Introduction
The incidence of cancer-associated thromboembolism
(CAT) depends on the type of cancer and is relatively high in
gynecological cancers (1,2). CAT complications are associated with
worse prognosis in patients with epithelial ovarian cancer (EOC)
(3,4). The prevalence of pretreatment CAT in
patients with EOC ranges from 10.4 to 26.4%, and many cases are
asymptomatic (4-6).
As untreated CAT can lead to life-threatening conditions, early
detection and treatment are essential for the comprehensive care of
patients with EOC.
D-dimer testing has been recommended as a
cost-effective and rapid screening tool for CAT in EOC (7,8);
however, D-dimer levels reflect a variety of conditions, including
inflammation caused by cancer cells and cancer treatments such as
surgery and chemotherapy (9-11).
In addition, cancer cells and EOC treatment may create an
environment for recurrent inflammation, where the level of
inflammation is influenced by the histological subtype and
International Federation of Gynecology and Obstetrics (FIGO) stage
(12-14).
This condition can affect the effectiveness of D-dimer as a
screening method (15,16).
Clear cell carcinoma (CCC) and high-grade serous
carcinoma (HGSC) are two histological subtypes of ovarian cancer
that exhibit several differences in biological and clinical
characteristics. CCC is more common in Asia than in Western
countries, often detected in earlier stages, and has a
platinum-resistant nature (17).
In contrast, HGSC is the most common histological type, frequently
discovered at an advanced stage, and is sensitive to platinum
(18). Although HGSC is often
complicated with CAT, CCC exhibits the highest complication rate
among all histological subtypes (3-6).
In CCC, CAT formation is caused by hypercoagulation due to
overexpression of tissue factor (TF) and interleukin-6 (IL-6)
(19,20). CAT formation in HGSC is naturally
related to hypercoagulation, but it may also be associated with
dehydration and inflammation due to massive ascites and
malnutrition because HGSC is usually discovered at a more advanced
stage than CCC (21). Thus, the
tumor microenvironment and status of patients with CCC differ from
those of patients with HGSC.
Considering these distinct characteristics, the
diagnostic accuracy of biomarkers for CAT may differ between
histolo-gical subtypes such as CCC and HGSC. However, this
possibility has not been systematically evaluated. If D-dimer does
not prove effective as a biomarker for CAT, a screening test for
CAT detection is required. Therefore, we aimed to examine the
effectiveness of CAT screening in patients with CCC compared with
HGSC to develop a new system for CAT detection.
Materials and methods
Patients and blood samples
Patients diagnosed with CCC or HGSC who underwent
primary surgery at our hospital between January 2009 and December
2023 were identified. Clinical data were retrospectively collected
from medical records. Patients with acute infections at the initial
visit were excluded because of strong inflammatory responses.
Additionally, patients with a history of thrombosis or
anticoagulation therapy at the initial visit, those with other
cancers, and those without medical records were excluded. This
study was approved by the Ethics Committee of National Defense
Medical College, Tokorozawa, Japan (No4933). Written informed
consent for all participation was obtained before the
treatment.
To identify the risk factors for CAT in CCC and
HGSC, the following variables were assessed: age at diagnosis, body
mass index, cardiovascular risk factors and comorbidities, the
World Health Organization Performance Status Scale (22), and 2014 FIGO (23). Additional factors included the
histological type diagnosed according to the 2020 WHO Health
Organization criteria (24), tumor
size measured using magnetic resonance imaging (MRI) before primary
treatment, and the presence of ascites, which was assessed
intraoperatively or through preoperative percutaneous abdominal
puncture. Cardiovascular risk factors and comorbidities included
previous or concurrent ischemic stroke, peripheral arterial
disease, coronary artery disease, hypertension, diabetes mellitus,
and hyperlipidemia.
Peripheral blood samples were obtained during the
initial visit. Data on white blood cell differential counts and
platelet, hemoglobin, C-reactive protein (CRP), albumin, D-dimer,
fibrinogen, and carbohydrate antigen 125 (CA 125) levels were
retrospectively obtained from medical records. D-dimer was measured
using a latex coagulation reaction by sensitization with an
anti-D-dimer mouse monoclonal antibody, and turbidity was assessed
using a spectrophotometer (CN-6500; SYSMEX, Hyogo, Japan). The
cutoff value for D-dimer was set at 1.0 µg/l. In addition, we
calculated the neutrophil-lymphocyte ratio (NLR), defined as the
absolute neutrophil count divided by the absolute lymphocyte count,
and the platelet-lymphocyte ratio (PLR), defined as the absolute
platelet count divided by the absolute lymphocyte count, as
biomarkers of inflammation (25).
CAT evaluation protocol
In our study, CAT was defined as CAT that developed
before primary treatment. CAT was classified as venous
thromboembolisms, including pulmonary embolism (PE) and deep vein
thrombosis (DVT). All patients underwent computed tomography before
the primary treatment to detect CAT. If patients had symptoms
suggestive of CAT, such as lower extremity edema, chest pain, and
dyspnea, we performed additional examinations, including
ultrasonography, MRI, and angiography.
Statistical analysis
All statistical analyses were performed using JMP
Pro 14 software (SAS Institute Inc., Cary, NC, USA). Chi-square
tests and Fisher's exact tests were used to compare clinical and
pathological characteristics between patients with and without CAT.
In addition, the incidence and risk factors for CAT in patients
with CCC and HGSC were examined. Univariate and multivariate
analyses were performed using logistic regression analysis.
Serologic and hematologic biomarkers were compared using the Mann
Whitney U test. Receiver operating characteristic (ROC) curves were
used to predict CAT development. The Youden index (sensitivity +
specificity-1) was calculated, and the cutoff value corresponding
to the highest Youden index was used as the optimal cutoff value.
Statistical significance was set at P<0.05.
Results
Clinical characteristics
In total, we analyzed the data of 79 patients with
CCC and 123 with HGSC. Of them, 20 patients with CCC (25.3%) and 15
with HGSC (12.2%) developed CAT (P=0.022). The CCC group included 6
patients (7.6%) with DVT, 6 (7.6%) with PE, and 8 (10.1%) with PE
and DVT, whereas the HGSC group included 8 patients (6.5%) with DVT
and 7 (5.7%) with PE.
Table I presents
the clinical characteristics of patients with and without CAT. In
the CCC group, patients with CAT were diagnosed at a more advanced
FIGO stage (P=0.004) and had larger tumors (P=0.030) and massive
ascites (P=0.013). However, no significant differences in CAT
complications were observed in the HGSC group. Table II summarizes the univariate and
multivariate analyses of risk factors for CAT in the CCC group.
Univariate analysis identified advanced stage [odds ratio (OR)
4.40, 95% confidence intervals (CI) 1.51 12.8, P=0.007], larger
tumor size (OR 4.46, 95% CI 1.18 16.9, P=0.028), and increased
ascites volume (OR 14.5, 95% CI 1.51 139.0, P=0.020) as significant
risk factors for CAT. Multivariate analysis confirmed that an
advanced disease stage (OR 3.28, 95% CI 1.04 10.3, P=0.042) was an
independent risk factor for CAT. Risk factors for CAT in the HGSC
group could not be determined.
 | Table ICharacteristics of patients with CCC
and HGSC with or without CAT. |
Table I
Characteristics of patients with CCC
and HGSC with or without CAT.
| | Patients with
CCC | Patients with
HGSC |
|---|
| Variables | CAT (+), n (%)
(n=20) | CAT (-), n (%)
(n=59) | P-value | CAT (+), n (%)
(n=15) | CAT (-), n (%)
(n=108) | P-value |
|---|
| Age at diagnosis,
years | | | 0.588 | | | 0.584 |
|
≥65 | 13 (65.0) | 42 (71.2) | | 5 (33.3) | 46 (42.6) | |
|
<65 | 7 (35.0) | 17 (28.8) | | 10 (66.6) | 62 (57.4) | |
| Body mass index,
kg/m2 | | | 0.999 | | | 0.739 |
|
≥25 | 3 (15.0) | 9 (15.3) | | 4 (26.7) | 23 (21.3) | |
|
<25 | 17 (85.0) | 50 (84.7) | | 11 (73.3) | 85 (78.7) | |
| Cardiovascular risk
factors and comorbidities | | | 0.490 | | | 0.720 |
|
Yes | 3 (15.0) | 7 (11.9) | | 3 (20.0) | 18 (16.7) | |
|
No | 17 (85.0) | 52 (88.1) | | 12 (80.0) | 90 (83.3) | |
| FIGO stage | | | 0.004 | | | 0.054 |
|
I | 8 (40.0) | 44 (74.6) | | 3 (20.0) | 18 (16.7) | |
|
II | 2 (10.0) | 7 (11.9) | | 7 (46.7) | 73 (67.6) | |
|
III | 9 (45.0) | 8 (13.5) | | 3 (20.0) | 9 (8.3) | |
|
IV | 1 (5.0) | 0 (0.0) | | 2 (13.3) | 8 (7.4) | |
| Tumor size, cm | | | 0.030 | | | 0.342 |
|
≥10 | 17 (85.0) | 33 (55.9) | | 5 (33.3) | 24 (22.2) | |
|
<10 | 3 (15.0) | 26 (44.1) | | 10 (66.7) | 84 (77.8) | |
| Ascites, ml | | | 0.013 | | | 0.382 |
|
≥1,000 | 4 (20.0) | 1 (1.7) | | 9 (60.0) | 61 (56.5) | |
|
<1,000 | 16 (80.0) | 58 (98.3) | | 6 (40.0) | 47 (43.5) | |
 | Table IIUnivariate and multivariate analysis
for the incidence of cancer-associated thromboembolism in clear
cell carcinoma. |
Table II
Univariate and multivariate analysis
for the incidence of cancer-associated thromboembolism in clear
cell carcinoma.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age at diagnosis
(≥65 vs. <65 years) | 1.33 | 0.45-3.91 | 0.588 | | | |
| Body mass index
(≥25 vs. <25 kg/m2) | 0.98 | 0.24-4.05 | >0.999 | | | |
| Cardiovascular risk
factors and comorbidities (yes vs. no) | 1.31 | 0.30-5.64 | 0.716 | | | |
| FIGO stage (II-IV
vs. I) | 4.40 | 1.51-12.76 | 0.007 | 3.28 | 1.04-10.32 | 0.042 |
| Tumor size (≥10 vs.
<10 cm) | 4.46 | 1.18-16.88 | 0.028 | 3.22 | 0.80-12.87 | 0.098 |
| Ascites (≥1,000 vs.
<1,000 ml) | 14.48 | 1.51-139.02 | 0.020 | 6.61 | 0.63-70.01 | 0.116 |
Biomarker analysis
Comparison of serological and hematological
biomarkers between patients with and without CAT is presented in
Table III. In the CCC group,
significant differences were observed in NLR, PLR, hemoglobin,
albumin, CRP, D-dimer, CA125, and CA19-9 levels. Conversely, in the
HGSC group, only D-dimer levels were significantly different.
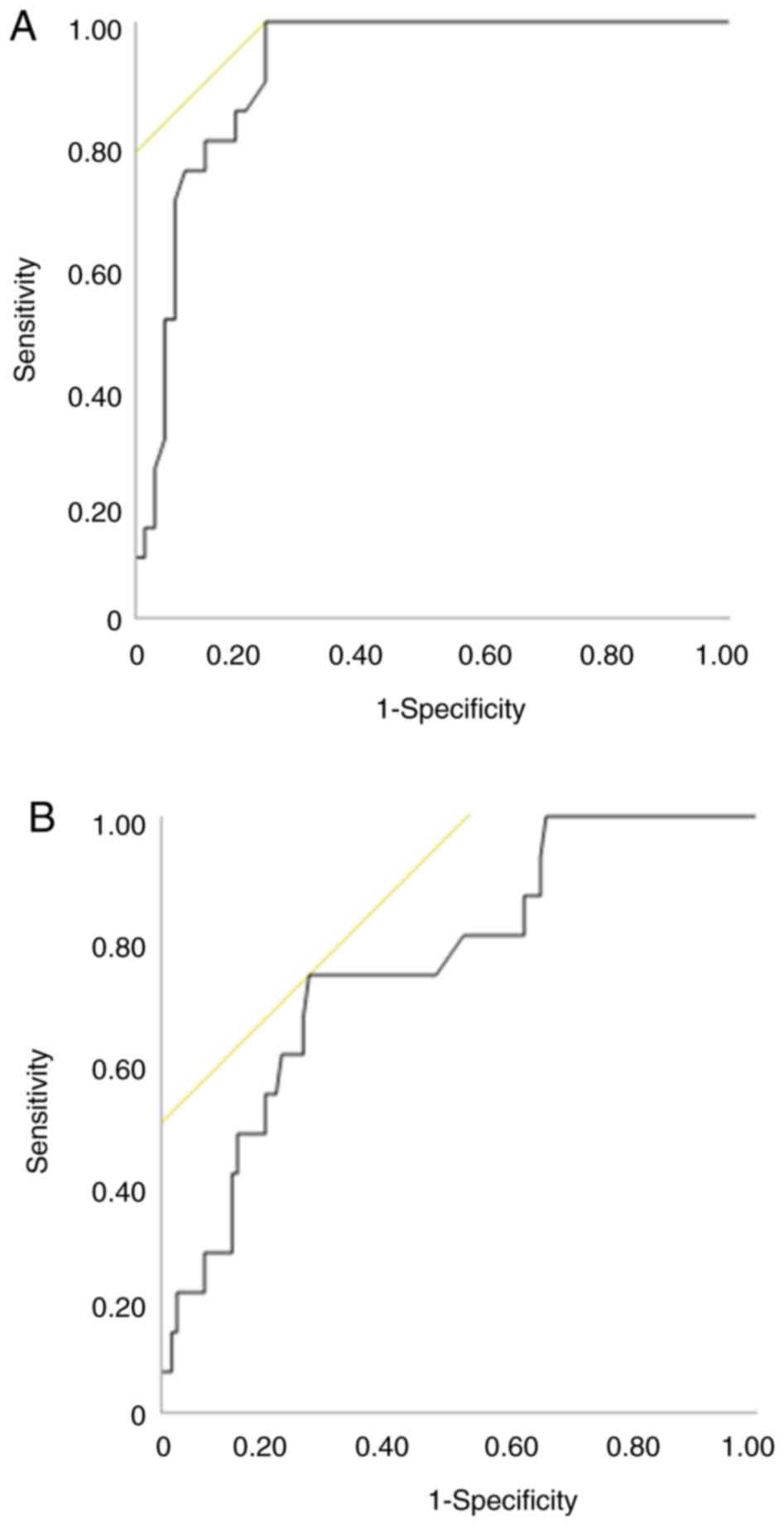
D-dimer had the highest area under the curve (AUC) (0.92,
P<0.001) in the CCC group and highest AUC (0.75, P=0.002) in the
HGSC group; the AUC in the HGSC group was lower than that in the
CCC group (Fig. 1). The cutoff
value of D-dimer as a predictor of CAT was 1.5 µg/ml in CCC and 5.8
µg/ml in HGSC.
 | Table IIIComparison of serologic and
hematologic biomarkers in patients with CCC and HGSC before primary
treatment. |
Table III
Comparison of serologic and
hematologic biomarkers in patients with CCC and HGSC before primary
treatment.
| | CCC | HGSC |
|---|
| Variable | CAT (+) (n=20) | CAT (-) (n=59) | P-value | AUC | CAT (+) (n=15) | CAT (-)
(n=108) | P-value | AUC |
|---|
| Neutrophil count,
109/l | 5.21
(3.05-27.28) | 4.82
(1.56-8.28) | 0.120 | 0.62 | 4.55
(1.69-12.11) | 5.41
(1.94-13.41) | 0.120 | 0.62 |
| Lymphocyte count,
109/l | 1.21
(0.49-2.20) | 1.47
(0.73-3.03) | 0.111 | 0.62 | 1.21
(0.42-1.92) | 1.25
(0.37-4.27) | 0.444 | 0.56 |
| NLR | 3.89
(2.37-25.77) | 3.15
(1.02-7.09) | 0.005 | 0.71 | 3.62
(1.13-22.73) | 4.18
(1.22-24.73) | 0.716 | 0.47 |
| Platelet count,
109/l | 32.65
(22.70-69.80) | 27.6
(10.50-57.50) | 0.099 | 0.62 | 28.70
(10.10-61.30) | 32.60
(16.00-69.20) | 0.152 | 0.61 |
| PLR | 281.50
(116.06-659.48) | 182.6
(74.12-576.51) | 0.015 | 0.68 | 202.02
(78.84-1293.89) | 265.48
(61.33-1217.98) | 0.599 | 0.46 |
| Hemoglobin,
g/l | 10.70
(8.20-13.70) | 12.30
(6.80-14.40) | 0.008 | 0.70 | 12.40
(7.50-15.30) | 12.70
(8.70-16.70) | 0.948 | 0.49 |
| Albumin, g/l | 3.50
(2.10-4.70) | 4.00
(2.60-5.10) | 0.002 | 0.73 | 3.30
(2.00-4.40) | 3.10
(2.00-4.90) | 0.278 | 0.59 |
| CRP, mg/dl | 2.25
(0.30-19.70) | 0.30
(0.30-8.70) | 0.004 | 0.71 | 1.70
(0.30-11.50) | 1.50
(0.14-27.10) | 0.651 | 0.54 |
| D-dimer, µg/l | 8.35
(1.60-28.10) | 0.70
(0.10-25.30) | <0.001 | 0.91 | 8.00
(2.00-27.50) | 3.50
(0.10-27.30) | 0.002 | 0.75 |
| Fibrinogen,
mg/dl | 454.50
(163.00-719.00) | 372.50 (174.00-847.
00) | 0.725 | 0.53 | 368.00
(217.00-744.00) | 412.00
(196.00-2,565.00) | 0.749 | 0.53 |
| CA125, U/ml | 400.30
(13.00-1,423.00) | 44.10
(5.40-2,206.00) | <0.001 | 0.80 | 1,367.10
(5.20-32,547.00) | 592.20
(6.60-15,665.00) | 0.243 | 0.59 |
Diagnostic performance of D-dimer
Tables IV and
V show the sensitivity,
specificity, positive predictive value (PPV), and negative
predictive value (NPV) of D-dimer in predicting CAT in the CCC and
HGSC groups. the AUC for predicting CAT was 0.75 in HGSC group, and
according to the cutoff values corresponding to the highest Youden
index, the sensitivity, specificity, PPV, and NPV were 73.3, 75.0,
28.9, and 95.3%, respectively. In contrast, the AUC, sensitivity,
specificity, PPV, and NPV for predicting CAT in the CCC group were
0.91, 100, 83.0, 50.0, and 100%, respectively. The combination of
FIGO stage I and D-dimer ≥1.5 µg/l improved the predictive ability
to an AUC of 0.97 in the CCC group.
 | Table IVDiagnostic performance of D-dimer
cutoffs and FIGO stage for detecting cancer-associated
thromboembolism in clear cell carcinoma before treatment. |
Table IV
Diagnostic performance of D-dimer
cutoffs and FIGO stage for detecting cancer-associated
thromboembolism in clear cell carcinoma before treatment.
| Marker
combination | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC |
|---|
| D-dimer (1.00
µg/ml)a | 100.0 | 61.0 | 46.5 | 100.0 | 0.91 |
| D-dimer (1.50
µg/ml)b | 100.0 | 78.0 | 60.6 | 100.0 | 0.91 |
|
Plus FIGO
stage I | 100.0 | 88.6 | 61.5 | 100.0 | 0.97 |
|
Plus FIGO
stage II-IV | 100.0 | 60.0 | 66.7 | 100.0 | 0.81 |
 | Table VDiagnostic performance of D-dimer
cutoffs for detecting cancer-associated thromboembolism in
high-grade serous carcinoma before treatment. |
Table V
Diagnostic performance of D-dimer
cutoffs for detecting cancer-associated thromboembolism in
high-grade serous carcinoma before treatment.
| Marker | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC |
|---|
| D-dimer (1.00
µg/ml)a | 100.0 | 13.9 | 13.9 | 100.0 | 0.75 |
| D-dimer (5.80
µg/ml)b | 73.3 | 75.0 | 28.9 | 95.3 | 0.75 |
Discussion
Our study showed that CCC was more frequently
complicated by CAT. Advanced stage was the only risk factor
identified for CAT in the CCC group, whereas no significant risk
factors were found in the HGSC group. Furthermore, D-dimer showed
the highest AUC for detecting CAT in both the CCC and HGSC groups,
with the CCC group having a higher AUC than the HGSC group.
Additionally, a model combining D-dimer levels and early-stage
disease exhibited improved sensitivity and specificity for
detecting CAT.
Our results indicated that CAT incidence before
treatment in patients with CCC and those with HGSC fell within the
range reported in previous studies, ranging from 13.4 to 33.1% in
CCC (3,4,6,26)
and 11.8 to 24.0% in HGSC (5,6,26).
Patients with EOC and CAT do not usually present with symptoms
(5), which were consistent with
the findings of our study. Therefore, imaging studies such as CT
angiography or ultrasound sonography are necessary for thrombosis
screening.
Some risk factors for CAT have been reported,
including age, massive ascites, FIGO stage, and cardiovascular
disease (4,6,26,27).
Our analysis revealed that advanced FIGO stage was an independent
risk factor for CAT formation in the CCC group. In contrast, no
significant risk factors were identified in the HGSC group.
Therefore, particular attention is needed for patients with CCC
experiencing risk factors and for all patients with HGSC.
Although we attempted to identify new biomarkers,
the D-dimer test remained the most useful tool for detecting CAT in
both the CCC and HGSC groups, consistent with previous findings
(4,15,16).
Notably, the D-dimer test was more effective in detecting CAT in
patients with CCC than in those with HGSC. We assumed that these
results were due to the different characteristics of CCC and HGSC.
Overexpression of TF and IL-6 plays a pivotal role in CAT
development in CCC (14,19,27).
TF, Factor III, initiates the extrinsic coagulation pathway by
binding to Factor VIIa and Factor Xa, leading to thrombin
generation. Meanwhile, IL-6 is a proinflammatory cytokine that
promotes coagulation by increasing TF expression and fibrinogen
synthesis (28). In HGSC, while
these factors also contribute to CAT, CAT is strongly associated
with ascites, tumor pressure on the veins, and dehydration
(6). Our reports suggest that a
strategy for detecting CAT can be established according to the
histological subtypes. The constructed predictive model, combining
disease stage and D-dimer levels, may be useful for detecting CAT
in CCC.
The limitations of our study included the
retrospective design, single-institution analysis, and small sample
size. Further studies with larger sample sizes are warranted to
confirm the clinical significance of the association between CCC
and CAT.
In conclusion, advanced stage is an independent risk
factor for the development of CAT in CCC. D-dimer is an excellent
screening tool for CAT in CCC, in particular when combined with
FIGO stage; however, it is less reliable in HGSC, suggesting a need
for histology-specific screening strategies. Further studies are
required for preoperative diagnosis using imaging, tumor markers,
and molecular biological methods for early detection of CAT.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TI, MM and MT contributed to the conception and
design of the study. Material preparation, and data collection and
analysis were carried out by TI, NK, JS and TH. TI, MM, RT, SN, JS,
TH, YO, KK, HS, KO, YH and MT contributed to the interpretation of
the data. The first draft of the manuscript was written by TI. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of the National Defense Medical College, Tokorozawa,
Saitama, Japan on January 20, 2024 (confirmation no. 4933). All
patient records and information were completely anonymized prior to
analysis to prevent the disclosure of their identities. Before the
treatment, written informed consent was obtained from all patients
with ovarian cancer who underwent surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gerotziafas GT, Taher A, Abdel-Razeq H,
AboElnazar E, Spyropoulos AC, El Shemmari S, Larsen AK and Elalamy
I: COMPASS-CAT Working Group. A predictive score for thrombosis
associated with breast, colorectal, lung, or ovarian cancer: The
prospective compass-cancer-associated thrombosis study. Oncologist.
22:1222–1231. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chandra A, Pius C, Nabeel M, Nair M,
Vishwanatha JK, Ahmad S and Basha R: Ovarian cancer: Current status
and strategies for improving therapeutic outcomes. Cancer Med.
8:7018–7031. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Matsuura Y, Robertson G, Marsden DE, Kim
SN, Gebski V and Hacker NF: Thromboembolic complications in
patients with clear cell carcinoma of the ovary. Gynecol Oncol.
104:406–410. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang W, Liu X, Cheng H, Yang Z and Zhang
G: Risk factors and treatment of venous thromboembolism in
perioperative patients with ovarian cancer in China. Medicine
(Baltimore). 97(e11754)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Satoh T, Oki A, Uno K, Sakurai M, Ochi H,
Okada S, Minami R, Matsumoto K, Tanaka YO, Tsunoda H, et al: High
incidence of silent venous thromboembolism before treatment in
ovarian cancer. Br J Cancer. 97:1053–1057. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tasaka N, Minaguchi T, Hosokawa Y, Takao
W, Itagaki H, Nishida K, Akiyama A, Shikama A, Ochi H and Satoh T:
Prevalence of venous thromboembolism at pretreatment screening and
associated risk factors in 2086 patients with gynecological cancer.
J Obstet Gynaecol Res. 46:765–773. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kawaguchi R, Furukawa N and Kobayashi H:
Cut-off value of D-dimer for prediction of deep venous thrombosis
before treatment in ovarian cancer. J Gynecol Oncol. 23:98–102.
2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bakhru A: Effect of ovarian tumor
characteristics on venous thromboembolic risk. J Gynecol Oncol.
24:52–58. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang N, Pan Y, Xu C and Li D:
Characteristics of emergency patients with markedly elevated
D-dimer levels. Sci Rep. 10(7784)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Beer JH, Haeberli A, Vogt A, Woodtli K,
Henkel E, Furrer T and Fey MF: Coagulation markers predict survival
in cancer patients. Thromb Haemost. 88:745–749. 2002.PubMed/NCBI
|
|
11
|
Shorr AF, Thomas SJ, Alkins SA,
Fitzpatrick TM and Ling GS: D-dimer correlates with proinflammatory
cytokine levels and outcomes in critically ill patients. Chest.
121:1262–1268. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Farolfi A, Petrone M, Scarpi E, Gallà V,
Greco F, Casanova C, Longo L, Cormio G, Orditura M, Bologna A, et
al: Inflammatory indexes as prognostic and predictive factors in
ovarian cancer treated with chemotherapy alone or together with
bevacizumab. A multicenter, retrospective analysis by the MITO
group (MITO 24). Target Oncol. 13:469–479. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shi J, Huo R, Li N, Li H, Zhai T, Li H,
Shen B, Ye J, Fu R and Di W: CYR61, a potential biomarker of tumor
inflammatory response in epithelial ovarian cancer microenvironment
of tumor progress. BMC Cancer. 19(1140)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang Y, Zong X, Mitra S, Mitra AK, Matei D
and Nephew KP: IL-6 mediates platinum-induced enrichment of ovarian
cancer stem cells. JCI Insight. 3(e122360)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shim H, Lee YJ, Kim JH, Lim MC, Lee DE,
Park SY and Kong SY: Preoperative laboratory parameters associated
with deep vein thrombosis in patients with ovarian cancer:
Retrospective analysis of 3,147 patients in a single institute. J
Gynecol Oncol. 35(e38)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu J, Fu Z, Liu G, Xu P, Xu J and Jia X:
Clinical significance of plasma D-dimer in ovarian cancer: A
meta-analysis. Medicine (Baltimore). 96(e7062)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Takano M, Goto T, Kato M, Sasaki N,
Miyamoto M and Furuya K: Short response duration even in responders
to chemotherapy using conventional cytotoxic agents in recurrent or
refractory clear cell carcinomas of the ovary. Int J Clin Oncol.
18:556–557. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rauh-Hain JA, Melamed A, Wright A, Gockley
A, Clemmer JT, Schorge JO, Del Carmen MG and Keating NL: Overall
survival following neoadjuvant chemotherapy vs primary
cytoreductive surgery in women with epithelial ovarian cancer:
Analysis of the national cancer database. JAMA Oncol. 3:76–82.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Matsuo K, Hasegawa K, Yoshino K, Murakami
R, Hisamatsu T, Stone RL, Previs RA, Hansen JM, Ikeda Y, Miyara A,
et al: Venous thromboembolism, interleukin-6 and survival outcomes
in patients with advanced ovarian clear cell carcinoma. Eur J
Cancer. 51:1978–1988. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sakurai M, Matsumoto K, Gosho M, Sakata A,
Hosokawa Y, Tenjimbayashi Y, Katoh T, Shikama A, Komiya H,
Michikami H, et al: Expression of tissue factor in epithelial
ovarian carcinoma is involved in the development of venous
thromboembolism. Int J Gynecol Cancer. 27:37–43. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gunderson CC, Thomas ED, Slaughter KN,
Farrell R, Ding K, Farris RE, Lauer JK, Perry LJ, McMeekin DS and
Moore KN: The survival detriment of venous thromboembolism with
epithelial ovarian cancer. Gynecol Oncol. 134:73–77.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982.PubMed/NCBI
|
|
23
|
Prat J: FIGO Committee on Gynecologic
Oncology. FIGO's staging classification for cancer of the ovary,
fallopian tube, and peritoneum: Abridged republication. J Gynecol
Oncol. 26:87–89. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
World Health Organization Classification
of Tumours Editorial Board: WHO classification of tumours 5th
edition female genital tumours. International Agency for Research
on Cancer, Lyon, pp34-167, 2020.
|
|
25
|
Kuplay H, Erdoğan SB, Bastopcu M,
Arslanhan G, Baykan DB and Orhan G: The neutrophil-lymphocyte ratio
and the platelet-lymphocyte ratio correlate with thrombus burden in
deep venous thrombosis. J Vasc Surg Venous Lymphat Disord.
8:360–364. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liang S, Tang W, Ye S, Xiang L, Wu X and
Yang H: Incidence and risk factors of preoperative venous
thromboembolism and pulmonary embolism in patients with ovarian
cancer. Thromb Res. 190:129–134. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Claussen C, Rausch AV, Lezius S,
Amirkhosravi A, Davila M, Francis JL, Hisada YM, Mackman N,
Bokemeyer C, Schmalfeldt B, et al: Microvesicle-associated tissue
factor procoagulant activity for the preoperative diagnosis of
ovarian cancer. Thromb Res. 141:39–48. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kerr R, Stirling D and Ludlam CA:
Interleukin 6 and haemostasis. Br J Haematol. 115:3–12.
2001.PubMed/NCBI View Article : Google Scholar
|