Introduction
Double-filtration plasmapheresis (DFPP) is a blood
purification process that uses specific filters to remove
pathogenic substances from the blood, such as immunoglobulin,
immune complexes, lipoproteins, etc. The DFPP system includes two
parts of filters. At the beginning of the surgery, the blood passes
through the first filter (plasma separator), which is used to
separate white blood cells, red blood cells and platelets from
plasma. Subsequently, the plasma without cellular components passes
through a second filter (plasma component separator) where
macromolecules are selectively removed and discarded (1).
Regional citrate anticoagulation (RCA) is an
anticoagulant regimen used for patients who are at a high risk of
bleeding. It provides excellent anticoagulation without increasing
the risk of bleeding and significantly reduces the occurrence of
clotting in the filter during treatment (2). At present, when RCA is performed for
DFPP, it is generally considered that only the first filter needs
to be anticoagulated, while the risk of coagulation in the second
filter is very rare, and anticoagulation is not required (2,3).
The present study describes the case of a patient
with anti-neutrophil cytoplasmic antibody (ANCA)-associated
vasculitis (AAV) treated with DFPP. During the course of treatment,
when conventional RCA was used for DFPP, the second filter became
clotted. Thus, segmented citrate anticoagulation (SCA) was then
performed and the treatment was successfully completed.
Case report
A 78-year-old Chinese female patient was admitted to
the Department of Cardiology, Chonggang General Hospital
(Chongqing, China), with a 3-day episode of dyspnea. A physical
examination revealed a temperature of 36.3˚C, a breathing rate of
22 breaths/min, a heart rate of 85 beats/min and a blood pressure
of 167/90 mmHg. She had edema of the lower limbs. A computed
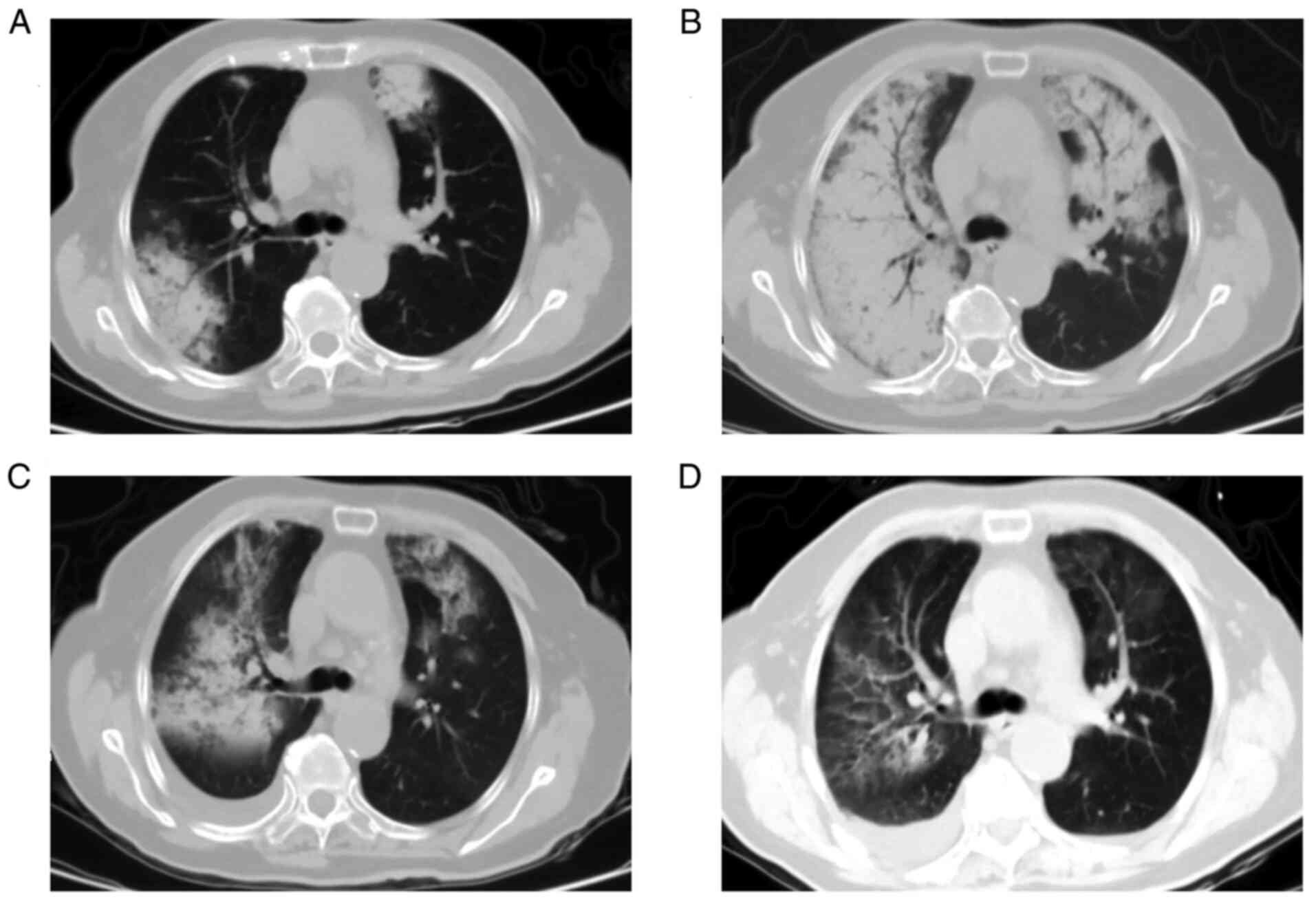
tomography (CT) scan revealed patchy opacities and shadows in the
bilateral lungs (Fig. 1A). The
laboratory test findings of the patient at the time of admission
are presented in Table I.
 | Table ILaboratory data of the patient upon
admission and on the 4th and 25th days after admission. |
Table I
Laboratory data of the patient upon
admission and on the 4th and 25th days after admission.
| Parameter | Upon admission | Day 4 after
admission | Day 25 after
admission |
|---|
| Complete blood
count | | | |
|
Hemoglobin
(g/dl) | 7.1 | 5.0 | 6.4 |
|
White blood
cells (/mm3) |
9.65x103 |
8.21x103 |
7.69x103 |
|
Platelets
(/mm3) |
57x103 |
69x103 |
86x103 |
|
hs-CRP
(mg/l) | 106 | 117 | 4.69 |
| Blood chemistry
tests | | | |
|
Alanine
aminotransaminase (IU/l) | 16 | 17 | 25 |
|
Aspartate
aminotransferase (IU/l) | 16 | 14 | 42 |
|
Total
protein (g/l) | 74.1 | 58.8 | 64.1 |
|
Albumin
(g/l) | 36.0 | 27.5 | 32.7 |
|
Total
cholesterol (mmol/l) | 3.82 | | |
|
Low-density
lipoprotein | 2.1 | | |
|
Cholesterol
(mmol/l) | | | |
|
High-density
lipoprotein | 1.36 | | |
|
Cholesterol
(mmol/l) | | | |
|
Triglycerides
(mmol/l) | 1.29 | | |
|
Scr
(mg/dl) | 5.07 | 5.63 | 3.90 |
|
eGFR
(ml/min/1.73m2) | 8.65 | 7.75 | 11.84 |
| Urinalysis | | | |
|
pH | 6.0 | | 6.0 |
|
Protein | (++) | | (++) |
|
Glucose | (-) | | (-) |
|
Red blood
cells (/U/l) | 503.85 | | 16.92 |
|
Urine
protein to creatine ratio | 647.1 | | 1387.9 |
|
(mg/g
creatinine) | | | |
| Serum
immunological | | | |
|
C3
(g/l) | | 1.1 | |
|
C4
(g/l) | | 0.3 | |
|
P-ANCA | | Negative | |
|
C-ANCA | | Positive | |
|
Myeloperoxidase-ANCA | | Negative | |
|
Proteinase
3-ANCA | | Positive | |
|
Anti-GBM
antibody | | Negative | |
|
Anti-nuclear
antibody | | Negative | |
|
Anti-dsDNA
antibody | | Negative | |
|
Anti-SM
antibody | | Negative | |
The patient had a 2-month history of hypertension
(of which the highest blood pressure value was 150/90 mmHg) and a
2-month history of renal disease (the serum creatinine level was 90
µmol/l, and the estimated glomerular filtration rate was 55
ml/min/1.73 m2). At the time of admission, the patient
was clinically diagnosed with the following: i) Grade 2
hypertension (severe); ii) heart failure (cardiac function grade
III); and iii) chronic kidney disease and renal anemia.
Following admission, the patient received
anti-hypertensive treatment (felodipine) and furosemide in the
Department of Cardiology, Chonggang General Hospital; however, the
symptoms did not markedly improve. On day 3, the patient was
transferred to the Department of Nephrology for further treatment
due to renal insufficiency. Considering that the patient presented
with rapidly deteriorating renal function, severe microscopic
hematuria and lung lesions, Goodpasture syndrome or ANCA-AAV was
suspected.
On day 4, the patient's dyspnea worsened, and
hemoptysis occurred. A re-examination of CT scan revealed the rapid
deterioration of bilateral lung lesions (Fig. 1B). The laboratory findings revealed
that hemoglobin decreased rapidly, renal function deteriorated, and
cytoplasmic pattern ANCA (C-ANCA, analyses performed by the
Guangzhou Kingmed Center For Clinical Laboratory, Co., Ltd.) and
anti-proteinase 3 ANCA (PR3-ANCA, analyses performed by the
Guangzhou Kingmed Center For Clinical Laboratory, Co., Ltd.) test
results were positive. Anti-glomerular basement membrane and
anti-nuclear antibody levels were normal (Table I). Combined with the clinical
manifestations, the imaging examination and laboratory test
results, a diagnosis of AAV was made.
There are currently no validated diagnostic criteria
for AAV and no precise or specific diagnostic tests (4). Classification criteria were established
in 1990 by the American College of Rheumatology (5). The 1994 Chapel Hill Consensus
Conference produced disease definitions for vasculitis and the
definitions were updated in 2012(6);
however, it should be noted that these are classification criteria
and nomenclature definitions, respectively, for use in clinical
trials and teaching.
A positive biopsy is strongly supportive of
vasculitis and the European League Against Rheumatism (EULAR)
recommend the procedure to assist diagnosis and further evaluation
for patients suspected of having vasculitis (7). However, the patient described herein
refused to undergo a tissue biopsy.
Despite the lack of tissue biopsy support, the
patient had a positive test result for a C-ANCA determined using
immunofluorescence and PR3-ANCA specificity determined using ELISA
(performed by the Guangzhou Kingmed Center For Clinical Laboratory,
Co., Ltd.), which has a high sensitivity and specificity for the
diagnosis of AAV (8). In addition,
the patient developed severe hematuria and proteinuria, and the
renal function deteriorated rapidly within two months. Diffuse
hemorrhage occurred in the lungs in a short period of time. Other
diseases, such as systemic lupus erythematosus, anti-glomerular
basement membrane disease and IgA vasculitis are not commonly
associated with the aforementioned symptoms. Therefore, it was
considered that the patient's condition was caused by AAV.
The patient was immediately treated with daily
intravenous methylprednisolone (500 mg) for 3 days. Considering the
critical condition of the patient, DFPP was initiated. The amount
of plasma per treatment was 1.5-fold the plasma volume of the
patient. The pulp waste was 400-500 ml. Sodium citrate (4%) was
used as the anticoagulant with a speed of 150 ml/h in the first
filter and 50 ml/h in the vein ampulla for DFPP. The second filter
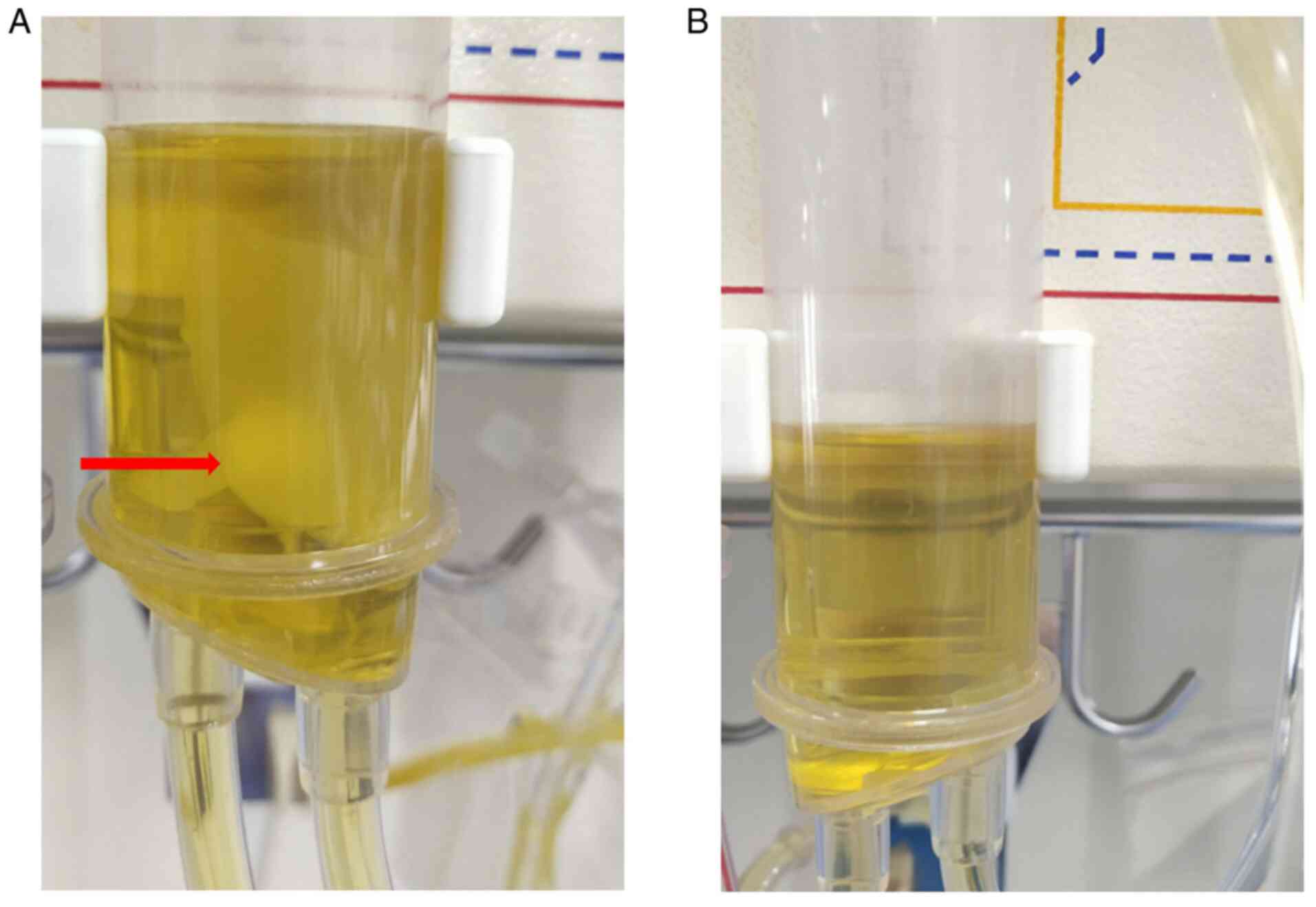
was clogged at ~1.5 h following the commencement of DFPP, and it
was found that a large number of fibrin clots were formed in the
second filter and plasma ampulla (Fig.
2A). As the coagulation of the second filter hindered the
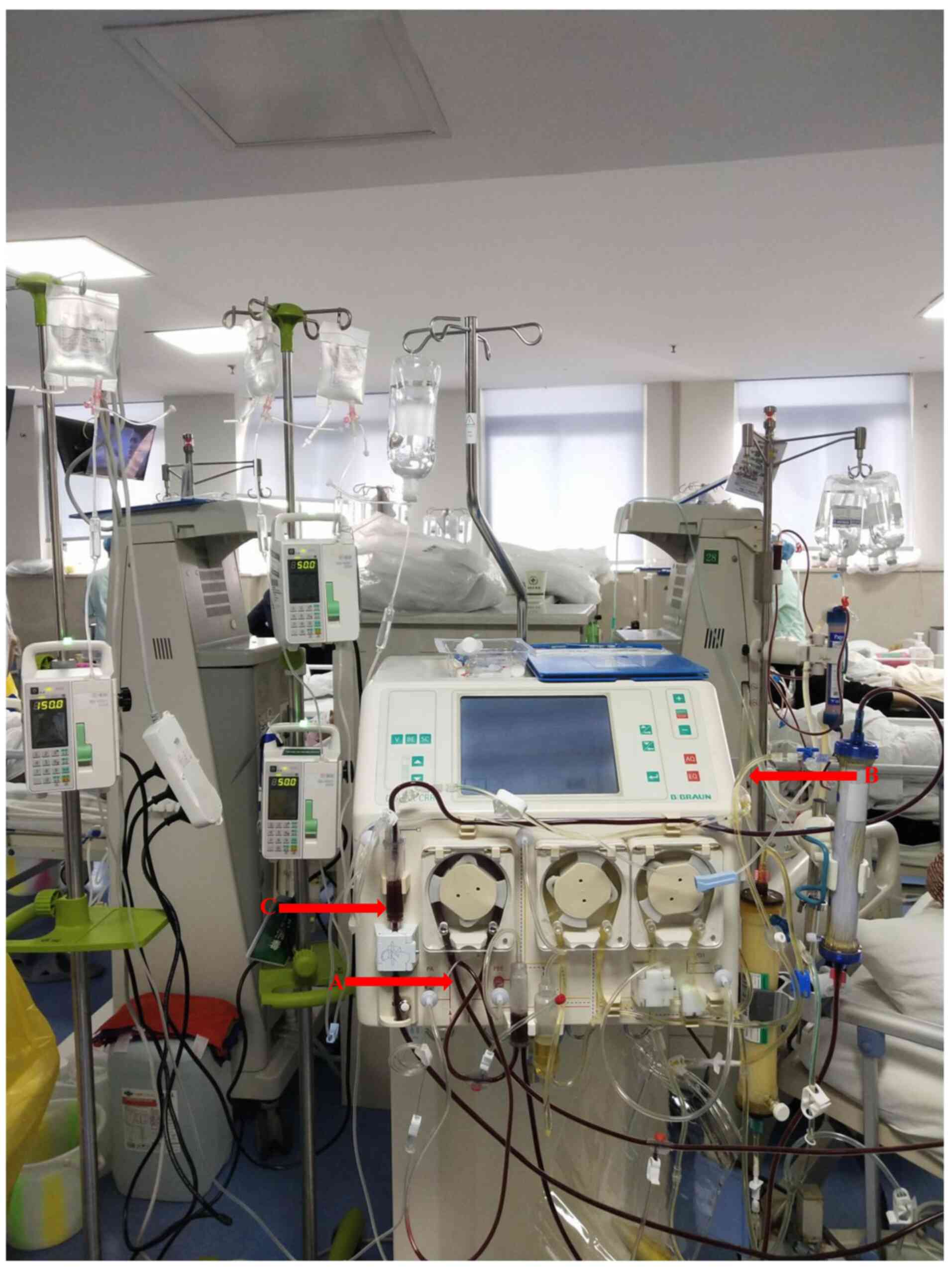
treatment process, citrate was administered to the first filter,
the second filter and vein ampulla at the same time. Sodium citrate
(4%) was administered at a speed of 150 ml/h in the first filter,
50 ml/h in the second filter and 50 ml/h in the vein ampulla
(Fig. 3). After adjusting the
anticoagulant regimen, no coagulation occurred in the second filter
or plasma ampulla (Fig. 2B).
Following the initiation of treatment, 0.9 g calcium
gluconate (10%) was administered intravenously every hour. Fresh,
frozen plasma was added during the treatment to maintain the
stability of blood volume and osmotic pressure. Following
continuous treatment with methylprednisolone and DFPP for 3 days,
the patient's lung lesions markedly improved (Fig. 1C). However, the patient developed
marked water and sodium retention; thus, DFPP was discontinued and
hemodialysis was initiated (three times) to remove the excess water
and sodium from the body. Following methylprednisolone pulse
therapy, intravenous cyclophosphamide (400 mg, every 15 days, 6
times in total) and oral prednisone (60 mg/day for 7 days) were
administered. After 1 week, the prednisone dosage was reduced to 40
mg/day for 7 days; after 1 week, the prednisone dosage was reduced
to 30 mg/day for 15 days; the drug was then tapered by 2.5 mg every
15 days. On the 25th day post-admission, the patient's pulmonary
hemorrhage further improved (Fig.
1D), and her kidney function also partially improved (Table I).
Discussion
AAV is complicated by diffuse alveolar hemorrhage in
~10% of patients, and once AAV is combined with pulmonary
hemorrhage, the risk of mortality significantly increases (9). The MEPEX study demonstrated that
although plasma exchange did not reduce the risk of mortality, it
increased the rate of renal recovery in patients with AAC that
presented with renal failure when compared with intravenous
methylprednisolone alone (10).
Another meta-analysis on 387 patients with AAV demonstrated that
plasma exchange may reduce the risk of progression to end-stage
renal disease or death in renal vasculitis (11). However, the PEXIVAS study found that
plasma exchange did not reduce the incidence of mortality or
end-stage renal disease among patients with severe AAV (12).
Although the efficacy of plasma exchange for AAV is
controversial, the 2021 update of the KDIGO guidelines still
recommends that plasma exchange should be considered in patients
with rapid deterioration of renal function and diffuse alveolar
hemorrhage (13). Therefore, in the
present study, considering the rapid deterioration of renal
function and diffuse alveolar hemorrhage in a short period of time,
DFPP we initiated for the patient.
For DFPP, the first issue to be resolved is the
selection of anticoagulation. As plasma exchange will rapidly
separate blood cells and plasma, it will easily coagulate in the
first filter; thus, some form of anticoagulation is necessary.
Heparin and low-molecular-weight heparin are commonly used
anticoagulant drugs; however, they can affect the coagulation
system (2,3). The patient had a rapid and severe
alveolar hemorrhage (Fig. 1 and
B), and hemoglobin levels also
rapidly decreased (from 71 to 50 g/l in 4 days). At this time, the
use of heparin or low-molecular-weight heparin poses a risk of
aggravating the pulmonary hemorrhage.
The question remains of whether regional
heparinization combining a prefilter dose of heparin with
post-filter neutralization with protamine can be used for
anticoagulation. Previous studies have indicated that regional
heparinization anticoagulation is a simple and safe procedure that
prevents increases in hemofilter transmembrane pressure and
increases circuit lifetime (14,15).
However, in clinical practice, regional heparinization does not
appear to be an ideal choice. First, the use of regional
heparinization for anticoagulation may expose the patient to the
side-effects of both heparin and protamine. Second, heparin has a
much longer half-life than protamine, and it is difficult to
titrate the optimal protamine dose required to neutralize heparin
(16). In view of this, the 2012
KDIGO guidelines for AKI directly recommended that local heparin
anticoagulation should be avoided in patients who are at a high
risk of bleeding and who need continuous renal replacement therapy
(17). Therefore, RCA was selected
for the patient described herein.
Citrate binds to ionized calcium in plasma and
induces local hypocalcemia, which inhibits the conversion of
prothrombin to thrombin, thereby inhibiting activation of the
coagulation cascade in vitro. When the blood is returned to
the patient, the citrate chelating calcium can release the same
amount of ionized calcium through the tricarboxylic acid cycle
metabolic pathway. Losses of calcium ions and citrate-chelating
calcium are then replenished to restore coagulation properties
(3,18-20).
When RCA is used for DFPP, citrate is generally only
administered in the first filter. This is due to the fact that
thrombus formation in the second filter is difficult due to the
absence of platelets and red blood cells in plasma (3). In addition, in the present study,
fresh-frozen plasma was used as the replacement fluid, which also
contains citrate as an anticoagulant. Therefore, in the initial
treatment, anticoagulation was performed only in the first filter
and the vein ampulla, which is also a site prone to coagulation in
extracorporeal circulation devices (21). However, the second filter was clogged
~1.5 h after the first DFPP treatment commenced. When examining the
circuit, it was found that a large number of fibrin clots were
formed in the second filter and plasma ampulla.
For the fibrin clot in the second filter and plasma
ampulla, it was initially speculated that the insufficient dose of
citrate may have caused the ionic calcium level in plasma not to
fall to the appropriate range (0.2-0.4 mmol/l), resulting in
thrombin activation and cleavage of fibrinogen to form a fibrin
clot (22). Indeed, it has been
reported that fibrin clots may be formed when the amount of
anticoagulant is deficient in the collection of blood by
plasmapheresis from normal donors (23). However, in this case, both filters
should be clotted at the same time (the risk of clotting of the
first filter is much greater than that of the second filter), and
no fibrin clots were found in the plasma separated from the first
filter in the case described herein. Therefore, it may be possible
that the membrane surface of the second filter plays a role similar
to platelets and cooperates with calcium ions and other coagulation
factors to form prothrombin activators, resulting in the formation
of fibrin clots. Therefore, citrate was administered to the first
filter, second filter and vein ampulla at the same time. Following
the adjustment of the anticoagulation regimen, no fibrin clots were
formed during the subsequent treatment.
It should be emphasized that this is the first time
SCA was used for DFPP, at least to the best of our knowledge. In
addition, there are no scientific reports on the use of SCA in
DFPP; thus, determining the dose of citric acid is difficult. A
research team proposed a new protocol that enables the
individualization of the use of RCA during therapeutic plasma
exchange: CiWB=(1-Hct) x (8.035 x pCai3-22.89 x pCai2+24.68 x
pCai-5.361), where CiWB represents the whole blood citrate
concentration in mmol/l, Hct represents the hematocrit, and pCai
represents the plasma concentration of ionized calcium in mmol/l)]
(24). However, this method is not
suitable for DFPP, and the calculation process is cumbersome.
Therefore, we refer to the protocol for RCA in critically ill
patients undergoing continuous renal replacement therapy; that is,
citric acid is infused at a fixed speed (25,26).
Combined with the authors' own experience in using citric acid, the
citric acid dose was set at 150 ml/h [first filter, 1.2-fold the
blood flow rate (130 ml/min)], 50 ml/h [second filter, 0.4-fold the
blood flow rate (130 ml/min)] and 50 ml/h [vein ampulla, 0.4-fold
the blood flow rate (130 ml/min)].
During the course of treatment, certain unexpected
evets occurred due to the lack of experience. First, the use of SCA
increases the input of citrate, which poses the risk of overloading
water and sodium. In fact, after the third DFPP treatment, severe
sodium and water retention occurred (Table II). Thus, emergency dialysis had to
be initiated to relieve the water overload. Second, a large amount
of citrate input will increase the risk of hypocalcemia or
metabolic alkalosis (24). Although
there was no severe hypocalcemia following the calcium
supplementation, the patient developed an alkaline overload due to
the use of citrate (Table II).
Therefore, the changes in serum calcium and bicarbonate in patients
need to be strictly monitored to adjust the dose of citrate over
time.
 | Table IIElectrolyte changes before and after
DFPP. |
Table II
Electrolyte changes before and after
DFPP.
| Electrolyte | Before DFPP | After the first
DFPP | After the second
DFPP | After the third
DFPP |
|---|
| Serum calcium
(mmol/l) | 1.91 | 1.96 | 2.06 | 2.02 |
| Serum potassium
(mmol/l) | 4.52 | 3.72 | 3.32 | 3.37 |
| Serum sodium
(mmol/l) | 131.8 | 137.8 | 147.5 | 155.3 |
| Serum chlorine
(mmol/l) | 98.9 | 95.1 | 92.2 | 94.7 |
| Bicarbonate
(mmol/l) | 15.90 | 25.10 | 36.65 | 43.70 |
When a patient requires DFPP for treatment, it often
means that the patient is in critical condition. At this time, the
use of SCA can not only ensure the successful completion of DFPP,
but may also avoid the bleeding tendency caused by the use of
heparin or low-molecular-weight heparin. There is a risk of sodium
and water retention, as well as hypocalcemia and metabolic
alkalosis when performing SCA. However, the hazard caused by
citrate can be reduced through appropriate measures, such as the
use of diuretics, calcium supplements or lowering the dose of
citrate, and if necessary, blood purification.
In conclusion, to the best of our knowledge, the
present reports for the first time that SCA can be used for
anticoagulation in DFPP. The regimen can more effectively prevent
clogging of the first and second filters, thereby maintaining the
continuity of treatment and avoiding treatment interruption and
increased treatment costs caused by filter replacement.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JH, CM and FW conceived the case report, wrote the
initial manuscript and reviewed the final manuscript. JH
interpreted and created the images and reviewed the final
manuscript. All authors have read and approved the final
manuscript. JH, CM and FW confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The patient provided informed consent for her
involvement in the present study.
Patient consent for publication
The patient provided informed consent for the
publication of all the relevant clinical data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marlu R, Malvezzi P, Seyve L, Jouve T,
Maurizi J, Defendi F, Carron PL, Christophe M, Le Gouellec A,
Polack B and Rostaing L: Effect of double-filtration plasmapheresis
for antibody-mediated rejection on hemostasis parameters and
thrombin generation. Thromb Res. 166:113–121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stucker F, Ponte B, Tataw J, Martin PY,
Wozniak H, Pugin J and Saudan P: Efficacy and safety of
citrate-based anticoagulation compared to heparin in patients with
acute kidney injury requiring continuous renal replacement therapy:
A randomized controlled trial. Crit Care. 19(91)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Peng X, Xie X, Yin J, Yu S, Zhang C and
Zhao S: Anticoagulation effect and safety observation of regional
citrate anticoagulation for double-filtration plasmapheresis in
critical patients. Blood Purif. 49:542–549. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mcgeoch L, Twilt M, Famorca L, Bakowsky V,
Barra L, Benseler SM, Cabral DA, Carette S, Cox GP, Dhindsa N, et
al: CanVasc recommendations for the management of antineutrophil
cytoplasm antibody-associated vasculitides. J Rheumatol. 43:97–120.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bloch DA, Michel BA, Hunder GG, McShane
DJ, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Fries JF,
Leavitt RY, et al: The American College of Rheumatology 1990
criteria for the classification of vasculitis, patients and
methods. Arthritis Rheum. 33:1068–1073. 1990.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jennette JC, Falk RJ, Bacon PA, Basu N,
Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen
EC, et al: 2012 revised international Chapel Hill consensus
conference nomenclature of vasculitides. Arthritis Rheum. 65:1–11.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yates M, Watts RA, Bajema IM, Cid MC,
Crestani B, Hauser T, Hellmich B, Holle JU, Laudien M, Little MA,
et al: EULAR/ERA-EDTA recommendations for the management of
ANCA-associated vasculitis. Ann Rheum Dis. 75:1583–1594.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Choi HK, Liu SM, Merkel PA, Colditz GA and
Niles JL: Diagnostic performance of antineutrophil cytoplasmic
antibody tests for idiopathic vasculitides: Metaanalysis with a
focus on antimyeloperoxidase antibodies. J Rheumatol. 28:1584–1590.
2001.PubMed/NCBI
|
|
9
|
Mohammad AJ, Mortensen KH, Babar J, Smith
R, Jones RB, Nakagomi D, Sivasothy P and Jayne DRW: Pulmonary
involvement in antineutrophil cytoplasmic antibodies
(ANCA)-associated vasculitis: The influence of ANCA Subtype. J
Rheumatol. 44:1458–1467. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jayne DR, Gaskin G, Rasmussen N,
Abramowicz D, Ferrario F, Guillevin L, Mirapeix E, Savage CO,
Sinico RA, Stegeman CA, et al: Randomized trial of plasma exchange
or high-dosage methylprednisolone as adjunctive therapy for severe
renal vasculitis. J Am Soc Nephrol. 18:2180–2188. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Walsh M, Catapano F, Szpirt W, Thorlund K,
Bruchfeld A, Guillevin L, Haubitz M, Merkel PA, Peh CA, Pusey C and
Jayne D: Plasma exchange for renal vasculitis and idiopathic
rapidly progressive glomerulonephritis: A meta-analysis. Am J
Kidney Dis. 57:566–574. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Walsh M, Merkel PA, Peh CA, Szpirt WM,
Puéchal X, Fujimoto S, Hawley CM, Khalidi N, Floßmann O, Wald R, et
al: Plasma exchange and glucocorticoids in severe ANCA-Associated
vasculitis. N Engl J Med. 382:622–631. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kidney Disease: Improving Global Outcomes
(KDIGO) Glomerular Diseases Work Group: KDIGO 2021 clinical
practice guideline for the management of glomerular diseases.
Kidney Int. 100:S1–S276. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fabbri LP, Nucera M, Malyan MA and Becchi
C: Regional anticoagulation and antiaggregation for CVVH in
critically ill patients: A prospective, randomized, controlled
pilot study. Acta Anaesthesiol Scand. 54:92–97. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Biancofiore G, Esposito M, Bindi L,
Stefanini A, Bisà M, Boldrini A, Consani G, Filipponi F and Mosca
F: Regional filter heparinization for continuous veno-venous
hemofiltration in liver transplant recipients. Minerva Anestesiol.
69:527–534, 534-8. 2003.PubMed/NCBI(In English, Italian).
|
|
16
|
Carr JA and Silverman NJ: The
heparin-protamine interaction. A review. Cardiovasc Surg (Torino).
40:659–666. 1999.PubMed/NCBI
|
|
17
|
KDIGO AKI: Kidney Disease Improving Global
Outcomes. AKI Work Group: Clinical Practice Guideline for Acute
Kidney Injury. Kidney International, pp1-138, 2012.
|
|
18
|
Gattas DJ, Rajbhandari D, Bradford C, Buhr
H, Lo S and Bellomo R: A randomized controlled trial of regional
citrate versus regional heparin anticoagulation for continuous
renal replacement therapy in critically Ill adults. Crit Care Med.
43:1622–1629. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Buturovic-Ponikvar J: Is regional citrate
anticoagulation the future of hemodialysis? Ther Apher Dial.
20:234–239. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Janssen MJ, Huijgens PC, Bouman AA, Oe PL
and van der Meulen J: Citrate anticoagulation and divalent cations
in hemodialysis. Blood Purif. 12:308–316. 1994.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Webb AR, Mythen MG, Jacobson D and Mackie
IJ: Maintaining blood flow in the extracorporeal circuit:
Haemostasis and anticoagulation. Intensive Care Med. 21:84–93.
1995.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Brandl M, Strobl K, Hartmann J, Kellner K,
Posnicek T and Falkenhagen D: A target-orientated algorithm for
regional citrate-calcium anticoagulation in extracorporeal
therapies. Blood Purif. 33:7–20. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kang JW, Seo DH, Oh YC, LEE HJ and KIM SI:
A case of a large fibrin clot formed in a plama unit collected by
donor plasmapheresis. Korean J Blood Trans. 11:183–187. 2000.
|
|
24
|
Kissling S, Legallais C, Pruijm M, Teta D,
Vogt B, Burnier M, Rondeau E and Ridel C: A new prescription model
for regional citrate anticoagulation in therapeutic plasma
exchanges. BMC Nephrol. 18(81)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yin Z, Xu Z, Zhu Q, Liu J, Qian J, You H,
Gu Y, Hao C, Jiao Z and Ding F: Citrate pharmacokinetics in
critically Ill patients with acute kidney injury. PLoS One.
8(e65992)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wen M, Küchle C, Steubl D, Satanovskji R,
Heemann U, Suttmann Y, Angermann S, Kemmner S, Rehbehn L, Huber M,
et al: A novel citrate-based protocol versus heparin
anticoagulation for sustained low-efficiency dialysis in the ICU:
Safety, efficacy, and cost. BMC Nephrol. 19(79)2018.PubMed/NCBI View Article : Google Scholar
|