Introduction
The standard of care for advanced non-small cell
lung cancer (NSCLC) is systemic therapy. Although cytotoxic agents
conferred an improved survival for NSCLC patients with a good
performance status (PS) (1), the
effectiveness was limited. In this context, molecular targeted
therapies and immune checkpoint inhibitors (ICIs) have been
developed to improve the prognosis of patients with NSCLC. The
effectiveness of ICIs for advanced NSCLC depends on tumor
programmed death-ligand 1 (PD-L1) expression. It has been shown
that the survival benefit of ICI monotherapy for pre-treated
non-squamous NSCLC (2) and
chemoimmunotherapy for untreated NSCLC (3) was greater in those with higher PD-L1
expression levels. Thus, tumor PD-L1 expression is considered an
indicator of the efficacy of ICI therapy for NSCLC (2).
Alternatively, thyroid transcription factor-1
(TTF-1) expression may be another biomarker for the prognosis of
NSCLC. TTF-1 is a transcription factor essential for the
development and differentiation of the lung and thyroid. In animal
models, its inactivation results in severe malformations (4). Gene amplification of TTF-1 has been
demonstrated to promote tumorigenesis in terminal respiratory unit
(TRU)-type lung adenocarcinomas (5). Conversely, the loss of TTF-1
transcriptional activity due to inactivating mutations, such as
frameshift or nonsense mutations, or promoter methylation has been
implicated in the pathogenesis of invasive mucinous adenocarcinomas
(6) and non-TRU-type lung
adenocarcinomas (7). Moreover,
studies employing genetically engineered mouse models have
demonstrated the inactivation of TTF-1 promotes the development of
mucinous adenocarcinomas and induces a phenotypic shift toward
gastric lineage differentiation (8,9).
Given these dual roles of TTF-1 in tumorigenesis,
its protein expression has been investigated as a prognostic marker
in NSCLC. It is reported that TTF-1 expression is associated with
survival after surgery (10),
targeted therapy (11), cytotoxic
agents (12), and ICI therapy
(13-17).
However, multiple antibodies for TTF-1, including 8G7G3/1, SP141,
and SPT24, are available, and the optimal antibody for the
evaluation of TTF-1 expression is undetermined. Furthermore,
opposite findings have been reported concerning the association
between TTF-1 expression and survival after the initiation of the
ICI therapy (12,18,19).
Thus far, the relationship between TTF-1 expression and the
therapeutic efficacy of ICIs in NSCLC remains unclear, with
existing studies yielding inconsistent or inconclusive results.
Immunohistochemistry for TTF-1 and napsin A using a
cocktail antibody (ADC Cocktail antibody, code: KT-17004,
prediluted, Pathology Institute, Toyama, Japan) is employed in
clinical practice in Japan. Herein, we analyzed the association
between TTF-1 expression assessed using a cocktail antibody and
survival after ICI therapy.
Patients and methods
Patient selection
We retrospectively analyzed the data of patients
with NSCLC. Inclusion criteria were specified as follows: i)
Patients who were diagnosed as having non-squamous NSCLC between
January 2019 and December 2023 at Toyama University Hospital
(Toyama, Japan); ii) patients with inoperable diseases, including
advanced, locally advanced, and recurrent NSCLC; iii) patients who
received first-line treatment with ICI monotherapy or
chemoimmunotherapy (hereafter, ICI therapy); and iv) patients for
whom the data regarding TTF-1 expression were evaluated using a
cocktail antibody (TTF-1/napsin A). Exclusion criteria were
specified as follows: i) Patients with NSCLC showing EGFR mutations
and ALK rearrangements; ii) patients with adenosquamous cell
carcinoma, sarcomatoid carcinoma, or neuroendocrine carcinoma; and
iii) patients for whom important data were unavailable, including
PS or PD-L1 expression.
This study was conducted in accordance with The
Declaration of Helsinki and the Guideline of Ministry of Health,
Labor and Welfare and was approved by the Ethics Committee,
University of Toyama (approval no. R2020067). Because this study is
retrospective and noninvasive, written informed consent was not
required.
Clinical information
Clinical information was collected from medical
charts at the initiation of the treatment with ICI therapy,
including age, PS, smoking history, disease stage, tumor PD-L1
expression, TTF-1 expression, driver mutation, and treatment
history. PD-L1 expression testing was performed by a commercial
laboratory (BML INC., Tokyo, Japan) and evaluated using the 22C3
antibody. The expression of TTF-1 was evaluated using a cocktail
antibody (code: KT-17004, clone; SPT24/TMU-Ad02, prediluted,
Pathology Institute, Toyama, Japan). We performed
deparaffinization, antigen retrieval treatment, and endogenous
peroxidase blocking using a VENTANA BenchMark ULTRA system (F.
Hoffmann-La Roche, Ltd. Basel, Switzerland) according to the
manufacturer's instructions.
Statistical analysis
The endpoints of the present study were
progression-free survival (PFS) and overall survival (OS) from the
initiation of ICI therapy. PFS was calculated from the initiation
of ICI therapy to the date of RECIST progressive disease, clinical
progression, or death, whichever occurred first, and censored on
the day when none of these events was noted. OS was calculated from
the initiation of ICI therapy to the date of death and censored on
the day of the last visit without death. Kaplan-Meier curve of PFS
and OS was drawn, and the median (95% confidence interval, CI) was
estimated. PFS and OS were compared using the log-rank test.
Multivariate analysis was performed using the Cox progression
hazard model to analyze the association between TTF-1 expression
and survival, adjusting for potential prognostic factors such as
PS, histology, PD-L1 expression, driver mutation, and chemotherapy.
Fisher's exact test or Wilcoxon's rank sum test were used to
compare patient characteristics. All statistical analyses were
performed using JMP version 17.0.0 (SAS, Cary, NC, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
Between January 2019 and December 2023, 218 patients
with advanced or recurrent NSCLC received first-line treatment with
ICI therapy. After excluding 79 patients with EGFR gene mutations
or ALK fusion gene positivity, 65 patients with squamous cell
carcinoma/adenosquamous carcinoma/sarcomatoid
carcinoma/neuroendocrine carcinoma, 20 patients with unknown
TTF-1/napsin A status, 4 patients with unknown PD-L1 status, and 1
patient with unknown PS, 49 patients were included in the
analysis.
The patient characteristics are summarized in
Table I. Among the 49 patients, 14
were negative for TTF-1, and 35 were positive. A higher proportion
of cases in the TTF-1-negative group exhibited PD-L1 TPS < 50%
than in the positive group. Among the patients with driver gene
mutations, there was 1 case of KRAS G12A, 4 cases of KRAS G12C, 1
case of KRAS G12D, 3 cases of KRAS G12V, and 1 case of MET exon 14
skipping mutation. The representative images of positive and
negative TTF-1 staining are shown in Fig. S1.
 | Table ICharacteristics of patients with
negative and positive TTF-1 expression. |
Table I
Characteristics of patients with
negative and positive TTF-1 expression.
| | TTF-1 | |
|---|
| Characteristic | Negative | Positive | P-value |
|---|
| Median age, years
(range) | 72 (60-85) | 73 (46-89) | 0.894 |
| Sex, n (%) | | | |
|
Male | 11 (78.6%) | 26 (74.3%) | >0.999 |
|
Female | 3 (21.4%) | 9 (25.7%) | |
| PS, n (%) | | | |
|
0-1 | 11 (78.6%) | 29 (82.9%) | 0.702 |
|
≥2 | 3 (21.4%) | 6 (17.1%) | |
| Smoking history, n
(%) | | | |
|
Yes | 12 (85.7%) | 32 (91.4%) | 0.616 |
|
No | 2 (14.3%) | 3 (8.6%) | |
| Histology, n (%) | | | |
|
Adenocarcinoma | 9 (64.3%) | 33 (94.3%) | 0.015 |
|
Other | 5 (35.7%) | 2 (5.7%) | |
| Driver mutation, n
(%) | | | |
|
Positive | 3 (21.4%) | 7 (20.0%) | >0.999 |
|
Negative/unknown | 11 (78.6%) | 28 (80.0%) | |
| PD-L1 TPS, n (%) | | | |
|
<50% | 12 (85.7%) | 16 (45.7%) | 0.013 |
|
≥50% | 2 (14.3%) | 19 (54.3%) | |
| Stage, n (%) | | | |
|
3B | 0 (0.0%) | 1 (2.9%) | 0.802 |
|
4A | 4 (28.6%) | 9 (25.7%) | |
|
4B | 5 (35.7%) | 16 (45.7%) | |
|
Recurrence | 5 (35.7%) | 9 (25.7%) | |
| ILD, n (%) | | | |
|
Yes | 2 (14.3%) | 2 (5.7%) | 0.568 |
|
No | 12 (85.7%) | 33 (94.3%) | |
| Liver metastases, n
(%) | | | |
|
Yes | 1 (7.1%) | 2 (5.7%) | >0.999 |
|
No | 13 (92.9%) | 33 (94.3%) | |
| Brain metastases, n
(%) | | | |
|
Yes | 2 (14.3%) | 9 (25.7%) | 0.475 |
|
No | 12 (85.7%) | 26 (74.3%) | |
| NLR, n (%) | | | |
|
<5 | 6 (42.9%) | 24 (68.6%) | 0.116 |
|
≥5 | 8 (57.1%) | 11 (31.4%) | |
| LDH, n (%) | | | |
|
<200
U/l | 5 (35.7%) | 21 (60.0%) | 0.205 |
|
≥200
U/l | 9 (64.3%) | 14 (40.0%) | |
| CRP, n (%) | | | |
|
<1.0
mg/dl | 7 (50.0%) | 23 (65.7%) | 0.346 |
|
≥1.0
mg/dl | 7 (50.0%) | 12 (34.3%) | |
| Treatment, n
(%) | | | |
|
Chemotherapy
+ ICI | 10 (71.4%) | 17 (48.6%) | 0.207 |
|
ICI | 4 (28.6%) | 18 (51.4%) | |
Survival time
Tables II and
III show the results of a
multivariate analysis adjusted for PS, histology, PD-L1 expression,
presence of genetic abnormalities, and the use of chemotherapy. The
relationship was examined between TTF-1 expression and both PFS and
OS. TTF-1 positivity was significantly associated with a decreased
risk of disease progression, independent of these factors; however,
no significant association was observed with a decreased risk of
death.
 | Table IICox proportional hazard model for the
risk of progression. |
Table II
Cox proportional hazard model for the
risk of progression.
| Characteristic | HR | 95% CI | P-value |
|---|
| PS | | | |
|
0-1 | 0.83 | 0.27-2.50 | 0.735 |
|
≥2 | 1.00 | | |
| Histology | | | |
|
Adenocarcinoma | 1.27 | 0.43-3.76 | 0.666 |
|
Other | 1.00 | | |
| PD-L1 TPS | | | |
|
≥50% | 1.28 | 0.47-3.47 | 0.633 |
|
<50% | 1.00 | | |
| Driver
mutation | | | |
|
Positive | 0.82 | 0.32-2.14 | 0.692 |
|
Negative/unknown | 1.00 | | |
| TTF-1 | | | |
|
Positive | 0.18 | 0.06-0.54 | 0.002 |
|
Negative | 1.00 | | |
| Treatment | | | |
|
Chemotherapy
+ ICI | 1.34 | 0.53-3.43 | 0.537 |
|
ICI | 1.00 | | |
 | Table IIICox proportional hazard model for the
risk of death. |
Table III
Cox proportional hazard model for the
risk of death.
| Characteristic | HR | 95% CI | P-value |
|---|
| PS | | | |
|
0-1 | 0.61 | 0.16-2.41 | 0.485 |
|
≥2 | 1.00 | | |
| Histology | | | |
|
Adenocarcinoma | 0.29 | 0.08-1.11 | 0.070 |
|
Other | 1.00 | | |
| PD-L1 TPS | | | |
|
≥50% | 1.15 | 0.36-3.65 | 0.818 |
|
<50% | 1.00 | | |
| Driver
mutation | | | |
|
Positive | 1.10 | 0.30-3.98 | 0.884 |
|
Negative/unknown | 1.00 | | |
| TTF-1 | | | |
|
Positive | 0.67 | 0.15-2.97 | 0.601 |
|
Negative | 1.00 | | |
| Treatment | | | |
|
Chemotherapy
+ ICI | 0.81 | 0.29-2.29 | 0.694 |
|
ICI | 1.00 | | |
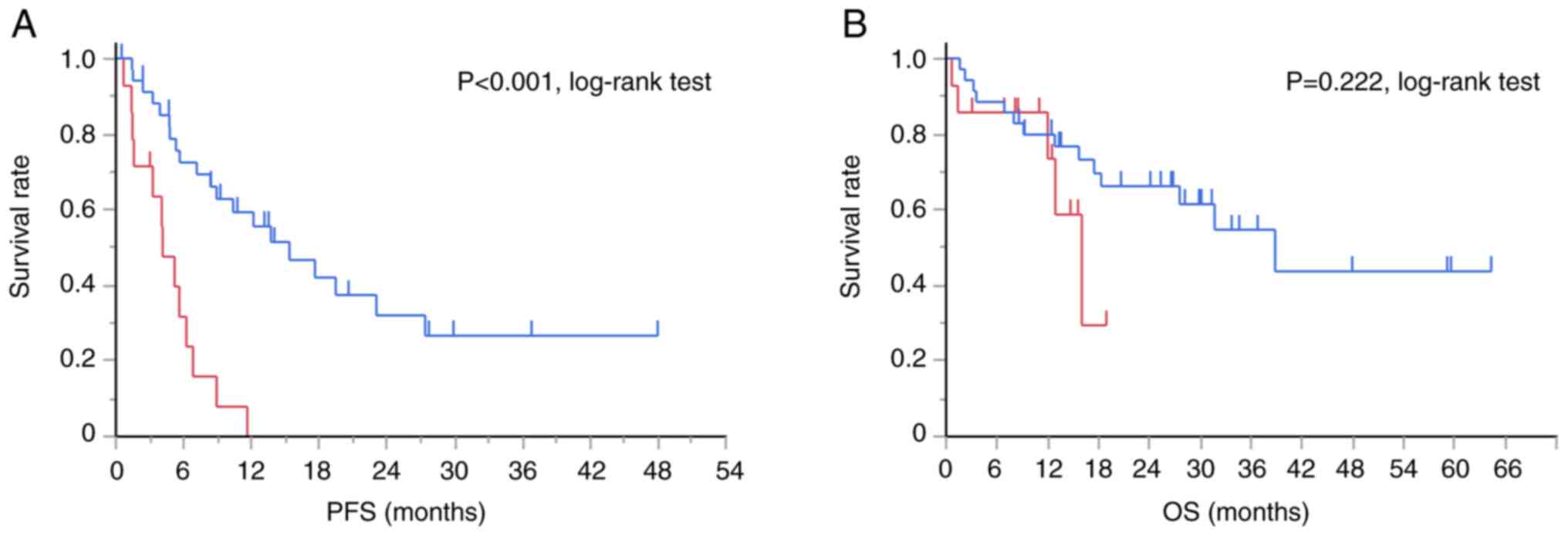
Fig. 1 presents the
Kaplan-Meier curves for PFS and OS in the TTF-1-positive and
-negative groups. The TTF-1-positive group demonstrated
significantly longer PFS (median PFS, 15.4 months vs. 4.1 months,
P<0.001, log-rank test) than the negative group, but not
significantly longer OS (median OS, 38.8 months vs. 16.0 months).
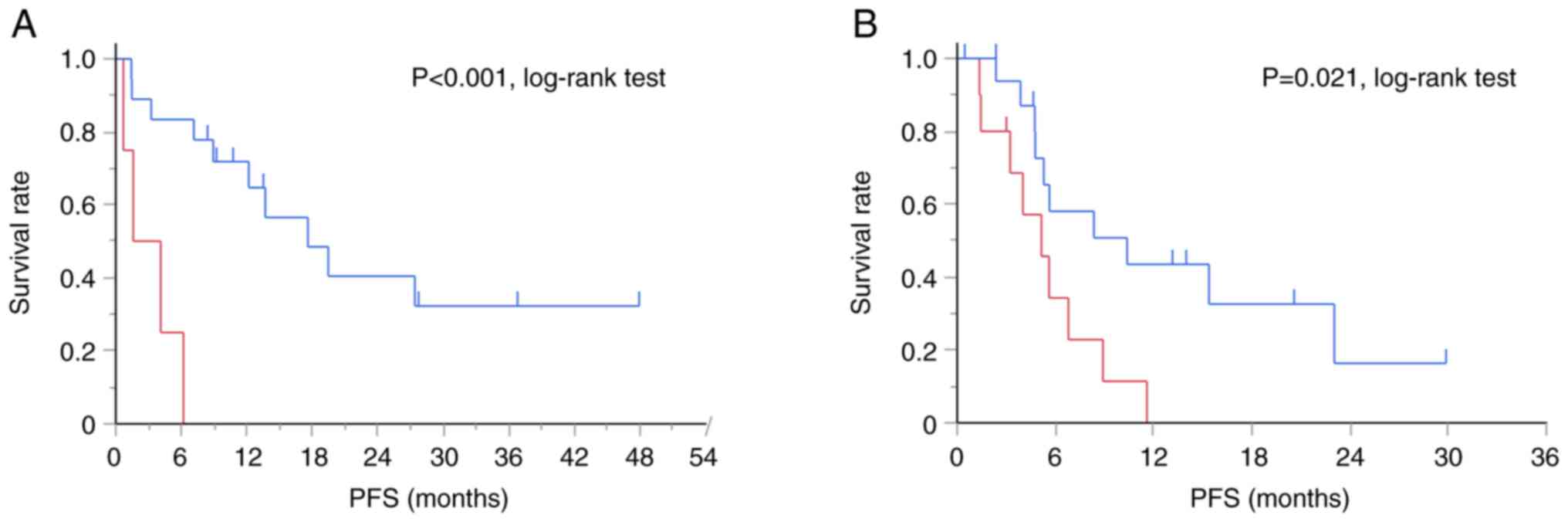
When patients were stratified by chemotherapy use, the
TTF-1-positive group exhibited significantly longer PFS than the
negative group in both the ICI monotherapy group (median PFS, 17.6
months vs. 2.9 months, P<0.001, log-rank test) and
chemoimmunotherapy group (median PFS, 10.4 months vs. 5.2 months,
P=0.021, log-rank test) (Fig.
2).
Discussion
TTF-1 is associated with the survival of NSCLC
patients as a prognostic factor. Recently, the relationship between
TTF-1 expression and survival after the initiation of ICI therapy
has garnered increasing interest, particularly in optimizing
treatment strategies for the poor prognostic population
characterized by negative TTF-1 expression (18). However, there are inconsistent
reports concerning the association between TTF-1 expression and
survival. TTF-1 has previously been analyzed using anti-TTF-1
antibody clone 8G7G3/1, SPT24, and SP141 (12,13,15,17-19).
The anti-TTF-1 antibody clone SPT24 is more sensitive but less
specific than 8G7G3/1(20). The
present study suggests that TTF-1 expression evaluated using a
cocktail antibody using SPT24/TMU-Ad02 antibody is associated with
PFS after ICI therapy.
TTF-1 is expressed on type II pulmonary epithelial
cells and club cells, contributing to lung development and the
homeostasis of surfactant protein. TTF-1 induces the expression of
myosin-binding protein H, which results in decreased cancer
invasion and metastasis (5).
Furthermore, several studies have proposed that TTF-1 may also
exert tumor-suppressive functions by maintaining alveolar
epithelial lineage identity and preventing dedifferentiation. The
loss of TTF-1 activity has been implicated in the development of
invasive mucinous adenocarcinoma and other non-TRU-type
adenocarcinomas (6,7). Thus, the favorable prognosis in
patients with TTF-1-positive tumors observed in our study could be
partially attributable to its tumor-suppressive function.
Conversely, in TTF-1-negative lung adenocarcinoma,
the inactivating mutation of kelch-like ECH-associated protein 1
(KEAP1) is enriched (21), which
results in the overexpression of nuclear factor-like 2 (Nrf2), a
master regulator of the antioxidant response. Nrf2 is associated
with poor prognosis in several cancers, including lung cancer
(21). Furthermore, the proportion
of PD-L1 positive tumors may be lower in TTF-1-negative NSCLC
(14,15), which was observed in the present
study. Tumor PD-L1 expression is induced either by oncogene
signaling or interferon γ secreted by T lymphocytes (22). Thus, it is hypothesized that
T-lymphocyte infiltration is deterred or the function is suppressed
in TTF-1-negative non-squamous NSCLC.
There is insufficient information on the association
between napsin A expression and clinical outcomes after ICI
therapy. Napsin A is an aspartic proteinase expressed on alveolar
type 2 cells, contributing to surfactant protein synthase.
Immunohistochemistry of napsin A is considered comparable to that
of TTF-1 for diagnosing primary lung adenocarcinoma, and dual
staining is likely the most beneficial (23,24).
Although it is difficult to explain the biological or immunological
significance of napsin A expression in relation to ICI therapy for
NSCLC, the selection of TTF-1/napsin A-negative non-squamous NSCLC
may result in the identification of more poorly differentiated
tumors.
The present study has several limitations. First,
the sample size was small, which may not sufficiently represent
NSCLC patients. Second, given the retrospective nature of the
study, the imbalance in patient backgrounds may have affected the
analysis, although multivariate analysis was performed to adjust
the potential prognostic factors.
In summary, we observed an association between TTF-1
expression, as evaluated using a cocktail antibody, and the
effectiveness of ICI therapy. Further accumulation of data
regarding TTF-1 evaluation methodologies and their association with
the prognosis of NSCLC patients is necessary.
Supplementary Material
Representative images of TTF-1
staining. (A) Hematoxylin-eosin staining findings of a
TTF-1-positive tumor. (B) TTF-1 staining findings of a
TTF-1-positive tumor. (C) Hematoxylin-eosin staining findings of a
TTF-1 negative tumor. (D) TTF-1 staining findings of a TTF-1
negative tumor. TTF-1-positivity was detected in normal alveolar
cells.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NM and MI designed the study and wrote the original
draft of the manuscript. NM, MI, DF, MH, NT, ZS, KTo, SO, SI, TM
and RH contributed to the acquisition of data. KTa and KH
contributed to the immunohistochemistry. NM and MI confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with The
Declaration of Helsinki and the Guideline of Ministry of Health,
Labor and Welfare and was approved by the Ethics Committee,
University of Toyama (approval no. R2020067). Because this study is
retrospective and noninvasive, written informed consent was not
required.
Patient consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or
non-financial interests to disclose.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript, and
subsequently, the authors revised and edited the content produced
by the AI tools as necessary, taking full responsibility for the
ultimate content of the present manuscript.
References
|
1
|
Socinski MA: Cytotoxic chemotherapy in
advanced non-small cell lung cancer: A review of standard treatment
paradigms. Clin Cancer Res. 10:4210s–4214s. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Garassino MC, Gadgeel S, Speranza G, Felip
E, Esteban E, Dómine M, Hochmair MJ, Powell SF, Bischoff HG, Peled
N, et al: Pembrolizumab plus pemetrexed and platinum in nonsquamous
non-small-cell lung cancer: 5-Year outcomes from the phase 3
KEYNOTE-189 study. J Clin Oncol. 41:1992–1998. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Minoo P, Su G, Drum H, Bringas P and
Kimura S: Defects in tracheoesophageal and lung morphogenesis in
Nkx2.1(-/-) mouse embryos. Dev Biol. 209:60–71. 1999.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hosono Y, Yamaguchi T, Mizutani E,
Yanagisawa K, Arima C, Tomida S, Shimada Y, Hiraoka M, Kato S,
Yokoi K, et al: MYBPH, a transcriptional target of TTF-1, inhibits
ROCK1, and reduces cell motility and metastasis. EMBO J.
31:481–493. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hwang DH, Sholl LM, Rojas-Rudilla V, Hall
DL, Shivdasani P, Garcia EP, MacConaill LE, Vivero M, Hornick JL,
Kuo FC, et al: KRAS and NKX2-1 mutations in invasive mucinous
adenocarcinoma of the lung. J Thorac Oncol. 11:496–503.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Matsubara D, Soda M, Yoshimoto T, Amano Y,
Sakuma Y, Yamato A, Ueno T, Kojima S, Shibano T, Hosono Y, et al:
Inactivating mutations and hypermethylation of the NKX2-1/TTF-1
gene in non-terminal respiratory unit-type lung adenocarcinomas.
Cancer Sci. 108:1888–1896. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Maeda Y, Tsuchiya T, Hao H, Tompkins DH,
Xu Y, Mucenski ML, Du L, Keiser AR, Fukazawa T, Naomoto Y, et al:
Kras(G12D) and Nkx2-1 haploinsufficiency induce mucinous
adenocarcinoma of the lung. J Clin Invest. 122:4388–4400.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Snyder EL, Watanabe H, Magendantz M,
Hoersch S, Chen TA, Wang DG, Crowley D, Whittaker CA, Meyerson M,
Kimura S and Jacks T: Nkx2-1 represses a latent gastric
differentiation program in lung adenocarcinoma. Mol Cell.
50:185–199. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Barletta JA, Perner S, Iafrate AJ, Yeap
BY, Weir BA, Johnson LA, Johnson BE, Meyerson M, Rubin MA, Travis
WD, et al: Clinical significance of TTF-1 protein expression and
TTF-1 gene amplification in lung adenocarcinoma. J Cell Mol Med.
13:1977–1986. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chung KP, Huang YT, Chang YL, Yu CJ, Yang
CH, Chang YC, Shih JY and Yang PC: Clinical significance of thyroid
transcription factor-1 in advanced lung adenocarcinoma under
epidermal growth factor receptor tyrosine kinase inhibitor
treatment. Chest. 141:420–428. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nishioka N, Hata T, Yamada T, Goto Y,
Amano A, Negi Y, Watanabe S, Furuya N, Oba T, Ikoma T, et al:
Impact of TTF-1 expression on the prognostic prediction of patients
with non-small cell lung cancer with PD-L1 expression levels of 1%
to 49%, treated with chemotherapy vs chemoimmunotherapy: A
multicenter, retrospective study. Cancer Res Treat. 57:412–421.
2025.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ibusuki R, Yoneshima Y, Hashisako M,
Matsuo N, Harada T, Tsuchiya-Kawano Y, Kishimoto J, Ota K,
Shiraishi Y, Iwama E, et al: Association of thyroid transcription
factor-1 (TTF-1) expression with efficacy of PD-1/PD-L1 inhibitors
plus pemetrexed and platinum chemotherapy in advanced non-squamous
non-small cell lung cancer. Transl Lung Cancer Res. 11:2208–2215.
2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nakahama K, Kaneda H, Osawa M, Izumi M,
Yoshimoto N, Sugimoto A, Nagamine H, Ogawa K, Matsumoto Y, Sawa K,
et al: Association of thyroid transcription factor-1 with the
efficacy of immune-checkpoint inhibitors in patients with advanced
lung adenocarcinoma. Thorac Cancer. 13:2309–2317. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Katayama Y, Yamada T, Morimoto K, Fujii H,
Morita S, Tanimura K, Takeda T, Okada A, Shiotsu S, Chihara Y, et
al: TTF-1 expression and clinical outcomes of combined
chemoimmunotherapy in patients with advanced lung adenocarcinoma: A
prospective observational study. JTO Clin Res Rep.
4(100494)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Terashima Y, Matsumoto M, Iida H,
Takashima S, Fukuizumi A, Takeuchi S, Miyanaga A, Terasaki Y,
Kasahara K and Seike M: Predictive impact of diffuse positivity for
TTF-1 expression in patients treated with platinum-doublet
chemotherapy plus immune checkpoint inhibitors for advanced
nonsquamous NSCLC. JTO Clin Res Rep. 4(100578)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Uhlenbruch M and Krüger S: Effect of TTF-1
expression on progression free survival of immunotherapy and
chemo-/immunotherapy in patients with non-small cell lung cancer. J
Cancer Res Clin Oncol. 150(394)2024.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Iso H, Hisakane K, Mikami E, Suzuki T,
Matsuki S, Atsumi K, Nagata K, Seike M and Hirose T: Thyroid
transcription factor-1 (TTF-1) expression and the efficacy of
combination therapy with immune checkpoint inhibitors and cytotoxic
chemotherapy in non-squamous non-small cell lung cancer. Transl
Lung Cancer Res. 12:1850–1861. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nishioka N, Kawachi H, Yamada T, Tamiya M,
Negi Y, Goto Y, Nakao A, Shiotsu S, Tanimura K, Takeda T, et al:
Unraveling the influence of TTF-1 expression on immunotherapy
outcomes in PD-L1-high non-squamous NSCLC: A retrospective
multicenter study. Front Immunol. 15(1399889)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vidarsdottir H, Tran L, Nodin B, Jirström
K, Planck M, Mattsson JSM, Botling J, Micke P, Jönsson P and
Brunnström H: Comparison of three different TTF-1 clones in
resected primary lung cancer and epithelial pulmonary metastases.
Am J Clin Pathol. 150:533–544. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cardnell RJG, Behrens C, Diao L, Fan Y,
Tang X, Tong P, Minna JD, Mills GB, Heymach JV, Wistuba II, et al:
An integrated molecular analysis of lung adenocarcinomas identifies
potential therapeutic targets among TTF1-negative tumors, including
DNA repair proteins and Nrf2. Clin Cancer Res. 21:3480–3491.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Prelaj A, Tay R, Ferrara R, Chaput N,
Besse B and Califano R: Predictive biomarkers of response for
immune checkpoint inhibitors in non-small-cell lung cancer. Eur J
Cancer. 106:144–159. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Turner BM, Cagle PT, Sainz IM, Fukuoka J,
Shen SS and Jagirdar J: Napsin A, a new marker for lung
adenocarcinoma, is complementary and more sensitive and specific
than thyroid transcription factor 1 in the differential diagnosis
of primary pulmonary carcinoma: Evaluation of 1674 cases by tissue
microarray. Arch Pathol Lab Med. 136:163–171. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
El Hag M, Schmidt L, Roh M and Michael CW:
Utility of TTF-1 and Napsin-A in the work-up of malignant
effusions. Diagn Cytopathol. 44:299–304. 2016.PubMed/NCBI View
Article : Google Scholar
|