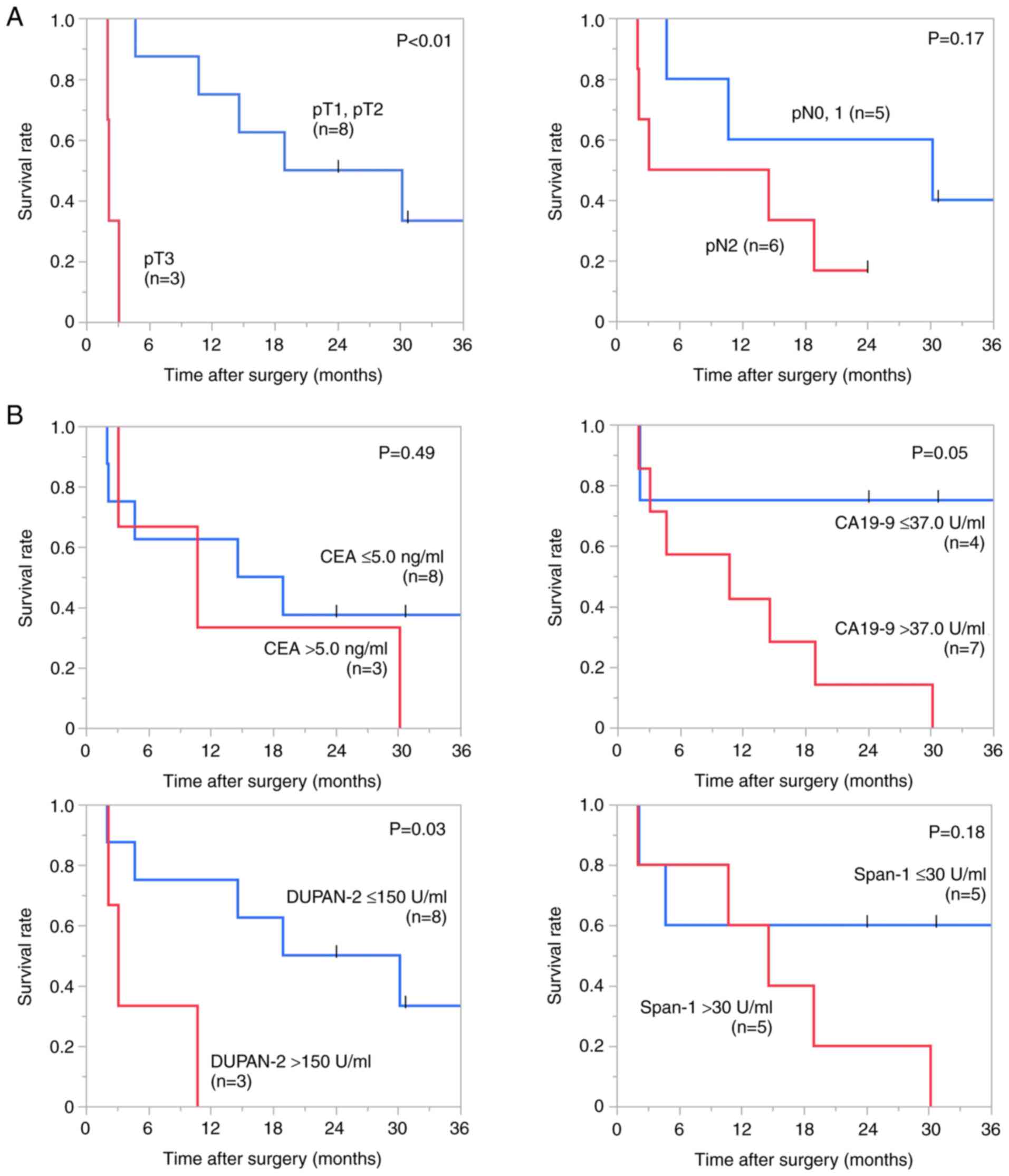
that with pT1 or pT2 (2.2 vs. 24.5 months, χ²=12.77, P<0.01),
whereas pathological lymph node metastases were not significantly
correlated to OS (Fig. 2A).
Preoperative prediction of survival is important for
selecting an optimal treatment strategy for pancreatic cancer. We
assessed whether tumor markers were associated with OS after
surgery with Kaplan-Meier survival analyses (Fig. 2B). The median OS time of the
patients with an elevated DUPAN-2 level have significantly shorter
OS than that of the patients with a normal range of DUPAN-2 (3.0
vs. 24.5 months, χ²=4.62, P=0.03), and there was a trend toward an
association between CA19-9 level and overall survival (χ²=3.77,
P=0.05).
Clinicopathological characteristics of
the patients with large ACP and poor prognosis
Our data so far indicated that pathological tumor
diameter may be one of the crucial factors correlating to
prognosis. Clinicopathological factors of the ACP cases with pT3
were compared to those of ACP cases with pT1 or pT2 to determine
the characteristics of large ACP which exhibited a poor prognosis
(Table III). No significant
difference was observed in patient characteristics, such as age,
gender, and tumor location among the groups. Regarding the
preoperative laboratory test, no difference was observed in the
preoperative tumor marker levels between the tumor with pT3 and the
tumor with pT1 or pT2. On the other hand, patients with pT3
demonstrated significantly higher levels of white blood cell (WBC),
C-reactive protein (CRP), and NLR than patients with pT1 or pT2
(P=0.04, P=0.04, and P=0.02). The results indicated that patients
with pT3 ACP suffered systemic inflammation at the time of
diagnosis. We then assessed the pathological findings of the tumor
with pT3. Most of the ACP with pT3 were concomitant with tumor
necrosis and intratumoral hemorrhage, both of which may be
attributed to the rapid tumor progression. Indeed, all ACP cases
with pT3 demonstrated high Ki-67 proliferative index.
 | Table IIICharacteristics of patients with
anaplastic carcinoma of the pancreas stratified by pathological
tumor diameter. |
Table III
Characteristics of patients with
anaplastic carcinoma of the pancreas stratified by pathological
tumor diameter.
| Parameters | pT1, 2 (n=8) | pT3 (n=4) | P-value |
|---|
| Age, years | 65 (53-83) | 64 (45-81) | 0.59 |
| Sex, n
(male/female) | 5/3 | 4/0 | 0.49 |
| Tumor location, n
(Ph/Pbt) | 5/3 | 1/3 | 0.55 |
| Preoperative
laboratory test | | | |
|
CEA,
U/ml | 3.4 (1.4-11.1) | 3.5 (0.7-5.5) | 0.93 |
|
CA19-9,
U/ml | 107.2
(24.1-1,621.0) | 241.2
(0.4-780.0) | >0.99 |
|
DUPAN-2,
U/ml | 32 (25-1,300) | 215 (86-1,000) | 0.15 |
|
Span-1,
U/ml | 34 (12-290) | 13 (1-100) | 0.41 |
|
WBC,
/µl | 7,350
(4,500-10,500) | 11,800
(6,400-14,400) | 0.04 |
|
Hb,
g/dl | 11.3
(9.3-15.9) | 8.8 (5.5-14.9) | 0.17 |
|
CRP,
mg/dl | 0.3 (0.1-0.7) | 4.9 (0.6-20.8) | 0.04 |
|
NLR | 2.92
(1.53-5.30) | 15.45
(5.89-182.00) | 0.02 |
|
PNI | 47.8
(38.3-56.7) | 18.9
(14.3-46.7) | 0.08 |
| Pathological
findings | | | |
|
Tumor
necrosis, n (+/-) | 3/5 | 4/0 | 0.08 |
|
Intratumoral
hemorrhage, n (+/-) | 1/7 | 3/1 | 0.07 |
|
Ki-67
proliferative index, n (high/low) | 2/6 | 4/0 | 0.06 |
Discussion
This study elucidated the oncological outcomes of
the surgical resection for ACP and examined the histopathological
features associated with prognoses. As previous reports
demonstrated the aggressive biological behavior of ACP (8), even patients with resectable ACP
experienced significantly worse survival compared to those with
resectable PDAC. 63.6% of the patients with radical resection had
early recurrence within 6 months after surgery and all of the pT3
cases in our cohort had poor prognosis postoperatively. Conversely,
limited cases that experienced prolonged survival without
recurrence included two cases with undifferentiated carcinoma with
OCGC and two pT1 cases with undifferentiated carcinoma. We
demonstrated that tumor necrosis and intratumoral hemorrhage, both
indicative of rapid growth, are associated with tumor diameter,
which may influence patient prognosis. The results of this study
are useful for predicting postoperative prognosis for ACP.
ACP frequently exhibits activation of
epithelial-mesenchymal transition and is clinically characterized
by rapid progression as well as concomitant lymphatic and distant
metastases (8,20). Therefore, the tumor diameter of ACP
upon diagnosis is relatively large and the prognosis of ACP is
worse compared to that of PDAC (5,20,21).
Since only a few reports investigated the oncological outcome of
the surgery for ACP, the survival benefits and optimal candidates
of surgery for ACP remain unclear.
The postoperative survival time of the patients with
ACP varies in our cohort. Of 11 patients who underwent
macroscopical curative pancreatic resection, 7 experienced early
recurrence within 6 months after surgery, resulting in poor
prognosis. Conversely, 4 could survived over 2 years without
recurrence after surgery. These results indicated that many
patients experienced early tumor recurrence after surgery and poor
prognosis, but there was a patient cohort with long-term survival
without recurrence postoperatively. Previous studies also revealed
that the survival of the patients with ACP during the first year
after operation was worse than that of the patients with PDAC, but
some ACP cases experienced long-term survival (13).
The WHO classification recognizes the
undifferentiated carcinoma with OCGC as a distinct subtype of
undifferentiated carcinoma of the pancreas. In our cohort, both
patients with undifferentiated carcinoma with OCGC achieved
long-term survival without recurrence even though these cases had
concomitant lymph node metastases. Previous studies have reported
that the prognosis of undifferentiated carcinoma with OCGC was more
favorable than that of PDAC with a 5-year survival rate of 59.1%
following surgical resection (15,22).
Furthermore, the majority of the ACP patients with long-term
survivors were reported to be undifferentiated carcinoma with OCGC
(21,23). These findings suggest that tumor
subtype may be associated with prognosis following radical
resection. Additionally, we revealed that two cases with
undifferentiated carcinoma experienced long-term survival. Tumor
necrosis and intratumoral hemorrhage are frequently observed in ACP
caused by the rapid progression (24). However, two long-term survival
cases with undifferentiated carcinoma were pathological T1 tumor
with low Ki-67 proliferative index and did not have tumor necrosis
or intratumoral hemorrhage, indicating that these cases may not
have highly proliferative potential and were diagnosed in the early
diseases stage. These data suggested that early disease detection
and surgical resection of the ACP at an early stage may contribute
to prolong survival. Conversely, the patients with pT3 had short OS
postoperatively in our cohort. These cases frequently demonstrated
tumor necrosis and intratumoral hemorrhage with a high Ki-67
proliferative index, indicating that the patients with large tumors
had poor prognosis possibly caused by the rapid tumor progression.
We could not observe the oncological survival benefits of the
surgery for these cases, but surgical resection achieved control of
tumor-related symptoms, such as tumor bleeding, in two
patients.
Preoperative prediction of prognosis after surgery
is important to select the appropriate candidates for surgical
resection of pancreatic cancer. Among the patients with
unresectable ACP, performance status, CRP level, and age were
reported to be associated with poor prognoses (25). Conversely, the prognostic factors
for the resected ACP remain unknown. This study indicated that
tumor diameter may affect the prognosis of patients with ACP after
surgical resection. Specifically, patients with pT3 in our cohort,
who experienced poor prognosis, exhibited tumor necrosis in the
tumor with systemic inflammation. These results indicated that high
serum inflammatory marker levels, such as WBC, CRP, and NLR, may be
associated with the poor oncological outcome after surgical
resection for ACP. Our results also suggested that hypodense
lesions with peripheral contrast enhancement in MDCT may be
important radiological findings that reflect tumor necrosis
observed in histopathological examination. In PDAC, tumor markers
were also associated with OS postoperatively and important
decision-making factors for the treatment. Previous reports
revealed that increased CEA and CA19-9 levels were not commonly
observed in patients with ACP (26). In this study, the patients with
elevated DUPAN-2 levels experienced poor survival and CA19-9 tended
to be associated with overall survival after surgery. These results
indicated that tumor markers may also be potential candidates for
predicting prognosis of the patients with ACP. Further research is
required to identify robust preoperative prognostic markers for ACP
patients undergoing surgical resection.
Moreover, this study included critical information
regarding preoperative diagnosis and preoperative treatment for
patients with ACP. Clinical diagnosis may be important in
determining the treatment strategy for patients with ACP,
particularly in the era of multidisciplinary treatment for
pancreatic cancer. According to the radiological results of MDCT
and MRI, the differential diagnosis includes PDAC, solid
pseudopapillary neoplasm, and neuroendocrine tumor. EUS-TA enables
the diagnosis of pancreatic cancer with high sensitivity and
specificity (27). In our cohort,
5 cases underwent EUS-TA of the pancreatic tumor and 3 cases were
preoperatively diagnosed as ACP. Recent studies have also reported
that EUS-TA facilitated accurate diagnosis of ACP before treatment
(28,29). Our survival analyses revealed that
ACP cases had a high frequency of early recurrence even in the
resectable state according to the UICC criteria, indicating that
multidisciplinary treatment may be necessary to improve the
prognosis of the patients with ACP. In our cohort, adjuvant
chemotherapy was administered whenever the patient's condition was
considered suitable for treatment based on the clinical trials for
pancreatic cancer (30,31). There are few reports on the
efficacy of neoadjuvant therapy for ACP. Two of three patients in
our cohort who underwent surgical resection after neoadjuvant
chemotherapy experienced a prolonged OS without recurrence.
Neoadjuvant chemotherapy may prolong the survival and also select
the optimal candidates for operation by excluding patients with
rapid growth and distant metastases (32,33).
Paclitaxel-containing regimens have been reported to be effective
for ACP (34). The indication of
neoadjuvant therapy and appropriate multidisciplinary treatment
warrants further investigation.
This study has a couple of potential limitations.
First, this study investigated a small number of patients and
multivariate analysis for evaluating prognostic factors was not
feasible in this study. However, a limited number of reports have
analyzed the outcome of surgical resection for ACP. This is the
first study to conduct detailed analyses regarding the
clinicopathological features of long-term survivors and the
patients with poor prognosis. Further studies with a large number
of patients are required to confirm our results. Second, patients
who received neoadjuvant therapy were analyzed together with those
who underwent upfront surgery for survival analysis. But there is
currently no established evidence demonstrating the survival
benefit of neoadjuvant therapy for ACP. While the efficacy of
neoadjuvant therapy in resectable PDAC remains controversial, some
clinical trials have shown potential benefits recently (35). The impact of neoadjuvant therapy on
the prognosis of ACP should be investigated in future studies. In
addition, this study included patients with ACP over an extended
period from 2003 to 2021, despite recent advances in
multidisciplinary treatment for pancreatic cancer. However, our
survival data on upfront surgery followed by adjuvant therapy for
ACP are crucial in determining whether preoperative therapy should
be introduced. Furthermore, aside from our study, there are few
case series reporting ACP patients who received neoadjuvant
chemotherapy followed by surgical resection.
In conclusion, ACP cases frequently exhibited early
recurrence after surgery, resulting in poor survival, whereas a
limited number of cases achieved long-term survival. Tumor subtype
and pathological features, such as tumor diameter, tumor necrosis,
and intratumoral hemorrhage, may be crucial factors associated with
postoperative prognosis for ACP.
Supplementary Material
Representative histological images of
each subtype of anaplastic carcinoma of the pancreas. Original
magnification, x200.
Representative histopathological
images for the present study. (A) Tumor necrosis and intratumoral
hemorrhage observed within the tumor. Original magnification, x100.
(B) Ki-67 immunohistochemical staining. Arrows indicate
Ki-67-positive tumor cells. Original magnification, x200.
Characteristics of patients with ACP
and patients with resectable PDAC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested form corresponding author.
Authors' contributions
IS, TK, ST and MO contributed to the study design.
IS, TK and KS contributed to the data acquisition. ST and TM
confirmed the authenticity of all the raw data. IS, TK, ST, TT, DS,
NS, IH, HN, TM and SN contributed to analysis and interpretation of
data. IS and TK drafted the manuscript. ST and MO critically
revised the manuscript. All authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Committee on
Human Research of Chiba University School of Medicine (approval no.
3302; Chiba, Japan). Informed consent was obtained using an opt-out
method, as approved by the institutional ethics committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoshimoto S, Matsui J, Miyata R, Takigawa
Y and Miyauchi J: Anaplastic carcinoma of the pancreas: Case report
and literature review of reported cases in Japan. World J
Gastroenterol. 22:8631–8637. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sakakida T, Ishikawa T, Doi T, Morita R,
Kataoka S, Miyake H, Yamaguchi K, Moriguchi M, Sogame Y, Yasuda H,
et al: Genomic landscape and clinical features of rare subtypes of
pancreatic cancer: Analysis with the national database of Japan. J
Gastroenterol. 58:575–585. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ishida M, Fujii T, Kishiwada M, Shibuya K,
Satoi S, Ueno M, Nakata K, Takano S, Uchida K, Ohike N, et al:
Japanese classification of pancreatic carcinoma by the Japan
pancreas society: Eighth edition. J Hepatobiliary Pancreat Sci.
31:755–768. 2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Organization. WH: WHO classification of
tumours: Digestive System Tumours. International Agency for
Research on Cancer 2020.
|
|
5
|
Clark CJ, Graham RP, Arun JS, Harmsen WS
and Reid-Lombardo KM: Clinical outcomes for anaplastic pancreatic
cancer: A population-based study. J Am Coll Surg. 215:627–634.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Clark CJ, Arun JS, Graham RP, Zhang L,
Farnell M and Reid-Lombardo KM: Clinical characteristics and
overall survival in patients with anaplastic pancreatic cancer. Am
Surg. 80:117–123. 2014.PubMed/NCBI
|
|
7
|
Ishida K, Yamashita R, Osakabe M, Uesugi
N, Yamada N, Nitta H, Fujishima F, Motoi F, Suzuki H, Shimamura H,
et al: Expression of epithelial-mesenchymal transition proteins in
pancreatic anaplastic (Undifferentiated) carcinoma. Pancreas.
48:36–42. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miura K, Kimura K, Amano R, Yamazoe S,
Ohira G, Nishio K, Kametani N, Hirakawa K and Ohira M: Analysis of
the origin of anaplastic pancreatic cancer and the mechanism of its
dedifferentiation. J Hepatobiliary Pancreat Sci. 24:176–184.
2017.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Sano M, Homma T, Hayashi E, Noda H, Amano
Y, Tsujimura R, Yamada T, Quattrochi B and Nemoto N:
Clinicopathological characteristics of anaplastic carcinoma of the
pancreas with rhabdoid features. Virchows Arch. 465:531–538.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Oymaci E, Yakan S, Yildirim M, Argon A and
Namdaroglu O: Anaplastic carcinoma of the pancreas: A rare clinical
entity. Cureus. 9(e1782)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vinzens S, Zindel J, Zweifel M, Rau T,
Gloor B and Wochner A: Granulocyte colony-stimulating factor
producing anaplastic carcinoma of the pancreas: Case report and
review of the literature. Anticancer Res. 37:223–228.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nitta T, Fujii K, Kataoka J, Tominaga T,
Kawasaki H and Ishibashi T: A case of long-term 24-month survival
in pancreatic anaplastic carcinoma (giant cell type) after S1
postoperative adjuvant chemotherapy. Int J Surg Case Rep.
23:134–137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Strobel O, Hartwig W, Bergmann F, Hinz U,
Hackert T, Grenacher L, Schneider L, Fritz S, Gaida MM, Büchler MW
and Werner J: Anaplastic pancreatic cancer: Presentation, surgical
management, and outcome. Surgery. 149:200–208. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bukhari N and Joudeh A: Early stage
anaplastic sarcomatoid carcinoma of the pancreas, a case report. Am
J Case Rep. 20:597–601. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Muraki T, Reid MD, Basturk O, Jang KT,
Bedolla G, Bagci P, Mittal P, Memis B, Katabi N, Bandyopadhyay S,
et al: Undifferentiated carcinoma with osteoclastic giant cells of
the pancreas: Clinicopathologic analysis of 38 cases highlights a
more protracted clinical course than currently appreciated. Am J
Surg Pathol. 40:1203–1216. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Demetter P, Marechal R, Puleo F, Delhaye
M, Debroux S, Charara F, Galdon MG, Van Laethem JL and Verset L:
Undifferentiated pancreatic carcinoma with osteoclast-like giant
cells: What do we know so far? Front Oncol.
11(630086)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Konishi T, Takano S, Furukawa K,
Takayashiki T, Kuboki S, Suzuki D, Sakai N, Hosokawa I, Mishima T
and Ohtsuka M: Impact of resection margin status on survival after
operation for pancreatic head cancer with extrapancreatic nerve
plexus invasion. J Surg Oncol. 126:1038–1047. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Konishi T, Takano S, Takayashiki T, Suzuki
D, Sakai N, Hosokawa I, Mishima T, Nishino H, Suzuki K, Nakada S
and Ohtsuka M: Preoperative prediction of long-term survival after
surgery in patients with resectable pancreatic ductal
adenocarcinoma. Ann Surg Oncol. 31:6992–7000. 2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Motoi F, Satoi S, Honda G, Wada K, Shinchi
H, Matsumoto I, Sho M, Tsuchida A and Unno M: Study Group of
Preoperative therapy for Pancreatic cancer (PREP). A single-arm,
phase II trial of neoadjuvant gemcitabine and S1 in patients with
resectable and borderline resectable pancreatic adenocarcinoma:
PREP-01 study. J Gastroenterol. 54:194–203. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Paal E, Thompson LD, Frommelt RA,
Przygodzki RM and Heffess CS: A clinicopathologic and
immunohistochemical study of 35 anaplastic carcinomas of the
pancreas with a review of the literature. Ann Diagn Pathol.
5:129–140. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Paniccia A, Hosokawa PW, Schulick RD,
Henderson W, Kaplan J and Gajdos C: A matched-cohort analysis of
192 pancreatic anaplastic carcinomas and 960 pancreatic
adenocarcinomas: A 13-year North American experience using the
national cancer data base (NCDB). Surgery. 160:281–292.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Reid MD, Muraki T, HooKim K, Memis B,
Graham RP, Allende D, Shi J, Schaeffer DF, Singh R, Basturk O and
Adsay V: Cytologic features and clinical implications of
undifferentiated carcinoma with osteoclastic giant cells of the
pancreas: An analysis of 15 cases. Cancer Cytopathol. 125:563–575.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Christopher W, Nassoiy S, Marcus R, Keller
J, Chang SC, Fischer T, Bilchik A and Goldfarb M: Prognostic
indicators for undifferentiated carcinoma with/without
osteoclast-like giant cells of the pancreas. HPB (Oxford).
24:1757–1769. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ishigami K, Nishie A, Yamamoto T, Asayama
Y, Ushijima Y, Kakihara D, Fujita N, Morita K, Ohtsuka T, Kawabe K,
et al: Imaging features of undifferentiated carcinoma of the
pancreas. J Med Imaging Radiat Oncol. 63:580–588. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Imaoka H, Ikeda M, Maehara K, Umemoto K,
Ozaka M, Kobayashi S, Terashima T, Inoue H, Sakaguchi C, Tsuji K,
et al: Risk stratification and prognostic factors in patients with
unresectable undifferentiated carcinoma of the pancreas.
Pancreatology. 21:738–745. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Imaoka H, Ikeda M, Umemoto K, Sunakawa Y,
Ueno M, Ueno H, Ozaka M, Kuwahara T, Okano N, Kanai M, et al:
Comprehensive review of undifferentiated carcinoma of the pancreas:
From epidemiology to treatment. Jpn J Clin Oncol. 53:764–773.
2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen G, Liu S, Zhao Y, Dai M and Zhang T:
Diagnostic accuracy of endoscopic ultrasound-guided fine-needle
aspiration for pancreatic cancer: A meta-analysis. Pancreatology.
13:298–304. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Khashab MA, Emerson RE and DeWitt JM:
Endoscopic ultrasound-guided fine-needle aspiration for the
diagnosis of anaplastic pancreatic carcinoma: A single-center
experience. Pancreas. 39:88–91. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Oka K, Inoue K, Sugino S, Harada T, Tsuji
T, Nakashima S, Katayama T, Okuda T, Kin S, Nagata A, et al:
Anaplastic carcinoma of the pancreas diagnosed by endoscopic
ultrasound-guided fine-needle aspiration: A case report and review
of the literature. J Med Case Rep. 12(152)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Uesaka K, Boku N, Fukutomi A, Okamura Y,
Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto
H, et al: Adjuvant chemotherapy of S-1 versus gemcitabine for
resected pancreatic cancer: A phase 3, open-label, randomised,
non-inferiority trial (JASPAC 01). Lancet. 388:248–257.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Oettle H, Neuhaus P, Hochhaus A, Hartmann
JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J,
Arning MB, et al: Adjuvant chemotherapy with gemcitabine and
long-term outcomes among patients with resected pancreatic cancer:
The CONKO-001 randomized trial. JAMA. 310:1473–1481.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Miyasaka Y, Ohtsuka T, Kimura R, Matsuda
R, Mori Y, Nakata K, Kakihara D, Fujimori N, Ohno T, Oda Y and
Nakamura M: Neoadjuvant chemotherapy with gemcitabine plus
nab-paclitaxel for borderline resectable pancreatic cancer
potentially improves survival and Facilitates Surgery. Ann Surg
Oncol. 26:1528–1534. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Inoue Y, Saiura A, Oba A, Ono Y, Mise Y,
Ito H, Sasaki T, Ozaka M, Sasahira N and Takahashi Y: Neoadjuvant
gemcitabine and nab-paclitaxel for borderline resectable pancreatic
cancers: Intention-to-treat analysis compared with upfront surgery.
J Hepatobiliary Pancreat Sci. 28:143–155. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Imaoka H, Ikeda M, Maehara K, Umemoto K,
Ozaka M, Kobayashi S, Terashima T, Inoue H, Sakaguchi C, Tsuji K,
et al: Clinical outcomes of chemotherapy in patients with
undifferentiated carcinoma of the pancreas: A retrospective
multicenter cohort study. BMC Cancer. 20(946)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Unno M, Motoi F, Matsuyama Y, Sataoi S,
Matsumoto I, Aosasa S, Shirakawa H, Wada K, Fujii T, Yoshitomi H,
et al: Randomized phase II/III trial of neoadjuvant chemotherapy
with gemcitabine and S-1 versus upfront surgery for resectable
pancreatic cancer (Prep-02/JSAP-05). J Clin Oncol. 37:189.
2019.PubMed/NCBI View Article : Google Scholar
|