Introduction
Hemangiomas and vascular malformations (VMs) in the
head and neck regions have traditionally been referred to as
‘hemangiomas.’ Currently, the International Society for the Study
of Vascular Anomalies (ISSVA) classifies conventional cavernous
hemangiomas as VMs (1). VMs in the
head and neck region account for ~40% of all VMs, with an incidence
of 1:5,000 to 1:10,000(2). The
treatment options for VM include surgery, sclerotherapy, and laser
therapy (1). Usually, surgical
resection cannot be performed without causing functional or
aesthetic impairment and may only be suitable for small localized
lesions. Therefore, sclerotherapy has become a valuable alternative
to the surgical resection of VMs in the maxillofacial region
(3). Compared with conventional
liquid sclerosing agents, foam sclerotherapy has been reported to
be more effective at low concentrations and in small doses, thereby
reducing complications and enabling safe and effective treatment
(4). Although there have been some
reports (5,6) on the effectiveness of polidocanol
sclerotherapy without foam for VMs in the maxillofacial region, to
the best of our knowledge, no study to date has evaluated the
effectiveness and safety of foam sclerotherapy using only foam
polidocanol for VMs in the oral and maxillofacial region. The
present study thus aimed to examine its therapeutic effectiveness
in patients with oral VMs (OVMs).
Patients and methods
Study population and ethical
approval
The present study retrospectively examined the
medical records of all patients treated between January, 2018 and
March, 2023 at Kyoto University Hospital (Kyoto, Japan) who
underwent foam sclerotherapy with polidocanol for VMs of the oral
and maxillofacial regions. Patients who received foam polidocanol
sclerotherapy outside the aforementioned hospital were excluded
from the study. Foam sclerotherapy with polidocanol was used when
surgical treatment would lead to extensive resection and possible
functional or aesthetic impairment or when the patient preferred
non-surgical treatment. Data regarding sex, age at the time of the
initial visit, and clinical and photographic findings were
collected. All patients underwent magnetic resonance imaging (MRI)
to assess the extent and distribution of the lesions. The diagnosis
of VM was based on history and clinical and MRI findings.
The present study was approved by the Kyoto
University Graduate School and Faculty of Medicine Ethics Committee
(Approval no. R 3495) and was conducted in accordance with the
principles outlined in the Declaration of Helsinki. Informed
consent was obtained from all patients and, in the case of minor
patients, from their parents through an opt-out option on the
website of the department of Oral and Maxillofacial Surgery
(https://oms.kuhp.kyoto-u.ac.jp).
Process of foam sclerotherapy with
polidocanol
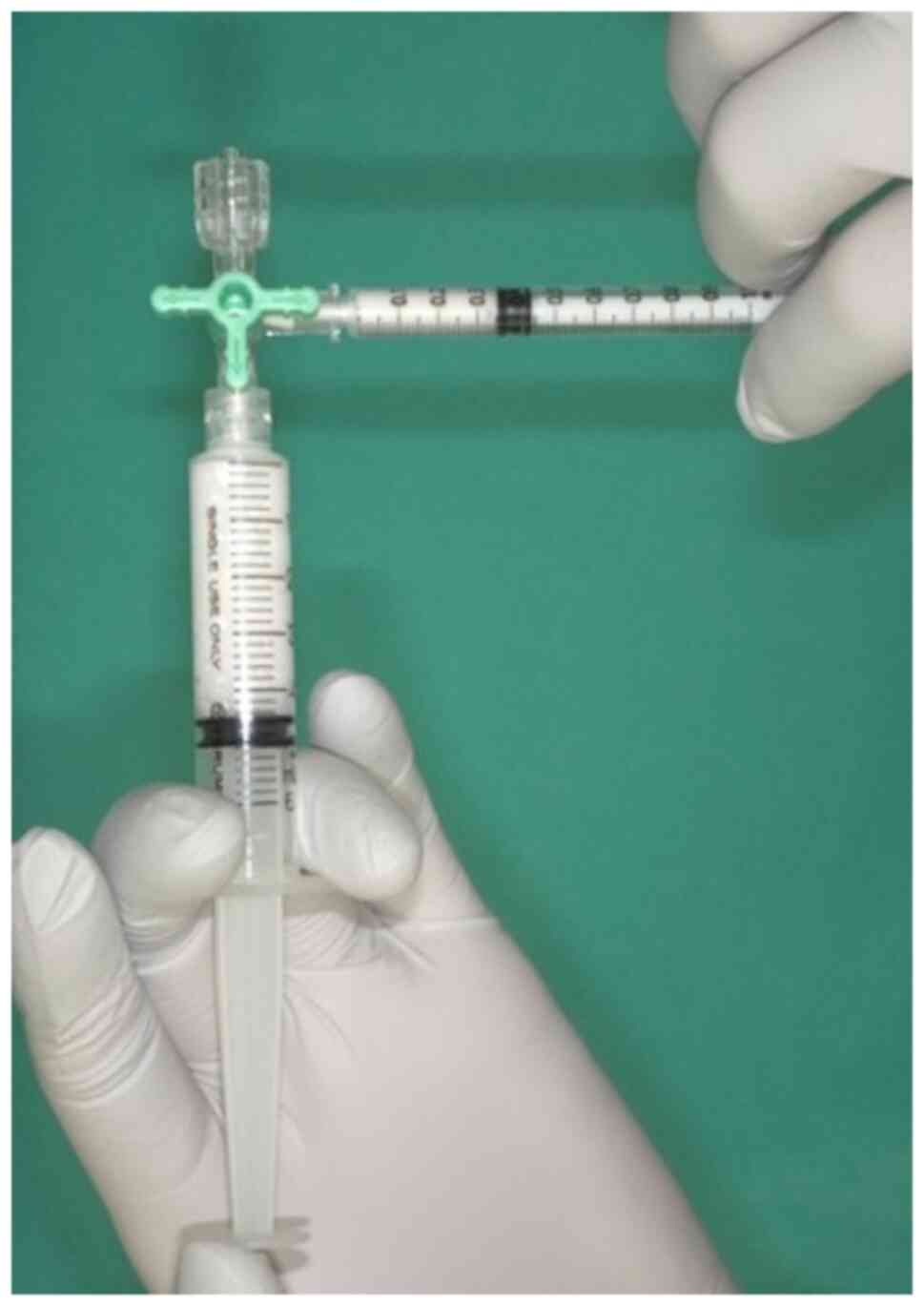
The curing material used was 1% polidocanol
(Chemical Factory Kreussler & Co. GmbH), obtained by mixing 1
ml polidocanol with air in a 1:4 ratio in two syringes attached to
a 3-way stopcock, as previously reported in the study by Tessari
et al (6) (Fig. 1). The resulting foam polidocanol was
then administered via a 22 G needle into the VM. The same site was
used during each treatment session, which was spaced at least 2
months apart. This treatment interval was selected in order to
allow for an accurate assessment of the disappearance of swelling
directly associated with sclerotherapy and to ensure the precise
evaluation of the clinical condition of patients. Sclerotherapy was
conducted at a maximum dosage of 2 mg/kg and terminated when the
lesions completely disappeared upon a visual examination or upon
patient satisfaction. The number of sclerotherapy sessions and
post-treatment complications were recorded. Complications did not
include acute swelling of the treated lesion or transient pain
exacerbation following sclerotherapy, as polidocanol typically
causes inflammation in and around venous malformations. Patients
with transient difficulty opening their mouths following
sclerotherapy were also excluded. The treatment response was
retrospectively evaluated by two surgeons, one oral surgeon, and
one plastic surgeon, using photographs and chart entries in the
medical records at follow-up.
Data analysis
Patients with the complete resolution of the OVM
were considered to have a complete response (CR), those with a
reduction in size from the initial diagnosis were considered to
have a partial response (PR), those with no change in size were
considered to have stable disease (SD), and those with an increase
in size were considered to have progressive disease (PD).
Statistical analysis
Characteristics were examined for all participants,
and group differences were assessed based on therapy evaluation,
with participants categorized into the CR and PR groups.
Differences in numerical variables (age and drug frequency) between
the subgroups were determined using a Student's t-test. The
difference in a nominal variable (sex) between the subgroups was
determined using a Fisher's exact test. A two-tailed P-value
<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using JMP 16.0
statistical software (SAS Institute).
Results
A total of 16 patients, comprising 4 male and 12
female patients, underwent foam sclerotherapy with polidocanol. The
mean age of the patients was 46.8 years (range, 9-80 years). Among
the patients, 4 patients had OVMs on multiple sites in the oral and
maxillofacial regions, while 12 patients had OVMs on a single site,
resulting in a total of 22 sclerotherapy sites. The following sites
were observed: A total of eight OVM sites were on the lips, six
were on the tongue, six on the buccal mucosa, and one each on the
masseter muscle and pharynx. Notably, the VMs were superficial
apart from those in the pharynx and masseter area. The average
number of polidocanol doses was four (ranging from one to nine
doses). The treatment outcome was CR in 6 cases and PR in 10 cases;
no patients were found to have SD or PD. Other than pain and
swelling at the injection site, no severe side effects, such as
circulatory dynamic fluctuations or skin necrosis, were observed in
the patients. In addition, no adverse events, such as blood clots,
were noted (Table I). Statistically
and descriptively, no obvious differences were found in various
factors, including age and the site of VM occurrence, by therapy
evaluation (Table II).
 | Table IPatients with OVMs treated with foam
polidocanol sclerotherapy. |
Table I
Patients with OVMs treated with foam
polidocanol sclerotherapy.
| Patient no. | Age, years | Sex | Site | Side | Size (mm) | Dosing frequency
(times) | Therapy
evaluation |
|---|
| 1 | 11 | F | Lip | R | 15 | 8 | PR |
| | | | Cheek | | | | |
| 2 | 77 | M | Tongue | L | 90 | 9 | PR |
| | | | Cheek | | | | |
| | | | Lip | | | | |
| 3 | 49 | F | Lip | R | 12 | 5 | CR |
| 4 | 71 | F | Tongue | R | 25 | 4 | CR |
| 5 | 30 | F | Masseter | L | 27 | 3 | CR |
| 6 | 33 | F | Lip | R | 30 | 3 | CR |
| 7 | 9 | F | Cheek | L | 52 | 5 | PR |
| 8 | 32 | F | Lip | R | | 7 | PR |
| | | | Tongue | | | | |
| 9 | 17 | F | Lip | R | 60 | 2 | PR |
| 10 | 76 | F | Lip | L | 5 | 1 | CR |
| 11 | 78 | M | Lip | R | 20 | 4 | PR |
| 12 | 53 | M | Tongue | B | 35 | 5 | PR |
| 13 | 54 | F | Cheek | L | 75 | 4 | PR |
| | | | Tongue | | | | |
| | | | Pharyngeal | | | | |
| 14 | 50 | F | Cheek | R | 12 | 2 | PR |
| 15 | 80 | M | Tongue | L | 10 | 1 | CR |
| 16 | 29 | F | Cheek | R | 14 | 1 | PR |
 | Table IIDifferences in parameters according to
the therapy evaluation. |
Table II
Differences in parameters according to
the therapy evaluation.
| | Therapy
evaluation | |
|---|
| Parameter | CR, n=6
(37.5%) | PR, n=10
(62.5%) | P-value |
|---|
| Age (years) | 56.5±22.2 | 41.0±25.4 | 0.236 |
| Sex (n, %) | | | NS |
|
Male | 1 (25.0) | 3 (75.0) | |
|
Female | 5 (41.7) | 7 (58.3) | |
| Drug frequency
(times) | 2.8±1.6 | 4.7±2.7 | 0.145 |
| Side (n, %) | | | |
|
Right | 3 (30.0) | 7 (70.0) | |
|
Left | 3 (42.9) | 4 (57.1) | |
|
Both | 0 (0.0) | 1 (100.0) | |
| Site (n, %) | | | |
|
Lip | 3 (37.5) | 5 (62.5) | |
|
Buccal
mucosa | 0 (0.0) | 5 (100.0) | |
|
Tongue | 2 (28.6) | 5 (71.4) | |
|
Masseter | 1 (100.0) | 0 (0.0) | |
|
Pharyngeal | 0 (0.0) | 1 (100.0) | |
Discussion
Hemangiomas are relatively common in the
maxillofacial regions. The majority of hemangiomas are considered
vascular malformations that do not exhibit neoplastic proliferation
of blood vessel-derived cells and cause local dysfunction,
bleeding, and aesthetic issues (7).
In 1982, Mulliken and Glowacki (8)
examined the presence of endothelial cell proliferation in diseases
previously considered as hemangiomas and performed a
histopathological classification of these diseases. They classified
conventional hemangiomas into two groups as follows: i) Those with
proliferative and quiescent phases of endothelial cells and ii)
those without proliferation of endothelial cells and with a normal
cell cycle. Vascular malformations were further subclassified into
arteriovenous, capillary, venous, and lymphatic. Currently, the
ISSVA classifies ‘hemangiomas’ and ‘vascular malformations’ as
separate disease entities (1).
The treatment for VMs in the oral cavity includes
surgery, sclerotherapy, and laser therapy (1). Surgical resection can result in
functional and aesthetic impairments. By contrast, sclerotherapy is
a minimally invasive treatment option for vascular malformations
that can be treated without causing functional or aesthetic
impairment (3). Sclerosing agents
damage vascular endothelial cells, causing thrombosis and
subsequent fibrosis (7). Anhydrous
ethanol, ethanolamine oleate, and polidocanol are frequently used
as sclerosing agents (9). A previous
study reported that anhydrous ethanol had the lowest recurrence
rate and was the most effective sclerosing agent (10). However, several complications
associated with anhydrous ethanol sclerotherapy have been reported,
and these include tissue necrosis, peripheral nerve damage, cardiac
arrhythmia, and pulmonary embolism (11). Monoethanolamine oleate has excellent
thrombogenic potential but needs to be administered under
fluoroscopic guidance, as leakage from the lesion can cause
hemolysis and acute renal failure (5).
Polidocanol is a non-ionic surfactant that lyses the
endothelial layer via absorption into the cell membrane (12). Polidocanol has been reported to be
effective in ~90% of OVMs and can be used for sclerotherapy without
unique administration methods with a limited number of
complications (5). Although
polidocanol is associated with relatively fewer complications than
other sclerosing agents, Marrocco-Trischitta et al (13) reported cases of cardiac arrest when
polidocanol was used in pediatric patients, suggesting the need for
careful administration when used in children.
In the present study, all patients achieved CR and
PR. However, multiple doses are necessary to achieve a sufficient
response for extensive lesions. Therefore, multiple treatment
cycles required for sclerotherapy with foam polidocanol are a vital
drawback to consider. To minimize the number of sclerotherapy and
achieve complete disappearance of the lesion, a possible treatment
strategy would be to use sclerotherapy to reduce the VM to a size
that does not compromise functionality and esthetics, followed by
surgical intervention.
Foam sclerotherapy is a type of sclerotherapy
wherein a sclerosing agent is mixed with air or carbon dioxide to
form a froth. This technique was first used by McAusland (14) in 1939 to treat telangiectasias by
shaking the sclerosing agent violently in a rubber stopper bottle
to form a froth. In 1944, Orbach (15) reported foam sclerotherapy as an
‘air-block technique’. Compared with conventional liquid sclerosing
agents, foam sclerotherapy has been reported to be more effective
at low concentrations and in small doses, thus reducing
complications and enabling safe and effective treatment (4). The smaller the foam formed, the more
effective the treatment (16).
According to the method described in the study by Tessari et
al (6), a 1:4 ratio of hardener
solution to air is the optimal mixture, and pumping >20 times
does not affect foam formation.
Cabrera et al (17) reported that foam sclerotherapy with
polidocanol and carbon dioxide was effective in 92% of patients and
eliminated VMs in 36% of patients. Yamaki et al (18) conducted a randomized controlled trial
of liquid sclerotherapy with polidocanol and ethanolamine oleate
vs. foam sclerotherapy with polidocanol and ethanolamine oleate.
They reported that foam sclerotherapy was significantly more
effective than liquid sclerotherapy, and significantly fewer
sclerosing agents were used in the former. Sclerotherapy with
polidocanol foam effectively achieved CR and PR in patients without
any severe complications. However, the present study did not
include cases involving infants. Thus, further studies are required
to explore the efficacy and safety of polidocanol sclerotherapy in
infants.
Given that polidocanol causes inflammatory reactions
in and around VMs, MRI imaging was not performed due to concerns
that accurate evaluation would not be possible as it would detect
not only the reduced venous malformation but also the surrounding
inflammatory reactions. Future considerations should focus on
refining image evaluation methods.
The limitations of the present study include the
small sample size, the lack of pediatric indications and
radiological evaluation of lesion size, and the lack of a defined
follow-up period. In the future, the authors aim to increase the
number of patients and conduct a multicenter collaborative
study.
In conclusion, the present study evaluated the
efficacy and safety of foam sclerotherapy with polidocanol for VMs
in the oral and maxillofacial region, demonstrating it as an
effective and safe treatment modality. However, further multicenter
collaborative studies are crucial in order to obtain more
comprehensive information.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analyzed in the current study
are available from the corresponding author upon reasonable
request.
Authors' contributions
TK and YK acquired the clinical data of the
patients, performed the search for relevant literature and edited
the manuscript. TK, YK, SF, TW, SY, KN and NM contributed
substantially to the conception and design of the study. NM, KN,
TW, SY and SF acquired data and provided clinical advice. TK, YK
and SF revised the manuscript. TK had a significant role in writing
the manuscript. TK and SF performed the statistical analyses. All
the authors have read and approved the final version of the
manuscript. TK, YK and SF confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The Kyoto University Graduate School and Faculty of
Medicine Ethics Committee (Kyoto, Japan) approved the present study
(approval no. R3495-2). Informed consent was obtained from all
patients and, in the case of minor patients, from their parents
through an opt-out option on the website of the department of Oral
and Maxillofacial Surgery (https://oms.kuhp.kyoto-u.ac.jp).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mimura H, Akita S, Fujino A, Jinnin M,
Ozaki M, Osuga K, Nakaoka H, Morii E, Kuramochi A, Aoki Y, et al:
Japanese clinical practice guidelines for vascular anomalies 2017.
J Dermatol. 47:e138–e183. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zheng JW, Mai HM, Zhang L, Wang YA, Fan
XD, Su LX, Qin ZP, Yang YW, Jiang YH, Zhao YF and Suen JY:
Guidelines for the treatment of head and neck venous malformations.
Int J Clin Exp Med. 6:377–389. 2013.PubMed/NCBI
|
|
3
|
Berenguer B, Burrows PE, Zurakowski D and
Mulliken JB: Sclerotherapy of craniofacial venous malformations:
Complications and results. Plast Reconstr Surg. 104:1–11;
discussion 12-5. 1999.PubMed/NCBI
|
|
4
|
Fukuzawa S, Yamagata K, Okubo-Sato M,
Terada K, Uchida F, Ishibashi-Kanno N and Bukawa H: Therapeutic
effect of polidocanol sclerotherapy on oral vascular malformations.
Dent J (Basel). 9(119)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ishikawa K, Sasaki S, Furukawa H, Maeda T,
Miura T, Sasaki Y, Yamamoto Y and Funayama E: Effectiveness and
safety of percutaneous sclerotherapy using absolute ethanol and/or
polidocanol for maxillofacial venous malformations involving the
masticatory muscles: A case series. Oral Surg Oral Med Oral Pathol
Oral Radiol. 135:355–362. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tessari L, Cavezzi A and Frullini A:
Preliminary experience with new sclerosing foam in the treatment of
varicose veins. Dermatol Surg. 27:58–60. 2001.PubMed/NCBI
|
|
7
|
Wiegand S, Eivazi B, Zimmermann AP,
Sesterhenn AM and Werner JA: Sclerotherapy of lymphangiomas of the
head and neck. Head Neck. 33:1649–1655. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mulliken JB and Glowacki J: Hemangiomas
and vascular malformations in infants and children: A
classification based on endothelial characteristics. Plast Reconstr
Surg. 69:412–422. 1982.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Horbach SE, Lokhorst MM, Saeed P, de
Goüyon Matignon de Pontouraude CM, Rothová A and van der Horst CM:
Sclerotherapy for low-flow vascular malformations of the head and
neck: A systematic review of sclerosing agents. J Plast Reconstr
Aesthet Surg. 69:295–304. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee BB, Kim DI, Huh S, Kim HH, Choo IW,
Byun HS and Do YS: New experiences with absolute ethanol
sclerotherapy in the management of a complex form of congenital
venous malformation. J Vasc Surg. 33:764–772. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li J, Chen J, Zheng G, Liao G, Fu Z, Li J,
Zhang T and Su Y: Digital subtraction angiography-guided
percutaneous sclerotherapy of venous malformations with
pingyangmycin and/or absolute ethanol in the maxillofacial region.
J Oral Maxillofac Surg. 68:2258–2266. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Parsi K: Interaction of detergent
sclerosants with cell membranes. Phlebology. 30:306–315.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Marrocco-Trischitta MM, Guerrini P, Abeni
D and Stillo F: Reversible cardiac arrest after polidocanol
sclerotherapy of peripheral venous malformation. Dermatol Surg.
28:153–155. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
McAusland S: The modern treatment of
varicose veins. Med press circular. 201:404–410. 1939.
|
|
15
|
Orbach EJ: Sclerotherapy of varicose
veins:utilization of an intravenous sir block. Am J Surg.
66:362–366. 1944.
|
|
16
|
Frullini A and Cavezzi A: Sclerosing foam
in the treatment of varicose veins and telangiectases: History and
analysis of safety and complications. Dermatol Surg. 28:11–15.
2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cabrera J, Cabrera J Jr, Garcia-Olmedo MA
and Redondo P: Treatment of venous malformations with sclerosant in
microfoam form. Arch Dermatol. 139:1409–1416. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yamaki T, Nozaki M, Sakurai H, Takeuchi M,
Soejima K and Kono T: Prospective randomized efficacy of
ultrasound-guided foam sclerotherapy compared with
ultrasound-guided liquid sclerotherapy in the treatment of
symptomatic venous malformations. J Vasc Surg. 47:578–584.
2008.PubMed/NCBI View Article : Google Scholar
|