Introduction
Recently, breast cancer incidence has increased and
total mastectomy was common surgical method for breast cancer
patients (1). In total mastectomy,
it is generally common to place drains. Although early drain
removal can reduce the length of hospital stay, a meta-analysis
pointed out that the frequency of adverse events (AE) such as
seroma increases due to the early removal of drains (2). The meta-analysis indicates that AE
increase when the drain is removed early, in practice, each
facility decides on its own criteria for removal. Particularly,
removing the drain at 30-50 ml/d is a relatively common criterion
in view of the benefits and disadvantages (3-7).
However, it is undeniable that we aim for fewer complications and
early discharge. Therefore, ensuring a reliable improvement in
surgical techniques becomes one of the key factors.
Recent advancements in surgical devices have
facilitated the sealing of blood vessels and reduced the burden of
the surgeon. In Japan, under the health insurance system, energy
devices (EDs) can be employed in axillary dissection. This is based
on positive evidence on the use of devices such as the Harmonic
Focus® and LigaSure Exact Dissector®
(8,9). Using EDs to dissect mammary fat
tissue from the pectoralis major muscle during total mastectomy may
help reduce surgical complications, including seroma formation.
Dissection of the pectoralis fascia in total mastectomy involves a
wide area of the posterior wall of the mammary gland, which making
it likely to benefit from the use of ED. In this study, we aimed to
evaluate the amount of drainage and seroma incidence in patients
undergoing total mastectomy with pectoralis fascia dissection using
EDs.
Materials and methods
Study design
This was a single-center, observational study, with
an exposure group comprising 41 consecutive patients with breast
cancer who underwent total mastectomy using an ED (Harmonic Focus
or LigaSure Exact) to separate breast fat tissue from the
pectoralis major muscle between January 1, 2020 and December 31,
2024. It is well known that breast cancer occurs predominantly in
women, while cases in men are rare. Therefore, all eligible cases
in this study are female. The historical control group comprised
117 consecutive patients who underwent total mastectomy for breast
cancer in Sapporo Medical University Hospital (Sapporo, Japan)
between January and December 2019, and conventional electrocautery
during chest wall dissection. A suction drain (5 mm SB VAC,
Sumitomo Bakelite, Tokyo, Japan) was inserted in all cases. The
suction drain was removed when the daily drainage volume was
approximately <40 ml.
The surgical procedure for total mastectomy is
described below. Electrocautery was conventionally used for skin
flap dissection in both groups. In both groups, using an ED,
axillary dissection was performed from the lateral chest wall to
axillary levels I and II. In the exposure group, an ED was used for
all pectoralis fascia dissection procedures, separating breast
tissue from the pectoralis major muscle, whereas electrocautery was
used in the control group (Fig.
1). Four breast specialists with >10 years of experience
performed the surgeries.
The study outcomes were as follows: primary outcome,
total amount until drain removal; secondary outcomes, daily
drainage fluid output [postoperative days (POD) 1-10], operative
time, blood loss, length of hospital stay, and frequency of AEs
requiring intervention.
This study adhered to the ethical tenets of The
Declaration of Helsinki and the Ethical Principles for Medical
Research Involving Human Subjects. It was approved by the Clinical
Trial Center of Sapporo Medical University (approval number
352-93), and registered with UMIN-CTR (UMIN000056764). The need for
informed consent was waived owing to the observational nature of
this study. An opt-out consent process was used, with disclosures
made on the university website (https://web.sapmed.ac.jp/byoin/rinshokenkyu/koukai/).
Statistical analysis
Statistical analyses were performed using JMP17 (SAS
Institute Inc., Cary, NC, USA). The unpaired Student s t-test
was used to compare the total amount of drain fluid, daily drainage
fluid output (postoperative days 1-10), operative time, blood loss,
and length of hospital stay between the ED and control groups. The
χ2 test or Fisher s exact test was used to analyze
cT stage (as a sequential variable) and the following nominal
variables: cN status (negative or negative), body mass index (BMI;
cutoff 25 kg/m2), prior chemotherapy, subtype, breast
surgery procedure (total mastectomy only), axillary surgery
procedure [axillary lymph node dissection (ALND) vs. sentinel lymph
node biopsy (SLNB)], and reconstructive surgery (presence or
absence). P<0.05 was considered to indicate a statistically
significant difference. Among these clinicopathological factors,
BMI, prior chemotherapy, and axillary surgery were treated as
covariates, and propensity score matching (PSM) was used to adjust
for these three factors. To identify independent factors associated
with AEs requiring intervention, a univariate analysis was
performed.
Results
Adjusting data using propensity score
matching
Patient characteristics and history for both groups
are listed in Table IA and B. As
shown in Table IA, key
characteristics included significantly higher BMI in the ED group
compared to the control group (P=0.0155), a higher proportion of
patients with prior preoperative chemotherapy (P=0.0001), and a
greater number of patients who had received ALND (P=0.0004). PSM
was performed using three parameters as covariates: BMI, presence
or absence of preoperative chemotherapy, and axillary surgery (ALND
vs. SLNB). The adjusted patient characteristics and history
post-PSM are summarized in Table
IB.
 | Table IPatient characteristics before and
after PSM. |
Table I
Patient characteristics before and
after PSM.
| A, Before PSM |
|---|
| Characteristic | Total (%) | ED (%) | Control (%) | P-value |
|---|
| Sample size | 158 | 41 | 117 | - |
| Age, years | | | | 0.5120 |
|
<50 | 48 (30.4) | 12 (29.3) | 36 (30.8) | |
|
≥50 | 110 (69.6) | 29 (70.7) | 81 (69.2) | |
| BMI,
kg/m2 | | | | 0.0155 |
|
<25 | 113 (71.5) | 23 (56.1) | 90 (76.9) | |
|
≥25 | 45 (28.5) | 18 (43.9) | 27 (23.1) | |
| cT | | | | 0.6034 |
|
0-1 | 71 (44.9) | 17 (41.5) | 54 (46.2) | |
|
≥2 | 87 (55,1) | 24 (58.3) | 63 (53.8) | |
| cN | | | | 0.1803 |
|
0 | 56 (35.4) | 11 (26.8) | 45 (38.5) | |
|
≥1 | 102 (64.5) | 30 (73.2) | 72 (61.5) | |
| Neoadjuvant
chemotherapy | | | | 0.0001 |
|
Yes | 31 (19.6) | 17 (41.5) | 14 (12.0) | |
|
No | 127 (80.4) | 24 (58.5) | 103 (88.0) | |
| Axillary surgery | | | | 0.0004 |
|
SLNB | 105(66.5) | 18 (43.9) | 87 (74.4) | |
|
ALND | 53(33.5) | 23 (56.1) | 30 (25.6) | |
| Reconstruction | | | | >0.9999 |
|
Yes | 8 (5.1) | 2 (4.9) | 6 (5.1) | |
|
No | 150 (94.9) | 39 (95.1) | 111 (94.9) | |
| ER/HER2 status | | | | 0.1039 |
|
ER+/HER2- | 93 (58.9) | 27 (65.9) | 66 (56.4) | |
|
ER+/HER2+ | 17 (10.7) | 5 (12.2) | 12 (10.3) | |
|
ER-/HER2- | 14 (8.9) | 5 (12.2) | 9 (7.7) | |
|
ER-/HER2+ | 22 (13.9) | 1 (2.4) | 21 (17.9) | |
|
DCIS | 12 (7.6) | 3 (7.3) | 9 (7.7) | |
| LigaSure Exact
Dissector | | 24 (58.5) | | - |
| Harmonic Focus | | 17 (41.5) | | - |
| B, After PSM |
|
Characteristics | Total (%) | ED (%) | Control (%) | P-value |
| Sample size | 76 | 38 | 38 | - |
| Age, years | | | | 0.1687 |
|
<50 | 17 (22.4) | 11 (28.9) | 6 (15.8) | |
|
≥50 | 59 (77.6) | 27 (71.1) | 32 (84.2) | |
| BMI,
kg/m2 | | | | 0.8154 |
|
<25 | 45 (59.2) | 22 (57.9) | 23 (60.5) | |
|
≥25 | 31 (40.8) | 16 (42.1) | 15 (39.5) | |
| cT | | | | 0.1536 |
|
0-1 | 28 (36.8) | 17 (44.7) | 11 (29.0) | |
|
≥2 | 48 (63.2) | 21 (55.3) | 27 (71.0) | |
| cN | | | | 0.7975 |
|
0 | 21 (27.6) | 11 (28.9) | 10 (26.3) | |
|
≥1 | 55 (72.6) | 27 (7.1) | 28 (73.3) | |
| Neoadjuvant
chemotherapy | | | | 0.8106 |
|
Yes | 27 (35.6) | 14 (56.8) | 13 (34.2) | |
|
No | 49 (63.4) | 24 (63.2) | 25 (65.8) | |
| Axillary
surgery | | | | 0.6445 |
|
SLNB | 34 (44.7) | 18 (47.4) | 16 (42.1) | |
|
ALND | 42 (55.3) | 20 (52.6) | 22 (57.9) | |
| Reconstruction | | | | 0.4933 |
|
Yes | 2 (2.6) | 2 (5.3) | 0 (0) | |
|
No | 74 (97.4) | 36 (94.7) | 38(100) | |
| ER/HER2 status | | | | 0.0391 |
|
ER+/HER2- | 43 (56.6) | 24 (64.3) | 19 (50.0) | |
|
ER+/HER2+ | 7 (9.2) | 5 (13.2) | 2 (5.3) | |
|
ER-/HER2- | 10 (13.1) | 5 (13.2) | 5 (13.1) | |
|
ER-/HER2+ | 11 (14.5) | 1 (2.6) | 10 (26.3) | |
|
DCIS | 3 (6.6) | 3 (7.6) | 2 (5.3) | |
| LigaSure Exact
Dissector | | 23 (60.5) | | - |
| Harmonic Focus | | 15 (39.5) | | - |
Surgical outcomes
As displayed in Table
II the mean total amount of drainage was 610 ml in the device
group and 812 ml in the control group, indicating a numerical
decrease without a statistically significant difference (P=0.1778).
No significant delay in operation time was observed with the use of
ED post-PSM [ED vs. control (mean ± standard deviation); 105±42 vs.
117±35 min; P=0.1960]. Moreover, no significant difference was
observed in the amount of bleeding (20±30 vs. 23±21 ml; P=0.6202),
POD of drain removal (7.3±2.7 vs. 7.9±3.3; P=0.3649), and length of
hospital stay (11.4±3.3 vs. 11.0±5.6 days; P=0.6737) post-PSM.
 | Table IISurgical outcome. |
Table II
Surgical outcome.
| A, Surgical outcome
(before PSM) |
|---|
| Variable | Total | ED | Control | P-value |
|---|
| Sample size | 158 | 41 | 117 | - |
| Operation time,
min | 109±39 | 104±41 | 111±39 | 0.3619 |
| Estimated blood
loss, ml | 24±28 | 19±29 | 26±28 | 0.1557 |
| Total amount of
drainage, ml | 570±513 | 610±398 | 556±548 | 0.5137 |
| Post operation day
of drain removal, days | 6.7±2.3 | 6.8±2.4 | 6.7±2.3 | 0.8390 |
| Duration of
hospital stay, days | 10.8±3.8 | 11.3±3.2 | 10.6±4.0 | 0.2303 |
| All adverse events
incidence, n (%) | | | | 0.0957 |
|
Presence of
adverse events | 47 (29.7) | 8 (19.5) | 39 (33.3) | |
|
Absence of
adverse events | 111 (70.3) | 33 (80.5) | 78 (66.7) | |
| Adverse events
requiring intervention, n (%) | | | | 0.0675 |
|
Presence of
adverse events | 40 (25.3) | 6 (14.6) | 34 (29.1) | |
|
Absence of
adverse events | 118 (74.7) | 35 (85.4) | 83 (70.9) | |
| B, Details and
breakdown of AEs (before PSM) |
| Variable | Total | ED | Control | P-value |
| Sample size | 40 | 6 | 34 | |
| AEs | | | | >0.9999 |
|
Seroma, n
(%) | 32 | 6(100) | 26 (76.5) | |
|
Skin
necrosis, n (%) | 2 | 0 (0) | 2 (5.9) | |
|
Hematoma, n
(%) | 0 | 0 (0) | 0 (0) | |
|
Surgical
site infection, n (%) | 1 | 0 (0) | 1 (2.9) | |
|
Seroma +
infection, n (%) | 4 | 0 (0) | 4 (11.8) | |
|
Hematoma +
infection, n (%) | 1 | 0 (0) | 1 (2.9) | |
| C, Surgical outcome
(after PSM) |
| Variable | Total | ED | Control | P-value |
| Sample size | 76 | 38 | 38 | - |
| Operation time,
min | 111±39 | 105±42 | 117±35 | 0.1960 |
| Estimated blood
loss, ml | 21±26 | 20±30 | 23±21 | 0.6202 |
| Total amount of
drainage, ml | 650±75 | 610±407 | 812±818 | 0.1778 |
| Post operation day
of drain removal, days | 7.1±2.4 | 6.8±2.4 | 7.5±2.3 | 0.1599 |
| Duration of
hospital stay, days | 11.6±4.6 | 11.4±3.3 | 11.9±5.6 | 0.6737 |
| All adverse events
incidence, n (%) | | | | 0.0805 |
|
Presence of
adverse events | 23 (30.3) | 8 (21.1) | 15 (39.5) | |
|
Absence of
adverse events | 53 (69.7) | 30 (78.9) | 23 (60.5) | |
| Adverse events
requiring intervention, n (%) | | | | 0.0372 |
|
Presence of
adverse events | 20 (26.3) | 6 (15.8) | 14 (36.8) | |
|
Absence of
adverse events | 56 (73.7) | 32 (84.2) | 24 (63.2) | |
| D, Details and
breakdown of AEs (after PSM) |
| | Total (%) | ED (%) | Control (%) | P-value |
| Sample size | 20 | 6 | 14 | |
| AEs | | | | >0.9999 |
|
Seroma, n
(%) | 17 (85.0) | 6(100) | 11 (78.6) | |
|
Skin
necrosis, n (%) | 0 (0) | 0 (0) | 0 (0) | |
|
Hematoma, n
(%) | 0 (0) | 0 (0) | 0 (0) | |
|
Surgical
site infection, n (%) | 1 (5.0) | 0 (0) | 1 (7.1) | |
|
Seroma +
infection, n (%) | 2 (10.0) | 0 (0) | 2 (14.3) | |
|
Hematoma +
infection, n (%) | 0 (0) | 0 (0) | 0 (0) | |
ED may reduce adverse events requiring
intervention and seroma incidence
The ED group had a significantly lower frequency of
AEs requiring intervention than the control group post-PSM [6/38
vs. 14/38 (15.4 vs. 36.8%); P=0.0372; Table II], although no difference was
observed between the two groups regarding all AE. Most AEs were
caused by seroma, with a smaller proportion attributed to
infection. In univariate analysis, the risk of AEs requiring
intervention was found to be reduced by the use of ED after PSM,
and there were no other confounding factors [OR 0.32, 95% CI
(0.10-0.93), P=0.0351; Table
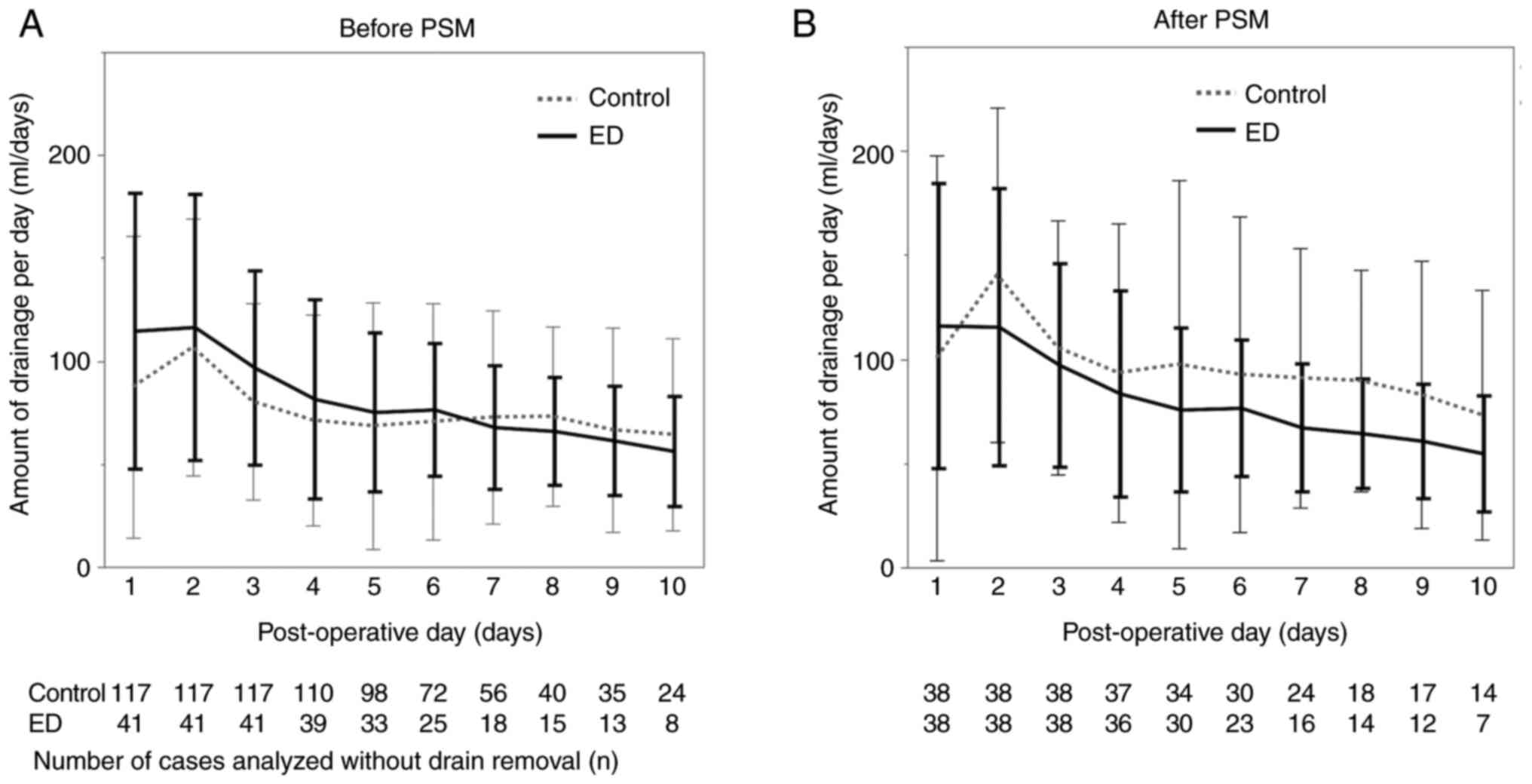
III]. Notably, the graph showing the average daily drainage
volume from POD 1 to POD 10 revealed a general downward trend in
drainage volume in the ED group after PSM. The drainage volume in
the ED group was markedly lower on POD 8 post-PSM (Fig. 2A and B).
 | Table IIIUnivariate analysis for AE requiring
interventions. |
Table III
Univariate analysis for AE requiring
interventions.
| A, Before PSM |
|---|
| Variable | Factor | OR | 95% CI | P-value |
|---|
| Age, years | ≥50 vs. <50 | 2.51 | 1.07-6.63 | 0.0335 |
| BMI,
kg/m2 | ≥25 vs. <25 | 1.09 | 0.51-2.29 | 0.8133 |
| cT | ≥2 vs. 0-1 | 1.14 | 0.56-2.38 | 0.7196 |
| cN | ≥1 vs. 0 | 0.89 | 0.42-1.90 | 0.7536 |
| Neoadjuvant
chemotherapy | Yes vs. no | 1.27 | 0.51-2.97 | 0.5998 |
| Axillary
surgery | ALND vs. SLNB | 1.26 | 0.59-2.65 | 0.5421 |
| ED/Control | With device vs.
without device | 0.42 | 0.15-1.02 | 0.0570 |
| B, After PSM |
| Variable | Factor | OR | 95% CI | P-value |
| Age, years | ≥50 vs. <50 | 3.29 | 0.81-22.28 | 0.1001 |
| BMI,
kg/m2 | ≥25 vs. <25 | 0.96 | 0.33-2.67 | 0.9333 |
| cT | ≥2 vs. 0-1 | 1.51 | 0.52-4.80 | 0.4554 |
| cN | ≥1 vs. 0 | 1.74 | 0.54-6.77 | 0.3633 |
| Neoadjuvant
chemotherapy | Yes vs. no | 1.72 | 0.60-4.93 | 0.3072 |
| Axillary
surgery | ALND vs. SLNB | 1.73 | 0.61-5.21 | 0.3042 |
| ED/Control | With device vs.
without device | 0.32 | 0.10-0.93 | 0.0351 |
The postoperative day of drain removal
correlates with the length of hospital stay
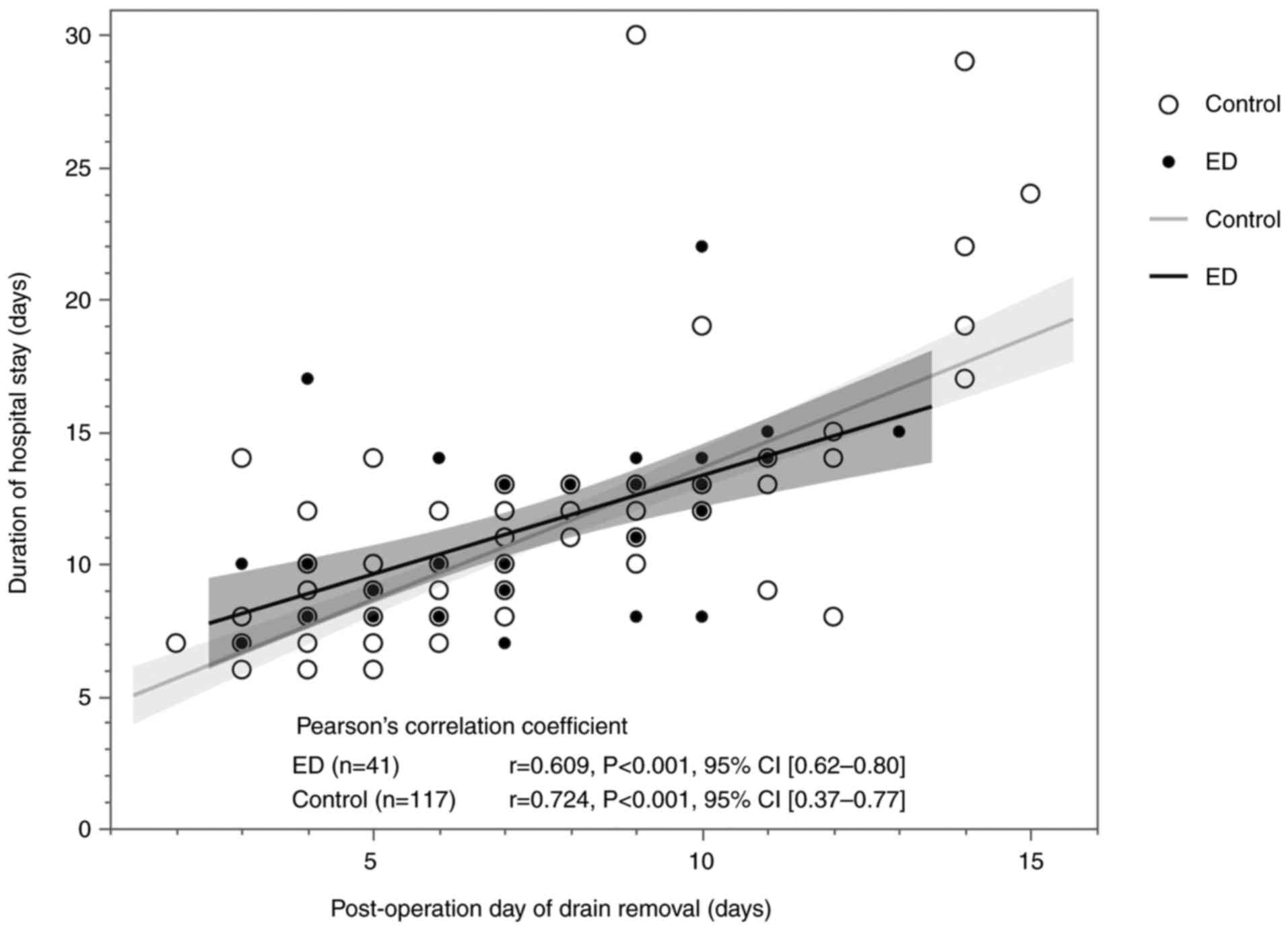
In this study, the Pearson s correlation
coefficient between the drain removal date and length of hospital
stay for the 158 cases analyzed was 0.701, indicating a positive
correlation between the two [95% CI (0.61-0.77)]. When divided into
the ED group (41 cases) and the control group (117 cases), the
respective coefficients were 0.609 and 0.724, with 95% CIs
(0.62-0.80) and (0.37-0.77). Both showed linear and positive
correlations (Fig. 3).
Discussion
This study examined whether incorporating a simple
procedure to seal the pectoralis fascia using an ED could reduce
the amount of drainage and frequency of seroma formation. This
technique is considered straightforward as it involves exposing the
pectoralis muscle during fascia resection and sealing the
microscopic lymphatic vessels within the fascia. Two types of EDs
were used in this study. The first was the LigaSure Exact Dissector
(Covidien Japan, Tokyo, Japan), a radiofrequency ablation device
that coagulates tissue using Joule heat and separates it with an
integrated knife. A randomized controlled study indicated that
using this ED could reduce the time until drain removal and amount
of drainage (8). The second was
Harmonic Focus (Harmonic, Ethicon Inc., Johnson & Johnson,
Tokyo, Japan), an ultrasonic coagulation and dissection device that
uses friction heat engendered by ultrasonic vibrations of the
blade. A meta-analysis reported that its use reduces operating
time, bleeding volume, drainage volume, and hospital stay (9). Both devices are covered by health
insurance in Japan and are widely used in clinical practice.
Furthermore, a recent meta-analysis observed no significant
differences in complications between the two EDs (10), confirming their comparability.
The pectoralis major muscle is the largest muscle
covering the chest wall (11). In
Asians, who are generally smaller than Westerners, the average
width of the pectoralis major muscle was 11.6 cm (12). The extent to which the mammary
gland tissue is separated from the pectoralis major muscle during
total mastectomy depends on the patient s physique; however,
it tends to be extensive. Moreover, lymphatic vessels that
penetrate the pectoralis fascia are not as major a drainage route
as those flowing to the axilla or parasternal region, although
their existence has been documented (13). Therefore, sealing the pectoralis
major fascia may help reduce lymphatic fluid accumulation in the
dead space created during surgery. Overall, numerically the ED
group tended to have lower drainage volumes than the control group,
and a markedly difference was likely detected only on POD 8. On the
other hand, in the ED group, the timing of drains removal was
numerically earlier than the control group. A reduction in drainage
volume after the 8th postoperative day may allow for earlier drain
removal. Therefore, it is suggested that the discharge is possibly
showing a slight decrease, and indirectly leading to anticipated
improvements in clinical course. It is also expected to potentially
contribute to the reduction in length of stay discussed later.
In this study, AE incidence was significantly
reduced in the ED group compared with the control group. Notably,
seroma was the most common AE requiring postoperative intervention
(14,15). Furthermore, surgical site
infections and hematomas were not observed, and ED use may
potentially reduce their frequency. As for seroma, which are the
most common adverse events. With the increasing frequency of breast
cancer surgeries, ED use may help minimize AEs. This study
suggested that extensive sealing due to ED potentially reduces
exudate and decreases seroma formation. Moreover, no disadvantages
were observed in terms of intraoperative blood loss. In addition,
although breast surgeons accustomed to dissecting with
electrocautery may be concerned about the additional time when
using ED, the overall duration of surgery was not extended. Since
ED is a simple technique that can reduce seroma incidence without
any significant disadvantages, such as an increased operative time,
it has potential to reduce patient discomfort, follow-up visits,
and events requiring intervention for complications, while also
alleviate the workload of surgeons.
The precise mechanism by which energy devices reduce
seroma formation remains unclear, but it has been suggested that
they suppress lymphatic fluid retention by sealing vessels. Sealing
integrity of the ED in vessels up to 7 mm approximates the burst
strength of ligation and clips, resists dislodgement, and is
unaffected by proximal thrombus (16,17).
Further investigation of the mechanism is necessary.
Most healthcare organizations allow patients to be
discharged only after drain removal, making the length of hospital
stay closely tied to the duration of drain use (2). Some reports recommend that patients
can be discharged with the drain in places with appropriate support
or that they are seen monitored as outpatients after discharge
(6,18-20).
At our hospital, patients are typically discharged the day after
drain removal. Earlier drain removal was positively correlated with
a shorter hospital stay in this study, and suggests that earlier
drain removal due to the use of ED may reduce the length of
hospital stay, potentially improving healthcare system efficiency
and cost-effectiveness. However, it is also argued that discharge
often occurs due to non-clinical reasons such as social factors
rather than the patient s clinical course. Discharge protocols
vary across studies, contributing to significant heterogeneity
(2).
This study has some limitations. First, this was an
observational study conducted at a single institution with a small
number of patients. And the use of historical controls might have
introduced bias due to time differences. However, precisely because
it is a single-center study, usage and techniques are performed
according to consistent methods. The surgeries were performed by a
well-trained board-certified breast surgeon and the procedures
themselves have not changed significantly. Despite the small sample
size, a clear trend was observed, suggesting that minor adjustments
in surgical technique may help prevent seroma formation. Future
research should involve prospective observational studies across
multiple institutions. Based on the foundational data from this
study, we aim to conduct a multicenter study comparing the use of
ED across various sites, including the axilla and anterior
pectoralis major muscle. Second, the primary focus of this study
was on surgical outcomes rather than oncological outcomes,
necessitating future studies for a more comprehensive
understanding. Nevertheless, definitive oncological resection was
achieved in all patients, with no pathologically positive margins.
Additionally, compared with non-removal, resection of the
pectoralis major fascia remains essential as it significantly
lowers the recurrence rate in the chest wall (3).
In conclusion, this study suggests that using an ED
during pectoralis fascia dissection in total mastectomy may reduce
the AEs incidence requiring the intervention and the incidence of
seroma. This relatively simple procedure has the potential to
enhance the quality of breast surgery. Therefore, further
investigation into the applications of EDs is justified.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors contributions
HS conceived and planned the present study in
detail. YK, KS, AN and AW extracted the entirety of patient data,
performed the data acquisition and inputted the data into the data
platform. NN, SU, DK, TN and TM performed analysis and
interpretation of the patient data with HS. Additionally, DK and TN
revised the manuscript critically for important intellectual
content. TM provided overall supervision and gave final approval
for the publishable version. HS and TN confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study adhered to ethical tenets of The
Declaration of Helsinki and Ethical Principles for Medical Research
Involving Human Subjects, was approved by the Clinical Trial Center
of Sapporo Medical University (approval number 352-93) and is
registered with UMIN-CTR (UMIN000056764). An opt-out consent
process was used, and disclosures were made on the
University s website (https://web.sapmed.ac.jp/byoin/rinshokenkyu/koukai/).
Patient consent for publication
An opt-out consent process was used, and disclosures
were made on the University s website (https://web.sapmed.ac.jp/byoin/rinshokenkyu/koukai/).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang-Petersen C, Sowden M, Chen J, Burns
J and Sprague BL: Changes to the US preventive services task force
screening guidelines and incidence of breast cancer. JAMA Netw
Open. 7(e2452688)2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shima H, Kutomi G, Sato K, Kuga Y, Wada A,
Satomi F, Uno S, Nisikawa N, Kameshima H, Ohmura T, et al: An
optimal timing for removing a drain after breast surgery: A
systematic review and meta-analysis. J Surg Res. 267:267–273.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dalberg K, Johansson H, Signomklao T,
Rutqvist LE, Bergkvist L, Frisell J, Liljegren G, Ambre T and
Sandelin K: A randomized study of axillary drainage and pectoral
fascia preservation after mastectomy for breast cancer. Eur J Surg
Oncol. 30:602–609. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ackroyd R and Reed MWR: A prospective
randomized trial of the management of suction drains following
breast cancer surgery with axillary clearance. The Breast.
6:271–274. 1997.
|
|
5
|
Gupta R, Pate K, Varshney S, Goddard J and
Royle GT: A comparison of 5-day and 8-day drainage following
mastectomy and axillary clearance. Eur J Surg Oncol. 27:26–30.
2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Peeters MJ, Kluit AB, Merkus JW and
Breslau PJ: Short versus long-term postoperative drainage of the
axilla after axillary lymph node dissection. A prospective
randomized study. Breast Cancer Res Treat. 93:271–275.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Seenivasagam RK, Gupta V and Singh G:
Prevention of seroma formation after axillary dissection-a
comparative randomized clinical trial of three methods. Breast J.
19:478–484. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Seki T, Hayashida T, Takahashi M, Jinno H
and Kitagawa Y: A randomized controlled study comparing a vessel
sealing system with the conventional technique in axillary lymph
node dissection for primary breast cancer. Springerplus.
5(1004)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cheng H, Clymer JW, Ferko NC, Patel L,
Soleas IM, Cameron CG and Hinoul P: A systematic review and
meta-analysis of Harmonic technology compared with conventional
techniques in mastectomy and breast-conserving surgery with
lymphadenectomy for breast cancer. Breast Cancer (Dove Med Press).
8:125–140. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Alharran AM, Alenezi YY, Hammoud SM,
Alshammari B, Alrashidi M, Alyaqout FB, Almarri A, Alharran YM,
Alazemi MH, Allafi F and Al*Sadder KA: Efficacy of ligasure versus
harmonic devices in laparoscopic sleeve gastrectomy: A systematic
review and meta-analysis. Cureus. 16(e57478)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gruber RP, Kahn RA, Lash H, Maser MR,
Apfelberg DB and Laub DR: Breast reconstruction following
mastectomy: A comparison of submuscular and subcutaneous
techniques. Plast Reconstr Surg. 67:312–317. 1981.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Baek WY, Byun IH, Kim YS, Jung BK, Yun IS
and Roh TS: Variance of the pectoralis major in relation to the
inframammary fold and the pectoralis minor and its application to
breast surgery. Clin Anat. 30:357–361. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Cieśla1 S, Wichtowski M, Poźniak-Balicka R
and Murawa D: The surgical anatomy of the mammary gland:
Vascularization, innervation, lymphatic drainage, the structure of
the axillary fossa (part 2.). J Oncol. 71:62–69. 2021.
|
|
14
|
He XD, Guo ZH, Tian JH, Yang KH and Xie
XD: Whether drainage should be used after surgery for breast
cancer? A systematic review of randomized controlled trials. Med
Oncol. 28 (Suppl 1):S22–S30. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Droeser RA, Frey DM, Oertli D, Kopelman D,
Peeters MJ, Giuliano AE, Dalberg K, Kallam R and Nordmann A:
Volume-controlled vs no/short-term drainage after axillary lymph
node dissection in breast cancer surgery: A meta-analysis. Breast.
18:109–114. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kennedy JS, Stranahan PL, Taylor KD and
Chandler JG: High-burst-strength, feedback-controlled bipolar
vessel sealing. Surg Endosc. 12:876–878. 1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Timm RW, Asher RM, Tellio KR, Welling AL,
Clymer JW and Amaral JF: Sealing vessels up to 7 mm in diameter
solely with ultrasonic technology. Med Devices (Auckl). 7:263–271.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Parikh HK, Badwe RA, Ash CM, Hamed H,
Freitas R Jr, Chaudary MA and Fentiman IS: Early drain removal
following modified radical mastectomy: a randomized trial. J Surg
Oncol. 51:266–269. 1992.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kopelman D, Klemm O, Bahous H, Klein R,
Krausz M and Hashmonai M: Postoperative suction drainage of the
axilla: For how long? Prospective randomised trial. Eur J Surg.
165:117–120. 1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Clegg-Lamptey JN, Dakubo JC and Hodasi WM:
Comparison of four-day and ten-day post-mastectomy passive drainage
in Accra, Ghana. East Afr Med J. 84:561–565. 2007.PubMed/NCBI View Article : Google Scholar
|